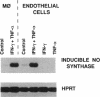
Abstract
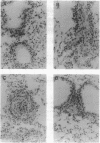
Like many pathogens that undergo an intravascular stage of development, larvae of the helminth parasite Schistosoma mansoni migrate through the blood vessels, where they are in close contact with endothelial cells. In vitro exposure of murine endothelial cells to various cytokines (interferon gamma, tumor necrosis factor alpha, and interleukin 1 alpha or 1 beta) resulted in their activation to kill schistosomula through an arginine-dependent mechanism involving production of nitric oxide (NO). Cytokine-treated endothelial cells showed increased expression of mRNA for the inducible form of the NO synthase, and both NO production and larval killing were suppressed by treatment with competitive inhibitors. The effector function of cytokine-treated endothelial cells was similar to that of activated inflammatory tissue macrophages, although activation appeared to be differentially regulated in these two cell types. Activated endothelial cells killed older (18-day) forms of the parasite, such as those currently thought to be a primary target of immune elimination in the lungs of mice previously vaccinated with radiation-attenuated cercariae, as well as newly transformed larvae. In C57BL/6 mice, which become resistant to S. mansoni infection as a result of vaccination with irradiated cercariae, endothelial cell morphology characteristic of activation was observed in the lung by 1-2 weeks after challenge infection. Similar endothelial cell changes were absent in P-strain mice, which do not become resistant as a result of vaccination. Together, these observations indicate that endothelial cells, not traditionally considered to be part of the immune system, may play an important role in immunity to S. mansoni and, by means of NO-dependent killing, could serve as effectors of resistance to other intravascular pathogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L., Helle M., Boeije L., Pascual-Salcedo D., de Groot E. Differential induction of interleukin-6 production in monocytes, endothelial cells and smooth muscle cells. Eur Cytokine Netw. 1991 Mar-Apr;2(2):115–120. [PubMed] [Google Scholar]
- Belloni P. N., Carney D. H., Nicolson G. L. Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvasc Res. 1992 Jan;43(1):20–45. doi: 10.1016/0026-2862(92)90004-9. [DOI] [PubMed] [Google Scholar]
- Bosse D., George V., Candal F. J., Lawley T. J., Ades E. W. Antigen presentation by a continuous human microvascular endothelial cell line, HMEC-1, to human T cells. Pathobiology. 1993;61(3-4):236–238. doi: 10.1159/000163800. [DOI] [PubMed] [Google Scholar]
- Cavender D. E. Interactions between endothelial cells and the cells of the immune system. Int Rev Exp Pathol. 1991;32:57–94. doi: 10.1016/b978-0-12-364932-4.50006-x. [DOI] [PubMed] [Google Scholar]
- Coulson P. S., Wilson R. A. Examination of the mechanisms of pulmonary phase resistance to Schistosoma mansoni in vaccinated mice. Am J Trop Med Hyg. 1988 May;38(3):529–539. doi: 10.4269/ajtmh.1988.38.529. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Wilson R. A. The role of pulmonary cellular reactions in the resistance of vaccinated mice to Schistosoma mansoni. Parasite Immunol. 1986 May;8(3):265–285. doi: 10.1111/j.1365-3024.1986.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Estrada C., Gómez C., Martín C., Moncada S., González C. Nitric oxide mediates tumor necrosis factor-alpha cytotoxicity in endothelial cells. Biochem Biophys Res Commun. 1992 Jul 15;186(1):475–482. doi: 10.1016/s0006-291x(05)80832-0. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Jaffe E. A., Levi R., Kilbourn R. G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991 Aug 15;178(3):823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- James S. L., Cook K. W., Lazdins J. K. Activation of human monocyte-derived macrophages to kill schistosomula of Schistosoma mansoni in vitro. J Immunol. 1990 Oct 15;145(8):2686–2690. [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- James S. L., Sher A. Cell-mediated immune response to schistosomiasis. Curr Top Microbiol Immunol. 1990;155:21–31. doi: 10.1007/978-3-642-74983-4_2. [DOI] [PubMed] [Google Scholar]
- James S. L. The effector function of nitrogen oxides in host defense against parasites. Exp Parasitol. 1991 Aug;73(2):223–226. doi: 10.1016/0014-4894(91)90025-r. [DOI] [PubMed] [Google Scholar]
- Kassim O. O., Dean D. A., Mangold B. L., Von Lichtenberg F. Combined microautoradiographic and histopathologic analysis of the fate of challenge Schistosoma mansoni schistosomula in mice immunized with irradiated cercariae. Am J Trop Med Hyg. 1992 Aug;47(2):231–237. doi: 10.4269/ajtmh.1992.47.231. [DOI] [PubMed] [Google Scholar]
- Li L. M., Kilbourn R. G., Adams J., Fidler I. J. Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res. 1991 May 15;51(10):2531–2535. [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–12234. [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Oswald I. P., Wynn T. A., Sher A., James S. L. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queluz T. T., Brunda M., Vladutiu A. O., Brentjens J. R., Andres G. Morphological basis of pulmonary edema in mice with cytokine-induced vascular leak syndrome. Exp Lung Res. 1991 Nov-Dec;17(6):1095–1108. doi: 10.3109/01902149109064337. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B., Vicart P., Delouis C., Paulin D. Mammalian cell lines can be efficiently established in vitro upon expression of the SV40 large T antigen driven by a promoter sequence derived from the human vimentin gene. Biol Cell. 1991;73(1):7–14. doi: 10.1016/0248-4900(91)90003-6. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Suschek C., Rothe H., Fehsel K., Enczmann J., Kolb-Bachofen V. Induction of a macrophage-like nitric oxide synthase in cultured rat aortic endothelial cells. IL-1 beta-mediated induction regulated by tumor necrosis factor-alpha and IFN-gamma. J Immunol. 1993 Sep 15;151(6):3283–3291. [PubMed] [Google Scholar]
- Woodman J. P., Dimier I. H., Bout D. T. Human endothelial cells are activated by IFN-gamma to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991 Sep 15;147(6):2019–2023. [PubMed] [Google Scholar]
- Wynn T. A., Eltoum I., Cheever A. W., Lewis F. A., Gause W. C., Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993 Aug 1;151(3):1430–1440. [PubMed] [Google Scholar]