Abstract
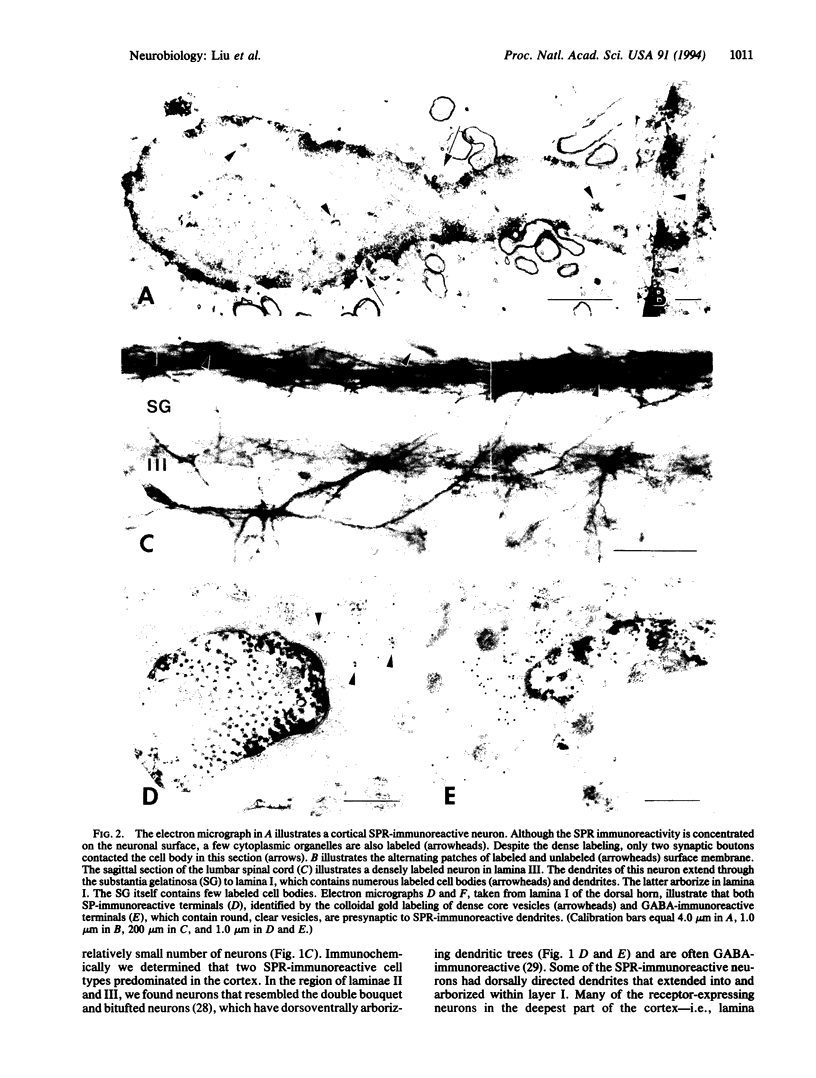
Light microscopic studies have demonstrated significant mismatches in the location of neuropeptides and their respective binding sites in the central nervous system. In the present study we used an antiserum raised against a synthetic peptide corresponding to the carboxyl-terminal tail of the substance P (SP) receptor (SPR) to further explore the relationship between a neuropeptide and its receptor. Light microscopy revealed an excellent correlation between the patterns of SPR immunoreactivity and of 125I-labeled SPR-binding sites in the central nervous system. The SPR appeared to be exclusively expressed by neurons; in fact, the SPR decorates the somatic and dendritic surface of neurons, producing Golgi-like images. Electron microscopic analysis in cortex, striatum, and spinal cord revealed that approximately 70% of the surface membrane of immunoreactive neurons is SPR laden. Simultaneous electron microscopic labeling of SP and SPR demonstrated significant mismatch at the synaptic level. Although some SP terminals contacted SPR-immunoreactive membrane, no more than 15% of the SPR-laden membrane apposed synaptic terminals. These results suggest that in contrast to more "classical" central and peripheral nervous system synapses, wherein the receptor immediately apposes the site of neurotransmitter storage and release, much of the surface of SPR-expressing neurons can be targeted by SP that diffuses a considerable distance from its site of release.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber R. P., Vaughn J. E., Saito K., McLaughlin B. J., Roberts E. GABAergic terminals are presynaptic to primary afferent terminals in the substantia gelatinosa of the rat spinal cord. Brain Res. 1978 Feb 3;141(1):35–55. doi: 10.1016/0006-8993(78)90615-7. [DOI] [PubMed] [Google Scholar]
- Beaudet A., Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3(10):851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Beaudet A., Woulfe J. Morphological substrate for neurotensin-dopamine interactions in the rat midbrain tegmentum. Ann N Y Acad Sci. 1992;668:173–185. doi: 10.1111/j.1749-6632.1992.tb27349.x. [DOI] [PubMed] [Google Scholar]
- Buma P., Roubos E. W. Ultrastructural demonstration of nonsynaptic release sites in the central nervous system of the snail Lymnaea stagnalis, the insect Periplaneta americana, and the rat. Neuroscience. 1986 Mar;17(3):867–879. doi: 10.1016/0306-4522(86)90051-5. [DOI] [PubMed] [Google Scholar]
- De Biasi S., Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y., Ribeiro-da-Silva A., Henry J. L., Cuello A. C. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5073–5077. doi: 10.1073/pnas.89.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Hope P. J., Jarrott B., Schaible H. G., Fleetwood-Walker S. M. Release, spread and persistence of immunoreactive neurokinin A in the dorsal horn of the cat following noxious cutaneous stimulation. Studies with antibody microprobes. Neuroscience. 1990;35(1):195–202. doi: 10.1016/0306-4522(90)90134-p. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Schaible H. G., Hope P. J., Lang C. W. Effect of peptidase inhibition on the pattern of intraspinally released immunoreactive substance P detected with antibody microprobes. Brain Res. 1992 May 8;579(2):261–269. doi: 10.1016/0006-8993(92)90059-i. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E., Beaudet A. Electron microscopic autoradiographic localization of opioid receptors in rat neostriatum. Nature. 1984 Nov 8;312(5990):155–157. doi: 10.1038/312155a0. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G., Emson P. C., Lawson D. E., Heizmann C. W., Streit P. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 1989;76(2):467–472. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- Henry J. L. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976 Sep 24;114(3):439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987 Oct;23(1):1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Jacob M. H., Lindstrom J. M., Berg D. K. Surface and intracellular distribution of a putative neuronal nicotinic acetylcholine receptor. J Cell Biol. 1986 Jul;103(1):205–214. doi: 10.1083/jcb.103.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. G. Neurotransmitters in the cerebral cortex. J Neurosurg. 1986 Aug;65(2):135–153. doi: 10.3171/jns.1986.65.2.0135. [DOI] [PubMed] [Google Scholar]
- Le Greves P., Nyberg F., Terenius L., Hökfelt T. Calcitonin gene-related peptide is a potent inhibitor of substance P degradation. Eur J Pharmacol. 1985 Sep 24;115(2-3):309–311. doi: 10.1016/0014-2999(85)90706-x. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith I. J., Minson J. B. Complete penetration of antibodies into vibratome sections after glutaraldehyde fixation and ethanol treatment: light and electron microscopy for neuropeptides. J Histochem Cytochem. 1992 Nov;40(11):1741–1749. doi: 10.1177/40.11.1431060. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Gates T., Mantyh C. R., Maggio J. E. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. J Neurosci. 1989 Jan;9(1):258–279. doi: 10.1523/JNEUROSCI.09-01-00258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Hunt S. P., Maggio J. E. Substance P receptors: localization by light microscopic autoradiography in rat brain using [3H]SP as the radioligand. Brain Res. 1984 Jul 30;307(1-2):147–165. doi: 10.1016/0006-8993(84)90470-0. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Johnson D. J., Boehmer C. G., Catton M. D., Vinters H. V., Maggio J. E., Too H. P., Vigna S. R. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Pinnock R. D., Downes C. P., Goedert M., Hunt S. P. Correlation between inositol phospholipid hydrolysis and substance P receptors in rat CNS. 1984 Jun 28-Jul 4Nature. 309(5971):795–797. doi: 10.1038/309795a0. [DOI] [PubMed] [Google Scholar]
- Mazella J., Leonard K., Chabry J., Kitabgi P., Vincent J. P., Beaudet A. Binding and internalization of iodinated neurotensin in neuronal cultures from embryonic mouse brain. Brain Res. 1991 Nov 15;564(2):249–255. doi: 10.1016/0006-8993(91)91460-i. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Giesler G. J., Jr, Besson J. M. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977 Jan 18;27(1):15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Merighi A., Polak J. M., Theodosis D. T. Ultrastructural visualization of glutamate and aspartate immunoreactivities in the rat dorsal horn, with special reference to the co-localization of glutamate, substance P and calcitonin-gene related peptide. Neuroscience. 1991;40(1):67–80. doi: 10.1016/0306-4522(91)90175-n. [DOI] [PubMed] [Google Scholar]
- Moussaoui S. M., Hermans E., Mathieu A. M., Bonici B., Clerc F., Guinet F., Garret C., Laduron P. M. Polyclonal antibodies against the rat NK1 receptor: characterization and localization in the spinal cord. Neuroreport. 1992 Dec;3(12):1073–1076. doi: 10.1097/00001756-199212000-00010. [DOI] [PubMed] [Google Scholar]
- Norregaard T. V., Moskowitz M. A. Substance P and the sensory innervation of intracranial and extracranial feline cephalic arteries. Implications for vascular pain mechanisms in man. Brain. 1985 Jun;108(Pt 2):517–533. doi: 10.1093/brain/108.2.517. [DOI] [PubMed] [Google Scholar]
- Pasquini F., Bochet P., Garbay-Jaureguiberry C., Roques B. P., Rossier J., Beaudet A. Electron microscopic localization of photoaffinity-labelled delta opioid receptors in the neostriatum of the rat. J Comp Neurol. 1992 Dec 8;326(2):229–244. doi: 10.1002/cne.903260206. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Quirion R., Shults C. W., Moody T. W., Pert C. B., Chase T. N., O'Donohue T. L. Autoradiographic distribution of substance P receptors in rat central nervous system. Nature. 1983 Jun 23;303(5919):714–716. doi: 10.1038/303714a0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Nakaya Y., Nomura S., Ogawa-Meguro R., Ohishi H., Kaneko T., Nakanishi S., Mizuno N. Immunocytochemical localization of rat substance P receptor in the striatum. Neurosci Lett. 1993 Apr 30;153(2):157–160. doi: 10.1016/0304-3940(93)90311-8. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Takagi H., Richards J. G., Mohler H. Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J Neurosci. 1989 Jun;9(6):2197–2209. doi: 10.1523/JNEUROSCI.09-06-02197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Pfeiffer F., Betz H., Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J Cell Biol. 1985 Aug;101(2):683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L., Jessell T. M., Gamse R., Mudge A. W., Leeman S. E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980 Jul 10;286(5769):155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- Zhu P. C., Thureson-Klein A., Klein R. L. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience. 1986 Sep;19(1):43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988 Feb;8(2):472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]