Abstract
Purpose of review
Dairy is a major food group with potential impact on cardiometabolic health. Self-reported dairy intake has limitations that can partly be avoided by using biomarkers. This review aims to summarize the evidence of odd-chain saturated fatty acids (OCFAs), that is, pentadecanoic acid (C15 : 0) and heptadecanoic acid (17 : 0), as biomarkers of dairy fat intake. In addition, the associations of OCFA biomarkers with cardiometabolic disease will be overviewed.
Recent findings
Adipose tissue 15 : 0 is the preferred biomarker but also circulating 15 : 0, and to a weaker extent 17 : 0, reflects both habitual and changes in dairy intake. Whereas results from studies assessing cardiovascular outcomes are inconsistent, OCFA biomarkers are overall associated with lower diabetes risk. Residual confounding should however be considered until interventional data and mechanisms are available. Although OCFA biomarkers mainly reflect dairy fat intake, recently proposed endogenous synthesis and metabolism do motivate further research.
Summary
Taking into account the study population diet and limitations of OCFA biomarkers, both adipose and circulating levels of 15 : 0, in particular, are useful for estimating total dairy fat intake. OCFA biomarkers are overall not linked to cardiovascular disease risk, but a possible beneficial role of dairy foods in diabetes prevention warrant further study.
Keywords: biomarkers, dairy food intake, milk fat, odd-chain saturated fatty acids, pentadecanoic acid
INTRODUCTION
Dairy food is a major source of energy and nutrients in many countries. To assess the impact of dairy food on preventable diseases such as type 2 diabetes (T2D) and cardiovascular disease (CVD), objective measures of intake are critical. Biomarkers of dairy food intake can add to self-reported data by e.g. avoiding memory bias, and under- and over-reporting, and food database errors [1]. Odd-chain saturated fatty acids (OCFAs) including pentadecanoic acid (C15 : 0) and heptadecanoic acid (17 : 0) are characteristic of dairy fat because they are synthesized through microbial fermentation in the ruminant gut from where they are absorbed and subsequently excreted in the milk [2]. The mean concentrations of 15 : 0 and 17 : 0 in milk are ∼1.2 and 0.54% of total fatty acids, respectively [3,4]. As further discussed, these fatty acids are also present in beef and lamb, and fish, but at lower concentrations. In humans, OCFAs can be measured in adipose tissue [5,6] and blood constituents (e.g., cholesterol esters [6]) and are commonly used as biomarkers of dairy fat intake.
The aim of this review was to provide a scientific update on the use of OCFAs as biomarkers of dairy fat intake. This review focus on the most commonly used specific milk fat biomarkers in the literature, 15 : 0 and 17 : 0, although other biomarkers (e.g., 14 : 0 and trans16 : 1n-7) of dairy fat intake exist [6,7]. In addition, we aimed to overview observational studies that have examined the associations of circulating and tissue 15 : 0 and 17 : 0 with risk of CVD-related outcomes and T2D.
Box 1.
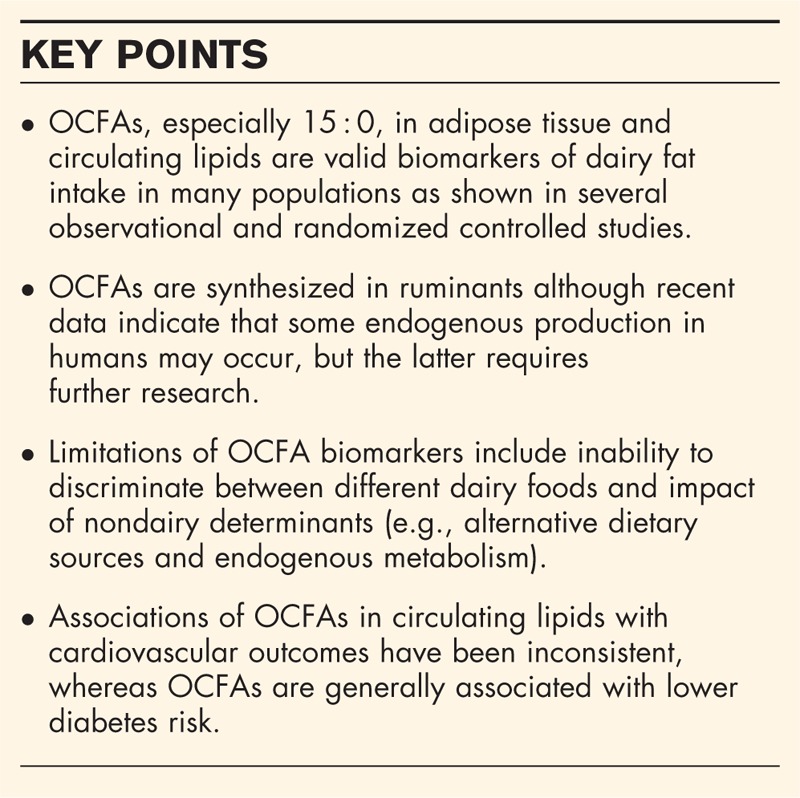
no caption available
BIOCHEMICAL ASSESSMENT OF 15 : 0 AND 17 : 0
From fasting blood samples (often stored in −70 to −80°C), 15 : 0 and 17 : 0 can be analyzed in most blood compartments and fractions by using thin-layer chromatography, as described previously by Wolk et al.[6]. Alternatively, they can be measured in adipose tissue triglycerides [6]. The fatty acids in the serum cholesterol esters and phospholipids as well as in adipose tissue triglycerides are commonly separated by gas-liquid chromatography after transmethylation, as described earlier [8]. The concentrations of the fatty acids are usually presented as percentages of the sum of all fatty acids analyzed, as weight% or mol% [9]. The within-analysis CV of 15 : 0 among studies has usually been 10% or less, which must be regarded as clearly acceptable. The CV may differ somewhat between lipid compartments, for example, it was shown to be ∼13% in cholesterol esters and ∼9% in phospholipids in one study [6].
BIOMARKERS OF DAIRY INTAKE: OBSERVATIONAL STUDIES
It was shown in Swedish women that adipose tissue OCFAs are valid biomarkers of long-term dairy fat intake [5]. The correlations were weaker when food intake was assessed by food frequency questionnaire (FFQ) compared with repeated food records [5]. Notably, dairy intake was more strongly correlated with adipose 15 : 0 than 17 : 0 [5,6,10,11], showing a clear dose–response relationships between intake and 15 : 0 [11]. In adipose tissue, the mean or median proportions of 15 : 0 range from 0.19 (Costa Rica) to 0.39 (Norway) and for 17 : 0 from 0.21 to 0.34 [5,6,10,12–14]. It was also elegantly shown by Wolk et al.[6] that 15 : 0 and 17 : 0 in serum and in adipose tissue among men were closely associated with dairy fat intake assessed by 24-h recalls. If adipose tissue is not available, 15 : 0 in serum cholesterol esters may be the best choice, because it has shown strong correlations (r = 0.58) with adipose tissue [10]. However, correlation coefficients between total dairy intake and biomarker 15 : 0 were comparable in phospholipids (r = 0.53) and cholesterol esters (r = 0.47) when assessed in the same population [6]. In addition, there is a strong correlation between 15 : 0 in cholesterol esters and phospholipids (r = 0.67) [15], and thus either of these compartments seems appropriate. When comparing 15 : 0 in total plasma lipids with that in erythrocytes, the former compartment was more strongly correlated to dairy fat intake assessed by FFQ [16,17].
Overall, the proportions of both 15 : 0 and 17 : 0 in human plasma, serum and erythrocytes correlate with intake of dairy fat [6,9,10,13,15,17–22], but in some populations (erythrocytes used) only weakly or not at all [17]. Even in young children, 15 : 0 in plasma phospholipids was recently shown to reflect dairy fat and dairy food intake [23▪], confirming a previous randomized study (intervention replaced regular dairy with low-fat dairy) in children where serum 15 : 0 (compared with 14 : 0 and 17 : 0) best reflected baseline total dairy fat intake as well as detecting changes in dairy fat intake [24].
However, OCFAs may not be good biomarkers of dairy fat intake when assessed in populations with low intake of dairy intake and concomitantly higher intake of beef or fish [5]. Thus, the background diet of the study population needs to be considered before interpreting the results from OCFA biomarkers.
BIOMARKERS OF DAIRY INTAKE: INTERVENTION STUDIES
Although the fairly strong correlations between biomarker 15 : 0 and dairy intake suggest the use of OCFAs as biomarkers of habitual dairy fat intake in various populations, it is perhaps even more important to also note that a number of randomized controlled studies have demonstrated that a change in dairy food intake is reflected by changes in 15 : 0 and 17 : 0, in various circulating and tissue lipid fractions [25–29]. Thus, such studies suggest a direct link between changes in dairy fat intake and change in OCFAs, also during weight-stable conditions. These strictly controlled studies have either provided all or some food to the participants, which presumably improve compliance and thus simplifies the interpretation of the data. For example, when butter and other dairy products are iso-calorically replaced with vegetable fats, there is a reduction of 15 : 0 and 17 : 0 in circulating lipids [25–29]. In addition, in mixed diets, which either increase or decrease of dairy fats, such intake variations are reflected by changes in circulating OCFAs [30–32]. Although these results support the use of 15 : 0 as dairy fat biomarker, the precise dose–response association between dairy fat intake and circulating 15 : 0 can only be obtained by strictly controlled feeding studies in which the quantity and quality of the dairy products are monitored and known [33].
NONDAIRY SOURCES OF 15 : 0 AND 17 : 0
In addition to dairy and ruminant meats, fish also contains 15 : 0 and 17 : 0 [34–37,38▪]. The proportions are clearly higher for 17 : 0 than 15 : 0 and varies dependent on fish type; the content range of 17 : 0 is ∼0.30 to 2% [39]. In a recent study that analyzed 27 freshwater fish species captured in the northeastern USA, mean content of 17 : 0 was 0.6% [38▪]. As the absolute intake of fish is overall considerably lower than total dairy intake in most Western populations, the contribution of 15 : 0 through fish is likely very limited. However, 17 : 0 may also reflect high fish intake in some populations including the USA [38▪], and especially in those where dairy intake is moderate or low. Some concern has recently been raised about the use of OCFA biomarkers of dairy fat intake in populations with high intake of fish [40]. Although correlations between plasma OCFAs and very long-chain n-3 fatty acids observed in some populations [15], the recent large EPIC-Interact case-cohort study found no associations between plasma OCFAs and fish intake, either lean or fatty fish [41]. In at least most Western populations, the levels of OCFAs in blood components and adipose tissue seemingly mainly reflect dairy fat intake, rather than fish or beef. For example, the significant associations between adipose tissue proportions of 15 : 0 and 17 : 0 and dairy intake were not noticeably affected by adjustment for either fish intake or meat intake in a population of Swedish men [19]. Importantly, randomized trials in Nordic populations (where diets are usually high in both fish and dairy) have suggested negligible effects of fish consumption on plasma OCFAs. For example, interventional prudent diets high in fish and low in high-fat dairy, cause significant relative reductions of plasma 15 : 0 and concurrent increases of EPA and DHA [30,31]. Overall 15 : 0 seems to be a solid dairy fat biomarker despite its content in fish, especially in populations with moderate and high dairy intake.
ENDOGENOUS SYNTHESIS OF ODD-CHAIN SATURATED FATTY ACIDS
Although OCFAs clearly reflect habitual dairy food intake, as well as capturing changes (both increases and decreases) caused by changes in dairy fat intake, emerging data indicate that 15 : 0 and 17 : 0 can also be endogenously synthesized in humans [42,43▪]. The potential evidence and hypotheses behind endogenous formation and metabolism of OCFAs are out of the scope of this review and have recently been described in detail elsewhere [42,43▪]. In brief, observations in vegans indicate plasma lipid levels of 15 : 0 and 17 : 0 comparable to those of dairy consumers [43▪,44], and 17 : 0 occurs in higher concentrations than 15 : 0 in human plasma despite higher intake of the latter [42]. This suggests that some metabolic regulation may occur, and that nondairy consumer could potentially have enhanced endogenous synthesis or metabolism of OCFAs. For example, it is believed that α-oxidation is one possible mechanism behind an endogenous production of OCFAs [42]. Indeed, α-oxidation may not only occur on branched chain fatty acids (e.g. phytanic acid) [45], but also on straight chain fatty acids to generate OCFAs [42]. In this context it can be noted that the branched-chain fatty acid phytanic acid, synthesized in ruminants from chlorophyll, has been correlated to dairy fat intake [46]. Taken together, one cannot exclude that circulating OCFAs, at least to a minor extent, are influenced by OCFAs’ metabolism [42,43▪]. Such influence, however, needs further investigation, and if evident, it will probably not have any major impact on the performance of OCFAs as valid biomarkers of dairy fat intake, given the correlations between intake and biomarker observed in diverse populations and randomized trials.
DAIRY FAT BIOMARKERS AND CARDIOMETABOLIC DISEASE
Several observational studies have been published on 15 : 0 and 17 : 0 with regard to risk of CVD or related health outcomes. The sum of 15 : 0 and 17 : 0 in phospholipids was associated with lower risk of incident coronary heart disease (CHD) in a case–control study nested in the British cohort EPIC-Norfolk [47]. Similarly, plasma phospholipid 15 : 0 was inversely associated with incident CVD and CHD in the Multi-ethnic Study on Atherosclerosis (MESA) [48], while both 15 : 0 and 17 : 0 in plasma phospholipids (but not in cholesterol esters) were associated with lower risk of heart failure in the Atherosclerosis Risk in Communities Study (ARIC) [49]. Further, the sum of 15 : 0 and 17 : 0 in phospholipids was associated with lower risk of myocardial infarction (especially among women [50]) in two Swedish nested case–control studies [50,51]. In contrast, plasma (but not erythrocyte) 15 : 0 was associated with higher risk of ischemic heart disease in a nested case–control study of the Nurses’ Health Study (NHS) [16] and in the US Women Health Initiative Observational Study, no association of plasma phospholipid 15 : 0 with CHD risk was evident [52]. Another proposed biomarker of dairy fat consumption, trans-palmitoleic acid (trans16 : 1n-7) measured in erythrocytes, was linked to lower risk of cardiovascular mortality in a German clinical cohort (the Ludwigshafen Risk and Cardiovascular Health Study) [53], but plasma phospholipid trans16 : 1n-7 was not associated with incident CVD or CHD in MESA [48].
In regard to stroke risk, 17 : 0 and the sum of 15 : 0 and 17 : 0 in plasma phospholipids were associated with lower stroke risk in a case–control study nested in two Swedish cohorts, Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) and Västerbotten Intervention Programme (VIP) [54]. In contrast, plasma and erythrocyte biomarkers of dairy fat intake were not associated with incident stroke in NHS and the Health Professional Follow-up Study (HPFS) [17]. Likewise, 15 : 0 in plasma cholesterol esters and phospholipids was not associated with risk of ischemic stroke in ARIC [55].
So far, only one cohort study has evaluated associations of OCFAs in adipose tissue with risk of a CVD-related outcome; in a Swedish cohort study on elderly men, 15 : 0 or 17 : 0 in adipose tissue were not associated with cardiovascular death [56▪]. Two studies have evaluated OCFAs measured in fat biopsies collected after a first myocardial infarction, but the results were inconsistent; 15 : 0 in adipose tissue was inversely associated with myocardial infarction among Norwegians [10] but not in Costa Ricans [11].
Findings from observational studies assessing associations of OCFA biomarkers with CVD-related outcomes are inconclusive which partly may be due to methodological differences between studies (e.g., study design and populations, outcome definitions, statistical models, biomarker compartment, and underlying diets). The inconsistencies between studies may also reflect that CVD risks associated with substitution of dairy fats with other dietary fats can differ substantially depending on the type of fat or food that replace the dairy fat; in NHS and HPFS, isocaloric replacement of dairy fat with vegetable fat was associated with lower risk of CVD whereas similar substitution of dairy fat with animal fat was linked with higher CVD risk [57]. The lack of clear relationship of OCFA biomarkers with CVD is in line with recent meta-analyses based on self-reported data, that show little or weak evidence for inverse dose–response relationships between total dairy fat and CHD, CVD or stroke in fully adjusted models [58,59▪].
Associations of circulating OCFAs with incident T2D have been assessed in several studies. In the large prospective case-cohort study EPIC-Interact (n = 12 132 cases), plasma phospholipid 15 : 0 and 17 : 0 were inversely associated with T2D risk [41]. Looking at individual studies of different EPIC centers, similar results were obtained in the Swedish VIP study [60], but not in the German EPIC-Potsdam [61] or in a small nested case-control study within the British EPIC-Norfolk [22]. In the Melbourne Consecutive Cohort Study, 15 : 0 in plasma phospholipids was associated with lower risk of T2D [62]. OCFAs in plasma phospholipids were not associated with T2D risk in the two American prospective cohorts Cardiovascular Health Study (CHS) [7] or MESA [63]. However, trans16 : 1n-7 was linked to lower risk of incident diabetes in both these cohorts [7,63]. In a pooled analysis of NHS and HPFS, plasma and erythrocyte levels of trans16 : 1n7 and 17 : 0 were associated with lower diabetes risk, while inverse associations of 15 : 0 were only observed in plasma [64▪]. Serum levels of 15 : 0 (but not 16 : 1n7t) were inversely associated with incident diabetes in the American cohort study Insulin Resistance and Atherosclerosis Study [65].
These results accord with cohort studies using self-reported intake data, showing weak but significant inverse associations between total dairy intake and T2D [66▪]. Importantly to note, however, is that reported intake of particularly yoghurt shows strong inverse association [66▪]. Yoghurt consumption (low-fat yoghurt, <3.9%) was also inversely associated with diabetes incidence in the EPIC-Norfolk study [67]. Furthermore, previous meta-analysis showed that low-fat dairy in particular was inversely linked with diabetes risk [68]. Thus a low-fat dairy food pattern may not be captured by OCFA biomarkers which presumably more reflect high-fat dairy. To date, there is no evidence for a direct role of OCFAs in improving glucose metabolism. Cause and effect needs to be confirmed by randomized trials, and the mechanisms behind a possible diabetes preventive effect of dairy food is unknown [69]. Several components in dairy foods may however be of interest e.g. short-chain fatty acids, fermentation (probiotic effect), high-quality proteins, calcium, and specific phospholipids.
BIOMARKERS OF HEALTHY LIFESTYLE?
It is possible that the inverse relationships between OCFAs and cardiometabolic disease, as observed in some studies, are explained by residual confounding of various lifestyle factors. Notably, alcohol intake has been strongly inversely related to both serum phospholipid and adipose tissue OCFAs in several populations [19,70], whereas intakes of fruit and vegetables [41] and fiber [19] have been correlated with OCFA biomarkers. Physical active vs. inactive Swedish men had ∼5% higher proportions of 15 : 0 and 17 : 0 in phospholipids and adipose tissue, although not statistically significant [19]. Adjustment for physical activity did however not affect the association between serum 15 : 0 and dairy intake, or with clinical characteristics [15]. In contrast, intake of various foods such as meat, vegetables, and beer, did influence an inverse association between serum 15 : 0 and various metabolic risk factors in men, suggesting potential confounding [15]. In support of this, the large pan-European EPIC-Interact study showed that apart from a strong correlation between OCFAs in plasma phospholipids and dairy food intake, OCFAs were simultaneously correlated with food groups that are typically included in prudent dietary patterns, for example fruits, vegetable and nuts, but inversely to red and processed meat [41].
CONCLUSION
Although not entirely specific for dairy foods, OCFAs and 15 : 0 in particular, are useful biomarkers of total dairy fat intake in many populations, for example, in Europe and the USA. In addition, they reflect changes in dairy fat intake and are thus important for monitoring dietary compliance in intervention studies. However, considering possible uncertainties of the dietary origin, endogenous metabolism, and intake of dairy in the population under study, caution is warranted in the interpretation of links between these biomarkers and disease risk. Regarding reported inverse relationships between OCFAs and diabetes, but not CVD, confounding lifestyle factors must be strongly considered until interventional data are available.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the Swedish Research Council and the Swedish Diabetes Fund.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol 2006; 17:22–27. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, Palmquist DL. Synthesis and biohydrogenation of fatty acids by ruminal microorganisms in vitro. J Dairy Sci 1991; 74:3035–3046. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell-Megaro AM, Barbano DM, Bauman DE. Survey of the fatty acid composition of retail milk in the United States including regional and seasonal variations. J Dairy Sci 2011; 94:59–65. [DOI] [PubMed] [Google Scholar]
- 4.Kusche D, Kuhnt K, Ruebesam K, et al. Fatty acid profiles and antioxidants of organic and conventional milk from low- and high-input systems during outdoor period. J Sci Food Agric 2015; 95:529–539. [DOI] [PubMed] [Google Scholar]
- 5.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr 1998; 68:291–295. [DOI] [PubMed] [Google Scholar]
- 6.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr 2001; 131:828–833. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Cao H, King IB, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010; 153:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boberg M, Croon LB, Gustafsson IB, Vessby B. Platelet fatty acid composition in relation to fatty acid composition in plasma and to serum lipoprotein lipids in healthy subjects with special reference to the linoleic acid pathway. Clin Sci (Lond) 1985; 68:581–587. [DOI] [PubMed] [Google Scholar]
- 9.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47:348–380. [DOI] [PubMed] [Google Scholar]
- 10.Biong AS, Veierod MB, Ringstad J, et al. Intake of milk fat, reflected in adipose tissue fatty acids and risk of myocardial infarction: a case-control study. Eur J Clin Nutr 2006; 60:236–244. [DOI] [PubMed] [Google Scholar]
- 11.Aslibekyan S, Campos H, Baylin A. Biomarkers of dairy intake and the risk of heart disease. Nutr Metab Cardiovasc Dis 2012; 22:1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 2002; 76:750–757. [DOI] [PubMed] [Google Scholar]
- 13.Brevik A, Veierod MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr 2005; 59:1417–1422. [DOI] [PubMed] [Google Scholar]
- 14.Iggman D, Arnlov J, Vessby B, et al. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia 2010; 53:850–857. [DOI] [PubMed] [Google Scholar]
- 15.Smedman AE, Gustafsson I-B, Berglund LG, Vessby BO. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr 1999; 69:22–29. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 2007; 86:929–937. [DOI] [PubMed] [Google Scholar]
- 17.Yakoob MY, Shi P, Hu FB, et al. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr 2014; 100:1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005; 162:373–381. [DOI] [PubMed] [Google Scholar]
- 19.Rosell M, Johansson G, Berglund L, et al. The relation between alcohol intake and physical activity and the fatty acids 14:0. 15:0 and 17:0 in serum phospholipids and adipose tissue used as markers for dairy fat intake. Br J Nutr 2005; 93:115–121. [DOI] [PubMed] [Google Scholar]
- 20.Hodge AM, Simpson JA, Gibson RA, et al. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Carbiovasc Dis 2007; 17:415–426. [DOI] [PubMed] [Google Scholar]
- 21.Wennberg M, Vessby B, Johansson I. Evaluation of relative intake of fatty acids according to the Northern Sweden FFQ with fatty acid levels in erythrocyte membranes as biomarkers. Public Health Nutr 2009; 12:1477–1484. [DOI] [PubMed] [Google Scholar]
- 22.Patel PS, Sharp SJ, Jansen E, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010; 92:1214–1222. [DOI] [PubMed] [Google Scholar]
- 23▪.Lund-Blix NA, Ronningen KS, Boas H, et al. Plasma phospholipid pentadecanoic acid, EPA, and DHA, and the frequency of dairy and fish product intake in young children. Food Nutr Res 2016; 60:31933. [DOI] [PMC free article] [PubMed] [Google Scholar]; This Norwegian study in children showed significant correlations between 15 : 0 and the intake of dairy foods and dairy fat as assessed by a food frequency questionnaire, suggesting that 15 : 0 can be used as dairy fat biomarker also in children.
- 24.Golley RK, Hendrie GA. Evaluation of the relative concentration of serum fatty acids C14:0, C15:0 and C17:0 as markers of children's dairy fat intake. Ann Nutr Metabol 2014; 65:310–316. [DOI] [PubMed] [Google Scholar]
- 25.Vessby B, Uusitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 2001; 44:312–319. [DOI] [PubMed] [Google Scholar]
- 26.Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr 2002; 76:1222–1229. [DOI] [PubMed] [Google Scholar]
- 27.Warensjö E, Risérus U, Gustafsson I-B, et al. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutr Metab Cardiovasc Dis 2008; 18:683–690. [DOI] [PubMed] [Google Scholar]
- 28.Wennersberg MH, Smedman A, Turpeinen AM, et al. Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study. Am J Clin Nutr 2009; 90:960–968. [DOI] [PubMed] [Google Scholar]
- 29.Bjermo H, Iggman D, Kullberg J, et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012; 95:1003–1012. [DOI] [PubMed] [Google Scholar]
- 30.Adamsson V, Reumark A, Fredriksson IB, et al. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (NORDIET). J Intern Med 2011; 269:150–159. [DOI] [PubMed] [Google Scholar]
- 31.Uusitupa M, Hermansen K, Savolainen MJ, et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome - a randomized study (SYSDIET). J Intern Med 2013; 274:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marklund M, Magnusdottir OK, Rosqvist F, et al. A dietary biomarker approach captures compliance and cardiometabolic effects of a healthy Nordic diet in individuals with metabolic syndrome. J Nutr 2014; 144:1642–1649. [DOI] [PubMed] [Google Scholar]
- 33.Djoussé L. Is plasma pentadecanoic acid a reasonable biomarker of dairy consumption? J Am Heart Assoc 2013; 2:e000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tocher DR, Sargent JR. Analyses of lipids and fatty acids in ripe roes of some Northwest European marine fish. Lipids 1984; 19:492–499. [DOI] [PubMed] [Google Scholar]
- 35.Linko RR, Kaitaranta JK, Vuorela R. Comparison of the fatty acids in Baltic herring and available plankton feed. Comp Biochem Physiol B 1985; 82:699–705. [DOI] [PubMed] [Google Scholar]
- 36.Aggelousis G, Lazos ES. Fatty acid composition of the lipids from eight freshwater fish species from Greece. J Food Compos Anal 1991; 4:68–76. [Google Scholar]
- 37.Arts MT, Ackman RG, Holub BJ. Essential fatty acids’ in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci 2001; 58:122–137. [Google Scholar]
- 38▪.Wang DH, Jackson JR, Twining C, et al. Saturated branched chain, normal odd-carbon-numbered, and n-3 (omega-3) polyunsaturated fatty acids in freshwater fish in the northeastern United States. J Agric Food Chem 2016; 64:7512–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined fatty acid composition in wild freshwater fish caught in northeastern US, and reports that the mean content of 15 : 0, and especially 17 : 0 were relatively high.
- 39.Ozogul Y, Ozogul F, Cicek E, et al. Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int J Food Sci Nutr 2009; 60:464–475. [DOI] [PubMed] [Google Scholar]
- 40.Lankinen M, Schwab U. Biomarkers of dairy fat. Am J Clin Nutr 2015; 101:1101–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forouhi NG, Koulman A, Sharp SJ, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014; 2:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 2015; 20:2425–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Pfeuffer M, Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr 2016; 7:730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review overviews the potential regulation and endogenous synthesis of OCFAs, and discuss the possible different sources of OCFAs in humans. The authors hypothesize a possible involvement in metabolic regulation from the assumption that there is a link between 15 : 0 and 17 : 0 and other OCFAs with different carbon chain length.
- 44.Kornsteiner M, Singer I, Elmadfa I. Very low n-3 long-chain polyunsaturated fatty acid status in Austrian vegetarians and vegans. Ann Nutr Metabol 2008; 52:37–47. [DOI] [PubMed] [Google Scholar]
- 45.Wanders RJ, Jansen GA, Lloyd MD. Phytanic acid alpha-oxidation, new insights into an old problem: a review. Biochim Biophys Acta 2003; 1631:119–135. [DOI] [PubMed] [Google Scholar]
- 46.Allen NE, Grace PB, Ginn A, et al. Phytanic acid: measurement of plasma concentrations by gas–liquid chromatography–mass spectrometry analysis and associations with diet and other plasma fatty acids. Br J Nutr 2008; 99:653–659. [DOI] [PubMed] [Google Scholar]
- 47.Khaw KT, Friesen MD, Riboli E, et al. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk Prospective Study. PLoS Med 2012; 9:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Oliveira Otto MC, Nettleton JA, Lemaitre RN, et al. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2013; 2:e000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warensjo E, Jansson JH, Cederholm T, et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr 2010; 92:194–202. [DOI] [PubMed] [Google Scholar]
- 51.Warensjo E, Jansson JH, Berglund L, et al. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case-control study. Br J Nutr 2004; 91:635–642. [DOI] [PubMed] [Google Scholar]
- 52.Matthan NR, Ooi EM, Van Horn L, et al. Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: a nested case-control study within the Women's Health Initiative observational study. J Am Heart Assoc 2014; 3:e000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleber ME, Delgado GE, Lorkowski S, et al. Trans-fatty acids and mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health Study. Eur Heart J 2016; 37:1072–1078. [DOI] [PubMed] [Google Scholar]
- 54.Warensjo E, Smedman A, Stegmayr B, et al. Stroke and plasma markers of milk fat intake – a prospective nested case-control study. Nutr J 2009; 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamagishi K, Folsom AR, Steffen LM. Plasma fatty acid composition and incident ischemic stroke in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis 2013; 36:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Iggman D, Arnlov J, Cederholm T, Riserus U. Association of adipose tissue fatty acids with cardiovascular and all-cause mortality in elderly men. JAMA Cardiol 2016; 1:745–753. [DOI] [PubMed] [Google Scholar]; This is the only prospective cohort study to date that has evaluated the associations of OCFAs in adipose tissue with CVD mortality. In a Swedish cohort study on elderly men, 15 : 0 or 17 : 0 in adipose tissue were not associated with cardiovascular mortality.
- 57.Chen M, Li Y, Sun Q, et al. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr 2016; 104:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr 2011; 93:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪.Alexander DD, Bylsma LC, Vargas AJ, et al. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr 2016; 115:737–750. [DOI] [PubMed] [Google Scholar]; This systematic review and meta-analysis have evaluated prospective cohorts of self-reported dairy food intake and risk of cardiovascular disease, including coronary heart disease and stroke. Although no biomarkers have been included, the data are of interest since it also examined some food groups in addition to total dairy intake.
- 60.Krachler B, Norberg M, Eriksson JW, et al. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2008; 18:503–510. [DOI] [PubMed] [Google Scholar]
- 61.Kroger J, Zietemann V, Enzenbach C, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2011; 93:127–142. [DOI] [PubMed] [Google Scholar]
- 62.Hodge AM, English DR, O’Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007; 86:189–197. [DOI] [PubMed] [Google Scholar]
- 63.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, et al. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013; 97:854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪.Yakoob MY, Shi P, Willett WC, et al. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016; 133:1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]; In two prospective US cohorts, higher plasma 15 : 0, 17 : 0 and trans16 : 1n-7 was associated with lower incident diabetes risk. Results were similar for erythrocyte 17 : 0, but not for erythrocyte 15 : 0 which were not significantly associated with diabetes risk.
- 65.Santaren ID, Watkins SM, Liese AD, et al. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr 2014; 100:1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪.Gijsbers L, Ding EL, Malik VS, et al. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016; 103:1111–1124. [DOI] [PubMed] [Google Scholar]; This meta-analysis in >500 000 individuals examined the associations between type 2 diabetes incidence and self-reported dairy food intake, at different levels of intake. The results showed that total dairy intake and low-fat dairy was inversely related, and among specific foods yoghurt intake was related to lower diabetes risk.
- 67.O’Connor LM, Lentjes MAH, Luben RN, et al. Dietary dairy product intake and incident type 2 diabetes: a prospective study using dietary data from a 7-day food diary. Diabetologia 2014; 57:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013; 98:1066–1083. [DOI] [PubMed] [Google Scholar]
- 69.Forouhi NG. Association between consumption of dairy products and incident type 2 diabetes – insights from the European Prospective Investigation into Cancer study. Nutr Rev 2015; 73 Suppl 1:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saadatian-Elahi M, Slimani N, Chajes V, et al. Plasma phospholipid fatty acid profiles and their association with food intakes: results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2009; 89:331–346. [DOI] [PubMed] [Google Scholar]


