Abstract
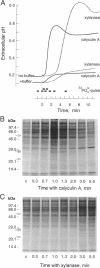
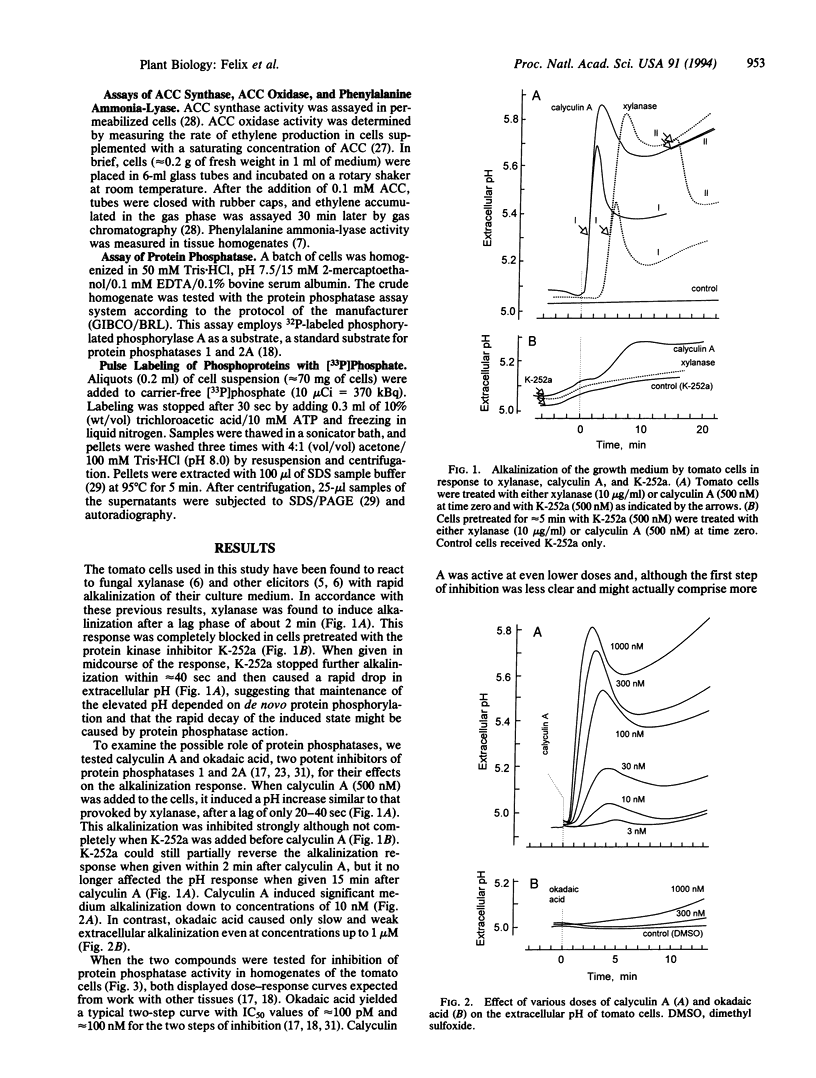
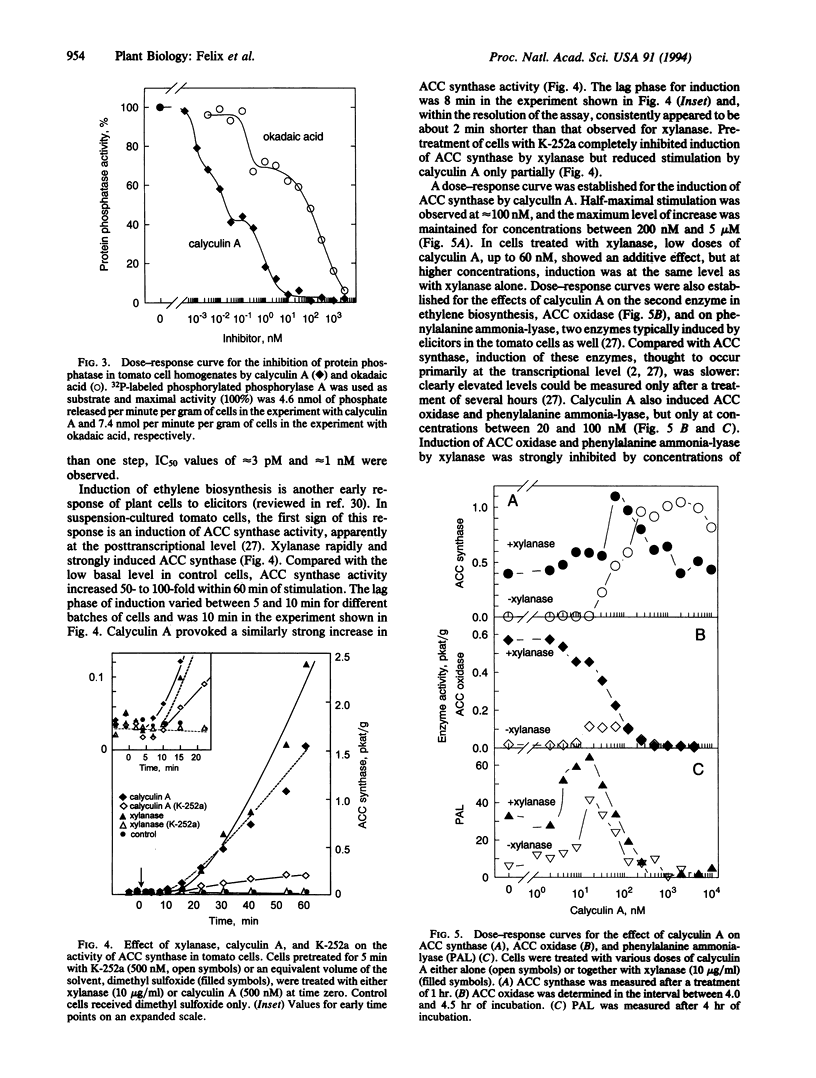
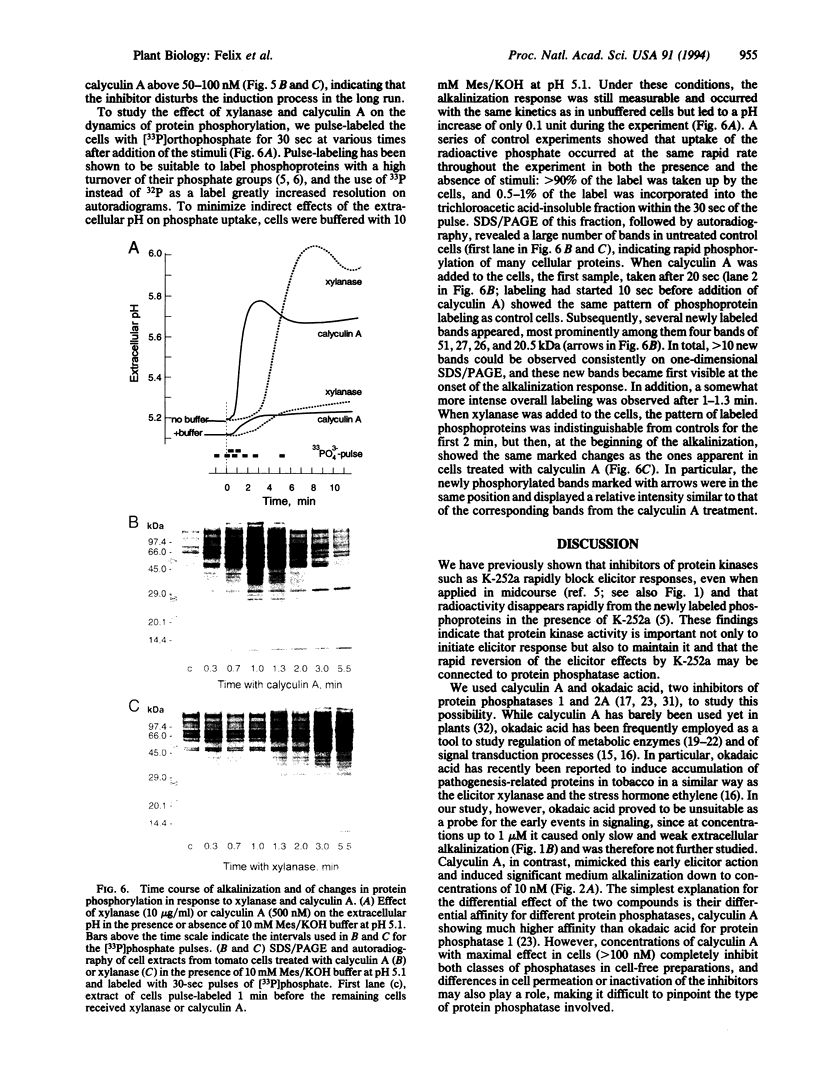
Suspension-cultured tomato cells react to microbial signals, so-called elicitors, with rapid alkalinization of the growth medium and increased biosynthesis of the stress hormone ethylene. These responses to elicitors can be blocked by staurosporine and K-252a, two specific inhibitors of protein kinases. Here we show that calyculin A, a potent inhibitor of protein phosphatases, mimics the action of elicitors and, at nanomolar concentrations, induces medium alkalinization as well as a strong increase in the activity of 1-aminocyclopropane-1-carboxylate synthase, the key enzyme of ethylene biosynthesis. Both responses were strongly inhibited by K-252a, and calyculin A induced both responses more rapidly than did a fungal elicitor, xylanase. For example, the lag phase for medium alkalinization was only 0.2-0.4 min for calyculin A, compared with 2 min for xylanase. To study changes in the dynamics of protein phosphorylation, cells were labeled with 30-sec pulses of [33P]orthophosphate. Calyculin A strongly increased phosphorylation of several polypeptide bands within 40 sec of treatment. The same phosphorylated bands also appeared in response to xylanase, but only after a lag phase of 2-3 min. These results show that the protein phosphatase inhibitor calyculin A leads to rapid hyperphosphorylation of specific proteins in cultured cells and indicate that elicitor action could be based on inhibition of a protein phosphatase as well as on activation of a protein kinase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey B. A., Korcak R. F., Anderson J. D. Alterations in Nicotiana tabacum L. cv Xanthi Cell Membrane Function following Treatment with an Ethylene Biosynthesis-Inducing Endoxylanase. Plant Physiol. 1992 Oct;100(2):749–755. doi: 10.1104/pp.100.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. J., Nimmo H. G., Fewson C. A., Wilkins M. B. Circadian rhythms in the activity of a plant protein kinase. EMBO J. 1991 Aug;10(8):2063–2068. doi: 10.1002/j.1460-2075.1991.tb07737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Conrath U., Jeblick W., Kauss H. The protein kinase inhibitor, K-252a, decreases elicitor-induced Ca2+ uptake and K+ release, and increases coumarin synthesis in parsley cells. FEBS Lett. 1991 Feb 11;279(1):141–144. doi: 10.1016/0014-5793(91)80269-9. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Dominov J. A., Stenzler L., Lee S., Schwarz J. J., Leisner S., Howell S. H. Cytokinins and auxins control the expression of a gene in Nicotiana plumbaginifolia cells by feedback regulation. Plant Cell. 1992 Apr;4(4):451–461. doi: 10.1105/tpc.4.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Basse C. W., Boller T. Elicitor-induced ethylene biosynthesis in tomato cells: characterization and use as a bioassay for elicitor action. Plant Physiol. 1991 Sep;97(1):19–25. doi: 10.1104/pp.97.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. Rhizobium--plant signal exchange. Nature. 1992 Jun 25;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Fuchs Y., Saxena A., Gamble H. R., Anderson J. D. Ethylene biosynthesis-inducing protein from cellulysin is an endoxylanase. Plant Physiol. 1989 Jan;89(1):138–143. doi: 10.1104/pp.89.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskopf D. G., Felix G., Boller T. K-252a inhibits the response of tomato cells to fungal elicitors in vivo and their microsomal protein kinase in vitro. FEBS Lett. 1990 Nov 26;275(1-2):177–180. doi: 10.1016/0014-5793(90)81466-2. [DOI] [PubMed] [Google Scholar]
- Huber J. L., Huber S. C. Site-specific serine phosphorylation of spinach leaf sucrose-phosphate synthase. Biochem J. 1992 May 1;283(Pt 3):877–882. doi: 10.1042/bj2830877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Coggins J., Cohen P. Plant protein phosphatases. Subcellular distribution, detection of protein phosphatase 2C and identification of protein phosphatase 2A as the major quinate dehydrogenase phosphatase. Biochem J. 1991 Feb 1;273(Pt 3):733–738. doi: 10.1042/bj2730733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C. Regulation of spinach-leaf nitrate reductase by reversible phosphorylation. Biochim Biophys Acta. 1992 Oct 6;1137(1):121–126. doi: 10.1016/0167-4889(92)90109-o. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Seuwen K. Transmembrane receptors and intracellular pathways that control cell proliferation. Annu Rev Physiol. 1992;54:195–210. doi: 10.1146/annurev.ph.54.030192.001211. [DOI] [PubMed] [Google Scholar]
- Raz V., Fluhr R. Ethylene Signal Is Transduced via Protein Phosphorylation Events in Plants. Plant Cell. 1993 May;5(5):523–530. doi: 10.1105/tpc.5.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J. 1993 Sep;12(9):3497–3505. doi: 10.1002/j.1460-2075.1993.tb06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu P., Felix G., Boller T. Inactivation of stress induced 1-aminocyclopropane carboxylate synthase in vivo differs from substrate-dependent inactivation in vitro. Plant Physiol. 1990 Aug;93(4):1482–1485. doi: 10.1104/pp.93.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten J. Chemosensory transduction in eukaryotic microorganisms. Annu Rev Physiol. 1992;54:639–663. doi: 10.1146/annurev.ph.54.030192.003231. [DOI] [PubMed] [Google Scholar]