Abstract
Peroxisome biogenesis disorders in the Zellweger spectrum (PBD-ZSD) are a heterogeneous group of genetic disorders caused by mutations in PEX genes responsible for normal peroxisome assembly and functions. As a result of impaired peroxisomal activities, individuals with PBD-ZSD can manifest a complex spectrum of clinical phenotypes that typically result in shortened life spans. The extreme variability in disease manifestation ranging from onset of profound neurologic symptoms in newborns to progressive degenerative disease in adults presents practical challenges in disease diagnosis and medical management. Recent advances in biochemical methods for newborn screening and genetic testing have provided unprecedented opportunities for identifying patients at the earliest possible time and defining the molecular bases for their diseases. Here, we provide an overview of current clinical approaches for the diagnosis of PBD-ZSD and provide broad guidelines for the treatment of disease in its wide variety of forms. Although we anticipate future progress in the development of more effective targeted interventions, the current guidelines are meant to provide a starting point for the management of these complex conditions in the context of personalized health care.
Keywords: Peroxisome biogenesis disorders, Zellweger spectrum disorder, Treatment guidelines, PEX genes, Very long-chain fatty acids, Sensorineural hearing loss, Retinal dystrophy
1. Definition, nomenclature, and epidemiology
Peroxisomes are membrane-bound organelles found within almost all eukaryotic cells [1]. They are formed through replication by fission (the major pathway for peroxisome formation) or can originate from the endoplasmic reticulum (ER) through a de novo process [2]. Contained within the peroxisome matrix of mammalian cells are over 70 distinct enzymes required for normal lipid metabolism and a host of other biochemical processes critical for normal health and development [3].
Peroxisome biogenesis disorders (PBDs) are autosomal recessive disorders that are characterized by defective peroxisome biosynthesis, assembly, and biochemical functions [4]. Although it is estimated that 1 in 50,000 births are affected by PBDs in North America [5], these estimates may increase with the introduction of newborn screening for peroxisomal disorders across the United States [6]. PBDs are primarily caused by mutations in any of 14 different PEX genes, which code for peroxins, proteins involved in peroxisome assembly [5,7]. While mutations in PEX1 account for nearly 70% of all PBD-ZSD cases, another 26% of cases are caused by mutations in PEX6, PEX10, PEX12, or PEX26, with the majority of these cases involving PEX6 mutations [8,9].
PBDs are divided into 2 groups: Zellweger spectrum disorder (PBD-ZSD) and rhizomelic chondrodysplasia punctata type 1 [10,11]. The treatment guidelines presented here will refer only to PBD-ZSD. Prior to the discovery of their shared peroxisomal basis, three different syndromes were historically described: Zellweger syndrome (ZS), also referred to as cerebrohepatorenal syndrome; neonatal adreno-leukodystrophy (NALD), and infantile Refsum disease (IRD) [12]. We recommend replacing these names with the overall classification of peroxisome biogenesis disorders in the Zellweger spectrum (PBD-ZSD), ranging from severe (ZS), intermediate (NALD), and mild (IRD) phenotypes, respectively. The purpose of this recommendation is to highlight the fact that the individual clinical pictures are along a spectrum of disease severity and often do not fit into the original assigned categories. Additionally, we now also recognize a group of PBD-ZSD patients who do not exhibit the vision and hearing loss usually described in PBD-ZSD, and instead present with peripheral neuropathy and/or cerebellar ataxia [13–15]. Other variant phenotypes continue to be described [16,17]. These patients would be diagnosed as intermediate or mild within the PBD-ZSD spectrum. Table 1 summarizes the clinical features observed in PBD-ZSD based on disease severity and age of symptom appearance. Symptom expression in most patients has an age-dependent component related to disease severity and considerable overlap exists among patients with severe, intermediate and milder phenotypes. Although the relative proportions of certain features were reported in one cohort with a subset of PEX genotypes [18], the prevalence and timing of all outcomes amongst PBD-ZSD patients is not yet adequately described, nor is the risk known for individual patients to develop various postnatal features.
Table 1.
Clinical features of PBD-ZSD: severity, age of onset, and suggested treatments.
| Clinical features | Neonate | 1–6 months | 6 months–4 years | >4 years | Suggested treatments (if available) |
|---|---|---|---|---|---|
| Neuronal migration disorder | S | ||||
| Chondrodysplasia punctata | S | ||||
| Renal cortical microcysts | S | ||||
| Respiratory compromise | S | S | Oxygen support | ||
| Craniofacial dysmorphism | S, I | I, M | |||
| Direct hyperbilirubinemia | S, I, M | I, M | |||
| Liver dysfunction, hepatomegaly | S | I, M | Vitamin K supplementation, primary bile acid therapy | ||
| Failure to thrive, small size, hypotonia and poor feeding | S, I | I, M | I, M | M | Feeding therapy, G-tube placement, vitamins A, D, E, and K |
| Seizures | S | I, M | I, M | M | Antiepileptic drugs |
| Adrenal insufficiency | S | I, M | I, M | M | Hydrocortisone (Cortef) |
| Cataracts | S | I, M | I, M | M | Cataract removal |
| Retinal degeneration | I, M | M | Corrective lenses | ||
| Sensorineural hearing loss | S | I, M | I, M | M | Hearing aid, cochlear implant |
| Psychomotor retardation | S | I, M | I, M | M | Physical/occupational therapy |
| Leukodystrophy | I | I | M | ||
| Osteopenia | I | I, M | Vitamin D, calcium, bisphosphonate treatment | ||
| Calcium oxalate renal stones | I, M | I, M | Increased fluid intake, urine alkalinization | ||
| Peripheral neuropathy | M | M | |||
| Cerebellar ataxia | M | M | |||
| Enamel hypoplasia | I, M | Bonding, repair of permanent teeth |
Abbreviations: S, severe; I, intermediate; M, mild; G-tube, gastrostomy tube.
2. Laboratory diagnostic criteria
2.1. Traditional biochemical testing
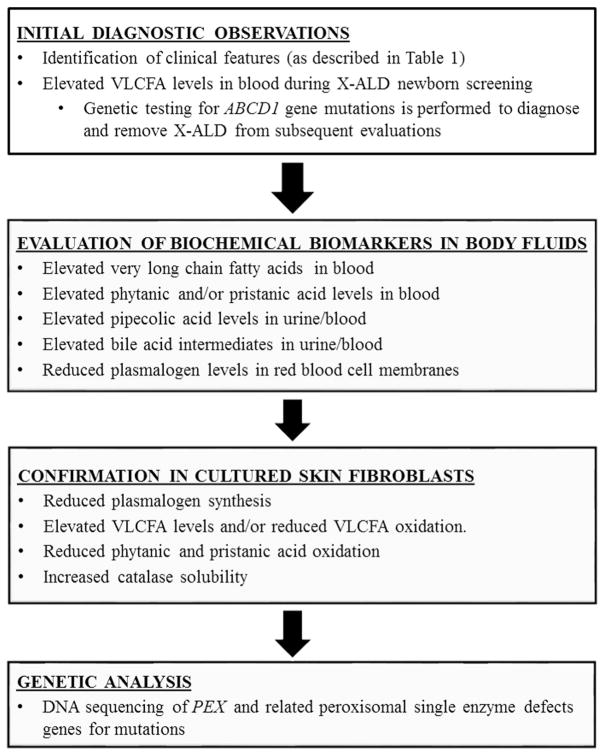
Since their initial discovery, an increasing number of biochemical functions have been ascribed to peroxisomes including β-oxidation of very long chain fatty acids (VLCFA, 24 carbons or longer) and pristanic acid, phytanic acid α-oxidation, pipecolic acid metabolism, ether glycerolipid (plasmalogen), bile acid biosynthesis, and subcellular localization of catalase (Table 2) [3]. PBDs can be diagnosed by demonstrating abnormalities in several peroxisome biochemical functions that can be monitored in bodily fluids (Fig. 1). The primary step in PBD-ZSD diagnosis generally involves the detection of elevated VLCFA in a fasting plasma sample [19]. Elevations of C26:0 and C26:1 fatty acids and the ratios of C24:0/C22:0 and C26:0/C22:0 are consistent with a peroxisomal fatty acid β-oxidation defect [5]. Although usually abnormal on a blood specimen drawn randomly during the day, equivocal results, for example, in the case of elevated C26:0 with normal or near normal ratios of 24:0/22:0 and 26:0/22:0, and a high total lipid fatty acid content, measurements should be repeated on a plasma sample after overnight fasting. False positive results have rarely been reported, although this can occur if patients are on a ketogenic diet [20,21]. Additional studies demonstrating defects in multiple peroxisome enzyme pathways are necessary to diagnose PBD-ZSD, such as measurement of the methyl-branched fatty acids phytanic and pristanic acids, erythrocyte plasmalogens, pipecolic acid in plasma and/or urine, and the bile acid intermediates dihydroxycholestanoic acid (DHCA) and trihydroxycholestanoic acid (THCA) in plasma and/or urine [11]. Reduced levels of erythrocyte plasmalogens, whose biosynthesis is dependent on peroxisome function, may be observed depending on disease severity [5]. It should be noted that pipecolic acid levels are more likely to be abnormal in urine in the newborn period, and more abnormal in plasma in later ages [22–24]. Additionally, phytanic and pristanic acids may not be elevated in newborn infants who are not consuming dairy products or other dietary sources of these fatty acids [19]. Owing to defective biosynthesis in liver peroxisomes of the final C24 bile acids, cholic and deoxycholic acids, there is elevation of C27 bile acid intermediates, DHCA and THCA, in blood and urine [3].
Table 2.
Diagnostically useful tests of peroxisome function in PBD-ZSD.
| Peroxisomal function | Tissue/cells tested | Findings in PBD-ZSD |
|---|---|---|
| β-Oxidation of VLCFA | Plasma/cultured fibroblasts | Elevated VLCFA in plasma, cells; deficient VLCFA oxidation in cells |
| β-Oxidation of branched-chain fatty acids (pristanic acid) | Plasma/cultured fibroblasts | Elevated pristanic acid in plasma; deficient pristanic acid oxidation in cells |
| α-Oxidation of branched-chain fatty acids (phytanic acid) | Plasma/cultured fibroblasts | Elevated phytanic acid in plasma; deficient phytanic acid oxidation in cells |
| Ether glycerolipid (plasmalogen) biosynthesis | RBC/cultured fibroblasts | Deficiency of RBC plasmalogens; deficient biosynthesis of plasmalogens in cells |
| Bile acid synthesis | Plasma/urine | Accumulation of C27 bile acids, DHCA and THCA |
| Pipecolic acid oxidation | Plasma/urine | Elevated pipecolic acid in plasma and urine |
| Catalase subcellular localization | Fibroblasts | Deficient peroxisomal catalase and elevated cytosolic catalase |
Abbreviations: PBD-ZSD, peroxisome biogenesis disorder-Zellweger spectrum disorder; RBC, red blood cell; VLCFA, very long-chain fatty acids; DHCA, dihydroxycholestanoic acid; THCA, trihydroxycholestanoic acid.
Fig. 1.
Diagnostic criteria flowchart for PBD-ZSD. Given the current availability of next generation sequencing panels, clinicians have moved from evaluation of biochemical markers to genetic analysis future reproductive options, carrier testing in relatives, eligibility purposes in clinical trials and for patients that are difficult to diagnose. In difficult cases, it may still be necessary to evaluate cultured fibroblasts, and this may be important also to ascertain responses of specific mutations for future interventional trials. Abbreviations: PBD-ZSD, peroxisome biogenesis disorder-Zellweger spectrum disorder; X-ALD, X-linked adrenoleukodystrophy; VLCFA, very long chain fatty acids.
Biochemical testing of skin fibroblasts is useful to confirm the metabolite abnormalities seen in the blood and urine and clarify questionable results in body fluids. The biochemical assays most frequently used in fibroblasts involve quantifying phytanic and pristanic acid oxidation, VLCFA accumulation and/or oxidation and plasmalogen biosynthesis [19]. Cultured skin fibroblasts are also valuable for establishing the subcellular localization of peroxisomal matrix proteins, such as catalase, which can distinguish PBD-ZSD from phenotypically similar peroxisomal single enzyme deficiencies [25].
Approximately 10–15% of suspected PBD-ZSD patients with elevated VLCFAs will not have PBD-ZSD, but a single β-oxidation enzyme defect in very long chain acyl-CoA oxidase (ACOX1) [26] or D-bifunctional protein (HSD17B4) [27]. The clinical phenotypes of these patients overlap that of PBD-ZSD. Other overlapping phenotypes include single enzyme/protein defects in branched chain fatty acid and bile acid metabolism, including α-methyl-acyl CoA racemase (AMACR) [28], phytanoyl-Coenzyme A hydroxylase (PHYH) [29], PEX7 [30] and sterol carrier protein X (SCPx) [31].
It is important to not rely on VLCFA screening alone for patients who are strongly suspected to have PBD-ZSD. In a small number of cases, mutations in PEX genes such as PEX2, PEX10, PEX12, PEX16 and PEX11B have been identified in patients with mild or absent elevations in VLCFA [9,13,32–35]. Consequently, testing for multiple biochemical functions in patients or obtaining biochemical studies on patient-derived fibroblasts and genetic testing may be necessary for proper diagnosis.
2.2. Genetic diagnostic testing
Next-generation sequencing panels for PEX genes are being used more frequently as a confirmatory test, and may be required for peroxisome disorders that are difficult to resolve by traditional biochemical methods [16,17,34,36–38]. These DNA tests are available on a clinical basis. Identification of mutations may have prognostic value [39]. For example, patients with two PEX null alleles generally have a severe phenotype, and those patients who carry the common PEX1-p.G843D allele are predicted to have a milder phenotype [40]. Homozygosity for PEX1-p.G843D typically predicts a milder phenotype, but even in this category there is a range of intellectual impairment to normal intellect, indicating that modifier genes, as yet to be identified, are influential [16,18]. The outcome of the combination of a PEX null allele with a missense allele can range from intermediate to milder, and this depends on the residual function of the missense allele. In a recent publication [17], certain missense alleles in PEX1 and PEX6, in combination with null alleles, defined a group of PBD-ZSD patients with normal intellect. In addition, patients with mutations in the region encoding the zinc finger domain of PEX2, PEX12 and PEX10, and certain mutations in PEX16 [13–15] exhibit variant phenotypes. In contrast to biochemical tests, mutation analysis will also identify heterozygous carriers, which will allow reliable genetic counseling of families and may assist with eligibility for future clinical trials.
2.3. Newborn screening
The combination of liquid chromatography and tandem mass spectrometry (LC–MS/MS) to detect elevated levels of VLCFAs in newborn blood spots has been validated as a diagnostic approach for X-linked adrenoleukodystrophy (X-ALD), a related peroxisomal disorder [6,41,42]. Legislation for X-ALD newborn screening has passed in New Jersey, Connecticut, Illinois, Tennessee and California and screening has begun in New York; continued legislative efforts are expected to expand through movements initiated by patient families and advocacy organizations to lobby their state legislatures. Recently, the Department of Health and Human Services Advisory Committee for Heritable Disorders for Newborns and Children voted to propose the addition of X-ALD screening in the Recommend Uniform Screening Panel. The implications of X-ALD newborn screening include the ability to perform clinical surveillance for early detection of symptom onset and treatment for affected males and counseling for carrier females [43]. Newborn screening for X-ALD should also detect the majority of PBD-ZSD cases that feature elevated blood VLCFA levels, thereby permitting early diagnosis and determination of accurate incidence estimates. As newborn screening expands in the future, the diagnostic approach for PBD-ZSDs will necessarily be revised toward more confirmatory testing as seen in other newborn screening diseases. It is anticipated that the clinical phenotype of PBD-ZSD will be expanded as variant patients are identified.
2.4. Prenatal diagnosis of PBD-ZSD
Prenatal diagnosis of PBD-ZSD can be accomplished in the first or second trimester using biochemical or genetic testing of chorionic villi cells or cultured amniocytes. Preimplantation genetic diagnosis can also be performed when the PEX gene mutations are known [44].
3. Management and treatment guidelines
PBD-ZSD is a multi-organ disease, as peroxisomes are involved in critical metabolic pathways in nearly all the cells of the body from fetal development throughout adult life [4]. The wide variation in clinical severity and rate of disease progression adds complexity to the medical management of the group as a whole. With the recognition that some manifestations of PBD-ZSD arise during fetal development and cannot be reversed, particularly any brain dysplasia, therapeutic expectations for some neurologic symptoms must be tempered. Nevertheless, additional medical issues arise postnatally that can benefit from current therapy. At this time, treatment of any manifestations of PBD-ZSD focuses largely on symptomatic or supportive therapies. The following guidelines are meant as a starting point for management of these complex conditions for personalized medical care.
3.1. Clinical evaluations following initial diagnosis
Table 3 summarizes recommended clinical evaluations at the time of the initial diagnosis of PBD-ZSD to establish the extent of disease and later in life as symptoms appear. It is likely that some of the recommended evaluations will have been completed by the time a PBD-ZSD diagnosis has been confirmed. Patients should be re-evaluated yearly (or more frequently) to detect progression of disease and begin timely therapy.
Table 3.
Recommended evaluations for PBD-ZSD patients.
| Symptoms | Specific examinations | Suspected findings in severe PBD-ZSD | Suspected findings in intermediate/mild PBD-ZSD |
|---|---|---|---|
| Growth failure | Height, weight and head circumference, nutritional evaluation | Poor growth, feeding difficulties, fat soluble vitamin deficiency | Poor growth, feeding difficulties, fat soluble vitamin deficiency |
| Deafness | Hearing testing, brainstem auditory evoked responses | Bilateral sensorineural deafness | Progressive bilateral sensorineural hearing loss, deafness |
| Visual impairment | Ophthalmologic exam, visual fields, fundus photography, OCT | Cataracts, glaucoma, optic nerve hypoplasia | Progressive retinal dystrophy, blindness, band keratopathy |
| Neurological | Brain MRI, EEG, nerve conduction studies | Hypotonia, neuronal migration defects on MRI, neonatal seizures | Hypotonia, leukodystrophy, cerebellar atrophy on MRI, seizures, peripheral neuropathy, ataxia |
| Hepatic dysfunction | AST, ALT, GGT, bilirubin, albumin, alkaline phosphatase, bile acids (intermediate C27 and mature C24 bile acids, PT, PTT, abdominal ultrasound | Hepatomegaly, elevated transaminases, cholestasis, defective synthetic functions, portal hypertension, | Same as severe ZSD, but milder |
| Renal insufficiency | Serum creatinine, BUN, abdominal ultrasound, urine oxalate | Renal cortical cysts | Calcium oxalate renal stones |
| Adrenal insufficiency, hyponatremia, hypotension, vomiting | Adrenal function tests, early morning (8 am) cortisol and ACTH, ACTH stimulation test | Progressive adrenal insufficiency | Progressive adrenal insufficiency |
| Skeletal abnormalities, fractures | X-rays, DXA scan, serum calcium and phosphorous, alkaline phosphatase | Chondrodysplasia punctata, hips and knees | Low bone mineral density, pathological fractures |
| Dental | Dental exam, X-rays | Enamel hypoplasia of secondary teeth | |
| Psychomotor retardation | Developmental assessment | Few developmental milestones gained | Delayed milestones with broad range of achievement from cognitive delay to normal cognition |
Abbreviations: PBD-ZSD, peroxisome biogenesis disorder-Zellweger spectrum disorder; ERG, electroretinogram; OCT, optical coherence tomography; MRI, magnetic resonance imaging; EEG, electrocephalogram; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; PT, prothrombin time; PTT, partial thromboplastin time; BUN, blood urea nitrogen; ACTH, adrenocorticotropic hormone; DXA, dual-energy x-ray absorptiometry.
Severe PBD-ZSD patients present in the neonatal period and have developmental malformations of the brain, kidneys and skeleton (Table 1). They have a more predictable clinical course than milder forms of PBD-ZSD. There is a characteristic craniofacial dysmorphology that includes an enlarged fontanelle, prominent forehead, epicanthal folds, hypertelorism, a broad, flat nasal bridge and migronathia [5,45]. Neonatal seizures, severe hypotonia and developmental delays are consequent to neuronal migrations defects that characteristically appear as polymicrogyria and heterotopias on brain magnetic resonance imaging [46]. Renal micronodular cortical cysts can be observed by renal ultrasound and are not usually symptomatic. An enlarged liver with dysfunction of the hepatocellular and biliary system is typically present. Due to the severe hypotonia, feeding difficulties are often prominent, as well as laryngomalacia and other respiratory dysfunction [45]. Developmental progress is usually minimal. For seizure control, standard antiepileptic drugs (AED) may be used. No type of AED is contraindicated, although certain medications that have respiratory suppressive effects must be avoided if respiratory compromise is present. Seizures may be difficult to control despite use of appropriate medication. Feeding problems may require the placement of a gastrostomy tube (G-tube). With regards to respiratory therapy, use of nasal cannula for oxygen may be necessary as the disease progresses. The transition to a more aggressive type of respiratory support is a decision that should be discussed between the family and medical care team with informed expectations about survival and quality of life. Overall, for severe PBD-ZSD, seizure control, feeding and respiratory support are often the main focus for management, although additional interventions as described below may also be valuable for quality of life.
For the majority of patients who present with intermediate or milder PBD-ZSD, the details of the management are discussed below.
3.2. Feeding and nutrition
Many PBD-ZSD children have significant food selectivity and the involvement of a behavioral feeding program is often indicated in the older PBD-ZSD child. Supplying adequate calorie intake for affected children may entail the placement of a G-tube to allow simpler home management. With many children having some degree of malabsorption due to bile acid deficiency, elemental formulas may be better tolerated.
Currently, there is no specific diet that is recommended for PBD-ZSD patients. Although VLCFA levels are elevated in the tissues and body fluids of PBD-ZSD patients, it is unclear as to whether a reduction in dietary VLCFA will prevent the progression of the disease or its associated symptoms [47]. A reduction in dietary VLCFA alone has not been shown to reduce blood VLCFA levels [48], as the body produces most VLCFA endogenously. Plasma VLCFA levels are decreased only by the combination of dietary reduction of VLCFA and supplementation with Lorenzo’s oil (a 4:1 mixture of glyceryl trioleate and glyceryl trierucate) in X-ALD patients [49], but this does not affect the progression of an already established leukodystrophy [50–52]. Moreover, increased dietary monounsaturated fatty acids in Lorenzo’s oil may be contraindicated in PBD-ZSD patients who already accumulate large amounts of C26:1 due to defective VLCFA oxidation [53]. Finally, the effects of these dietary interventions have not been studied in PBD-ZSD patients.
Phytanic acid is a methyl branched-chain fatty acid exclusively obtained from dietary sources such as ruminant fats, dairy products, and certain fish [54]. As such, it can be eliminated by dietary restriction [54]. There is a minor amount of phytanic acid in human breast milk [55]. Dietary restriction of phytanic acid might be considered, since high phytanic acid levels over time could contribute to disease through mechanisms similar to that observed in adult Refsum disease [56,57]. In contrast to most patients with adult Refsum disease, however, PBD-ZSD patients tend to have normal or lower plasma phytanic acid levels [58] and no studies have demonstrated specific effects of phytanic acid accumulation from other peroxisomal defects in PBD-ZSD patients. Until definitive studies are conducted, it seems reasonable to monitor plasma phytanic acid levels and consider dietary modification if levels become excessive.
Since PBD-ZSD patients have impaired endogenous synthesis of docosahexaenoic acid (DHA) [59], and DHA is important in brain and retinal development and function, supplementation with DHA, was previously recommended. However, a placebo-controlled study showed no clinical benefit of DHA supplementation in enrolled patients in the PBD-ZSD spectrum. [60]. Owing to defective bile acid synthesis, supplements of the fat-soluble vitamins, A, D, E, and K are recommended.
3.3. Liver
To help support liver function, supplementation of vitamin K at a dose of 2.5 mg–5 mg per day is recommended. Bile acid metabolism is altered in PBD-ZSD [61,62], and primary bile acid therapy (cholic acid and chenodeoxycholic acid) may improve liver function by reducing the accumulation of abnormal bile acid precursors, such as DHCA and THCA [63,64]. Recently, cholic acid (Cholbam) has been approved by the United States Food and Drug Administration to treat peroxisomal disorders, including PBD-ZSD [65]. The available studies evaluating the effectiveness of bile acid therapy in PBD-ZSD are limited and may have differential effects depending on the severity of the disease. Coagulation factors and other synthetic liver functions should be monitored. Persons with overt liver dysfunction require more frequent monitoring and may benefit from referral to a gastroenterologist. Liver dysfunction may lead to varices that respond to appropriate therapies.
3.4. Hearing
Many patients with PBD-ZSD have some degree of hearing loss [66]; auditory functions should therefore be evaluated annually in children affected with PBD-ZSD. Hearing aids should be used in children found to have substantive hearing loss. Cochlear implants have been effectively placed in PBD-ZSD children when hearing loss is severe and cannot be compensated by hearing aids. In such instances, improvements in environmental awareness, and in some circumstances, speech, have been frequently noted in other syndromes with congenital deafness [67].
3.5. Vision
Vision loss is commonly seen with PBD-ZSD due to retinal dystrophy and optic nerve abnormalities [16,68,69]. Therefore, periodic ophthalmologic evaluations are indicated. Although cataracts are rare, if present, their removal in early infancy may preserve vision with the understanding that retinal dysfunction may later develop. Glasses should be used, as needed, to correct refractive errors. In children with confirmed PBD-ZSD, there appears to be no value in performing multiple electroretinograms (ERG) to assess functional vision. ERG testing has not been demonstrated to be predictive of vision and does not provide an index of progression [60]. Performing optical coherence tomography in children who can cooperate by looking directly at a light source may be useful for defining and monitoring retinal health. For children with both hearing and vision impairment, enrollment in the deaf-blind community is strongly encouraged. Appropriate resources include the National Family Association for Deaf–Blind (http://www.nfadb.org) and the National Center on Deaf–Blindness (https://nationaldb.org), which can provide connections to individual state deaf-blind projects.
3.6. Neurological function
Seizures have been observed in the neonatal period in nearly all severely affected PBD-ZSD patients [70], and have been reported in 23% of less severe patients [18]. EEGs can determine the frequency and duration of seizures and should be performed whenever changes in seizure activity are suspected. Common medications used to control seizures in children affected by PBD-ZSD are levetiracetam, phenobarbital, clonazepam, topiramate, and lamotrigine.
PBD-ZSD patients can also develop a leukodystrophy [18,64], which can be silent, arrested or progressive. We recommend a baseline MRI of the brain, followed by additional studies if clinically indicated. Identification of white matter changes can have prognostic significance for changes in cognitive, behavioral and/or motor abilities.
Evaluation for early physical, occupational and speech therapy is recommended for all children with PBD-ZSD. Therefore, early intervention services should be provided.
3.7. Bone
Children with severe PBD-ZSD may have chondrodysplasia punctata or stippling seen at the growth plates. Decreased bone mineral density that worsens over time is associated with intermediate and milder forms of PBD-ZSD and pathologic fractures have occurred in some patients with no evidence of trauma. The incidence of bone disease in the course of PBD-ZSD has not been systematically studied. In patients who are older than 1 year and are non-weight bearing, or have had previous fractures, evaluation for bone disease should be considered. This should include dual-energy x-ray absorptiometry (DXA) that has been well-validated in pediatric patients. Evaluation of vitamin D status is also recommended. At the discretion of the clinician, markers of bone turnover such as phosphorus and parathyroid hormone levels may also be evaluated.
Regarding treatment of bone disease in PBD-ZSD, a recent study has reported successful treatment with bisphosphonate medications in a PBD-ZSD patient [71]. Bisphosphonate therapy should be carefully considered in consultation with an experienced metabolic bone specialist. Additionally, weight bearing physical activity has shown to slow bone loss in children and therefore prevent fractures [72].
3.8. Teeth
Dental examination should be performed every 6 months. Many children with PBD-ZSD have enamel abnormalities of permanent teeth and should receive appropriate dental care [73–75].
3.9. Adrenal insufficiency
As with other peroxisomal disorders, particularly X-ALD, primary adrenal insufficiency has occurred in PBD-ZSD. A recent study reported a high prevalence of primary adrenal insufficiency in a population of 29 PBD-ZSD patients [76]. It is recommended that after one year of age, yearly (or more frequent) adrenal monitoring with adrenocorticotropic hormone (ACTH) and morning cortisol be performed. Treatment with adrenal replacement using standard dosing should be instituted if abnormal. Families and clinicians should be aware of the possibility of adrenal insufficiency and consider stress dosing in periods of sudden severe illness, fever, and major surgical procedures.
3.10. Kidney
Children affected by PBD-ZSD, particularly older children (≥4–6 years), should be monitored for hyperoxaluria, which can lead to kidney stone formation and renal failure [77]. This can be determined by measuring oxalic acid and creatinine in the urine. Kidney ultrasound may be useful to detect renal stones.
3.11. Other recommendations
It is also recommended that all patients on the PBD-ZSD spectrum should be vaccinated against influenza and respiratory syncytial virus yearly, in addition to the normal course of vaccination for other childhood diseases.
4. Future directions
The treatment guidelines discussed herein provide a starting point for the personalized management of PBD-ZSD based on current medical practice; however, we anticipate these guidelines will evolve over time as emerging therapeutic strategies for PBDs are tested in laboratory settings and eventually in clinical trials. A robust portfolio of in vitro and whole organism models of PBD-ZSD provides the basis for laboratory research. Cultured patient cells, including skin fibroblasts, have provided invaluable for screening and testing drug therapies in vitro [78]. Most recently, PBD-ZSD patient-derived skin fibroblasts have been reprogrammed into induced pluripotent stem cells (iPSCs) that were differentiated into neural and hepatic cell models of disease that could be used in drug screening and testing efforts [79]. There are several genetically engineered mouse models of PEX gene defects [80], including a model of the common PEX1 p.G843D mutation [81]. In addition, a host of invertebrate models of PBD-ZSD exist including genetically engineered worms, fruit flies, and zebrafish [82]. All provide opportunities to screen for and/or test specific therapies on the scale of the whole organism.
The principal strategies being actively pursued include high-content screening of large chemical libraries for compounds that improve peroxisome assembly and function, as well as gene and cellular therapies. Seminal screening studies identified betaine as a potential molecular chaperone that can improve peroxisome assembly in cultured cells from PBD-ZSD patients with PEX1 p.G843D mutations [78]. Candidate drug screens identified arginine as another potential molecular chaperone in patient cell lines [83]. Larger-scale drug screens are currently being conducted at the National Center for Advancing Translational Sciences at the National Institutes of Health (Hacia, personal communication). Advances in gene therapy, including the emergence of adeno-associated virus (AAV) gene delivery systems, provide hope for the treatment of numerous genetic disorders, including PBD-ZSD. Multiple successful retinal gene augmentation trials for Leber congenital amaurosis (LCA) [84], a rare inherited eye disease, is of special relevance of PBD-ZSD. Currently, AAV9-mediated gene augmentation therapy for vision loss in PBD-ZSD is being developed and will be tested in mouse models of milder forms of PBD-ZSD (Bennett, personal communication). Given that PBD-ZSD is a multi-systemic disease, gene therapy aimed at correcting peroxisome assembly in other organs, most notably the central nervous system (CNS) and the liver, is of great interest to the medical research community. Finally, we recognize potential therapeutic opportunities for cellular therapies, including the transplant of cell types and cell lineages affected in PBD-ZSD patients.
5. Concluding remarks
With greater understanding of the full range of severity seen in PBD-ZSD, physicians can transition to a more targeted approach to supportive therapies. Vision and hearing interventions, nutrition provisions, along with monitoring for adrenal insufficiency, renal stones, bone density and dental enamel defects, can all enhance quality of life for PBD-ZSD patients. Further research into all of the variation in PBD-ZSD children is urgently needed in order to provide more evidence-based guidelines. Our established ongoing longitudinal natural history study on PBD-ZSD will help us acquire and disseminate information regarding this disease, and to identify accurate clinical endpoints for future interventional trials (https://clinicaltrials.gov/ct2/show/NCT01668186?term=NCT01668186&rank=1).
Acknowledgments
The authors wish to acknowledge Ms. Heidi Harris, Ms. Shannon Butalla and Ms. Melissa Gamble for their assistance in the preparation of this manuscript.
Abbreviations
- AAV
adeno-associated virus
- AED
anti-epileptic drug(s)
- DHA
docosahexaenoic acid
- DHCA
dihydroxycholestanoic acid
- ERG
electroretinogram
- G-tube
gastrostomy tube
- IRD
infantile Refsum disease
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- NALD
neonatal adrenoleukodystrophy
- OCT
optical coherence tomography
- PBD
peroxisome biogenesis disorder
- PBD-ZSD
peroxisome biogenesis disorder-Zellweger spectrum disorder
- THCA
trihydroxycholestanoic acid
- VLCFA
very long chain fatty acid(s)
- X-ALD
X-linked adrenoleukodystrophy
- ZS
Zellweger syndrome
References
- 1.Islinger M, Grille S, Fahimi HD, Schrader M. The peroxisome: an update on mysteries. Histochem Cell Biol. 2012;137(5):547–574. doi: 10.1007/s00418-012-0941-4. http://dx.doi.org/10.1007/s00418-012-0941-4. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal G, Subramani S. Emerging role of the endoplasmic reticulum in peroxisome biogenesis. Front Physiol. 2013;4:286. doi: 10.3389/fphys.2013.00286. http://dx.doi.org/10.3389/fphys.2013.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. http://dx.doi.org/10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 4.Braverman NE, D’Agostino MD, Maclean GE. Peroxisome biogenesis disorders: Biological, clinical and pathophysiological perspectives. Dev Disabil Res Rev. 2013;17(3):187–196. doi: 10.1002/ddrr.1113. http://dx.doi.org/10.1002/ddrr.1113. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW. Peroxisome biogenesis disorders. Biochim Biophys Acta. 2006;1763(12):1733–1748. doi: 10.1016/j.bbamcr.2006.09.010. http://dx.doi.org/10.1016/j.bbamcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Theda C, Gibbons K, Defor TE, Donohue PK, Golden WC, Kline AD, Gulamali-Majid F, Panny SR, Hubbard WC, Jones RO, Liu AK, Moser AB, Raymond GV. Newborn screening for X-linked adrenoleukodystrophy: further evidence high throughput screening is feasible. Mol Genet Metab. 2014;111(1):55–57. doi: 10.1016/j.ymgme.2013.10.019. http://dx.doi.org/10.1016/j.ymgme.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebberink MS, Koster J, Visser G, Spronsen F, Stolte-Dijkstra I, Smit GP, Fock JM, Kemp S, Wanders RJ, Waterham HR. A novel defect of peroxisome division due to a homozygous non-sense mutation in the PEX11 beta gene. J Med Genet. 2012;49(5):307–313. doi: 10.1136/jmedgenet-2012-100778. http://dx.doi.org/10.1136/jmedgenet-2012-100778. [DOI] [PubMed] [Google Scholar]
- 8.Yik WY, Steinberg SJ, Moser AB, Moser HW, Hacia JG. Identification of novel mutations and sequence variation in the Zellweger syndrome spectrum of peroxisome biogenesis disorders. Hum Mutat. 2009;30(3):E467–E480. doi: 10.1002/humu.20932. http://dx.doi.org/10.1002/humu.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebberink MS, Mooijer PA, Gootjes J, Koster J, Wanders RJ, Waterham HR. Genetic classification and mutational spectrum of more than 600 patients with a Zellweger syndrome spectrum disorder. Hum Mutat. 2011;32(1):59–69. doi: 10.1002/humu.21388. http://dx.doi.org/10.1002/humu.21388. [DOI] [PubMed] [Google Scholar]
- 10.Braverman NE, Moser AB, Steinberg SJ. Rhizomelic chondrodysplasia punctata type 1. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] University of Washington; Seattle, Seattle (WA): Nov 16, 2001. (1993–2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1270/, Updated 2012 Sep 13) [Google Scholar]
- 11.Steinberg SJ, Raymond GV, Braverman NE, Moser AB. Peroxisome biogenesis disorders, Zellweger syndrome spectrum. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] University of Washington; Seattle, Seattle (WA): Dec 12, 2003. (1993–2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1448/, Updated 2012 May 10) [Google Scholar]
- 12.Zellweger H, Maertens P, Superneau D, Wertelecki W. History of the cerebrohepatorenal syndrome of Zellweger and other peroxisomal disorders. South Med J. 1988;81(3):357–364. doi: 10.1097/00007611-198803000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Ebberink MS, Csanyi B, Chong WK, Denis S, Sharp P, Mooijer PA, Dekker CJ, Spooner C, Ngu LH, De Sousa C, Wanders RJ, Fietz MJ, Clayton PT, Waterham HR, Ferdinandusse S. Identification of an unusual variant peroxisome biogenesis disorder caused by mutations in the PEX16 gene. J Med Genet. 2010;47(9):608–615. doi: 10.1136/jmg.2009.074302. http://dx.doi.org/10.1136/jmg.2009.074302. [DOI] [PubMed] [Google Scholar]
- 14.Regal L, Ebberink MS, Goemans N, Wanders RJ, De Meirleir L, Jaeken J, Schrooten M, Van Coster R, Waterham HR. Mutations in PEX10 are a cause of autosomal recessive ataxia. Ann Neurol. 2010;68(2):259–263. doi: 10.1002/ana.22035. http://dx.doi.org/10.1002/ana.22035. [DOI] [PubMed] [Google Scholar]
- 15.Sevin C, Ferdinandusse S, Waterham HR, Wanders RJ, Aubourg P. Autosomal recessive cerebellar ataxia caused by mutations in the PEX2 gene. Orphanet J Rare Dis. 2011;6:8. doi: 10.1186/1750-1172-6-8. http://dx.doi.org/10.1186/1750-1172-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majewski J, Wang Z, Lopez I, Al Humaid S, Ren H, Racine J, Bazinet A, Mitchel G, Braverman N, Koenekoop RK. A new ocular phenotype associated with an unexpected but known systemic disorder and mutation: novel use of genomic diagnostics and exome sequencing. J Med Genet. 2011;48(9):593–596. doi: 10.1136/jmedgenet-2011-100288. http://dx.doi.org/10.1136/jmedgenet-2011-100288. [DOI] [PubMed] [Google Scholar]
- 17.Ratbi I, Falkenberg KD, Sommen M, Al-Sheqaih N, Guaoua S, Vandeweyer G, Urquhart JE, Chandler KE, Williams SG, Roberts NA, El Alloussi M, Black GC, Ferdinandusse S, Ramdi H, Heimler A, Fryer A, Lynch SA, Cooper N, Ong KR, Smith CE, Inglehearn CF, Mighell AJ, Elcock C, Poulter JA, Tischkowitz M, Davies SJ, Sefiani A, Mironov AA, Newman WG, Waterham HR, Van Camp G. Heimler syndrome is caused by hypomorphic mutations in the peroxisome-biogenesis genes PEX1 and PEX6. Am J Hum Genet. 2015;97(4):535–545. doi: 10.1016/j.ajhg.2015.08.011. http://dx.doi.org/10.1016/j.ajhg.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poll-The BT, Gootjes J, Duran M, De Klerk JB, Wenniger-Prick LJ, Admiraal RJ, Waterham HR, Wanders RJ, Barth PG. Peroxisome biogenesis disorders with prolonged survival: phenotypic expression in a cohort of 31 patients. Am J Med Genet A. 2004;126A(4):333–338. doi: 10.1002/ajmg.a.20664. http://dx.doi.org/10.1002/ajmg.a.20664. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg S, Jones R, Tiffany C, Moser A. Investigational methods for peroxisomal disorders. Curr Protoc Hum Genet. 2008;Chapter 17(Unit 17):6. doi: 10.1002/0471142905.hg1706s58. http://dx.doi.org/10.1002/0471142905.hg1706s58. [DOI] [PubMed] [Google Scholar]
- 20.Stradomska TJ, Bachanski M, Pawlowska J, Syczewska M, Stolarczyk A, Tylki-Szymanska A. The impact of a ketogenic diet and liver dysfunction on serum very long-chain fatty acids levels. Lipids. 2013;48(4):405–409. doi: 10.1007/s11745-013-3761-y. http://dx.doi.org/10.1007/s11745-013-3761-y. [DOI] [PubMed] [Google Scholar]
- 21.Theda C, Woody RC, Naidu S, Moser AB, Moser HW. Increased very long chain fatty acids in patients on a ketogenic diet: a cause of diagnostic confusion. J Pediatr. doi: 10.1016/s0022-3476(06)80013-2. [DOI] [PubMed] [Google Scholar]
- 22.Peduto A, Baumgartner MR, Verhoeven NM, Rabier D, Spada M, Nassogne MC, Poll-The BT, Bonetti G, Jakobs C, Saudubray JM. Hyperpipecolic acidaemia: a diagnostic tool for peroxisomal disorders. Mol Genet Metab. 2004;82(3):224–230. doi: 10.1016/j.ymgme.2004.04.010. http://dx.doi.org/10.1016/j.ymgme.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Waterham HR, Ebberink MS. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim Biophys Acta. 2012;1822(9):1430–1441. doi: 10.1016/j.bbadis.2012.04.006. http://dx.doi.org/10.1016/j.bbadis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg SJ, Snowden A, Braverman NE, Chen L, Watkins PA, Clayton PT, Setchell KD, Heubi JE, Raymond GV, Moser AB, Moser HW. A PEX10 defect in a patient with no detectable defect in peroxisome assembly or metabolism in cultured fibroblasts. J Inherit Metab Dis. 2009;32(1):109–119. doi: 10.1007/s10545-008-0969-8. http://dx.doi.org/10.1007/s10545-008-0969-8. [DOI] [PubMed] [Google Scholar]
- 25.Krause C, Rosewich H, Gartner J. Rational diagnostic strategy for Zellweger syndrome spectrum patients. Eur J Hum Genet. 2009;17(6):741–748. doi: 10.1038/ejhg.2008.252. http://dx.doi.org/10.1038/ejhg.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferdinandusse S, Denis S, Hogenhout EM, Koster J, van Roermund CWIJL, Moser AB, Wanders RJ, Waterham HR. Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Hum Mutat. 2007;28(9):904–912. doi: 10.1002/humu.20535. http://dx.doi.org/10.1002/humu.20535. [DOI] [PubMed] [Google Scholar]
- 27.Lieber DS, Hershman SG, Slate NG, Calvo SE, Sims KB, Schmahmann JD, Mootha VK. Next generation sequencing with copy number variant detection expands the phenotypic spectrum of HSD17B4-deficiency. BMC Med Genet. 2014;15:30. doi: 10.1186/1471-2350-15-30. http://dx.doi.org/10.1186/1471-2350-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haugarvoll K, Johansson S, Tzoulis C, Haukanes BI, Bredrup C, Neckelmann G, Boman H, Knappskog PM, Bindoff LA. MRI characterisation of adult onset alpha-methylacyl-coA racemase deficiency diagnosed by exome sequencing. Orphanet J Rare Dis. 2013;8:1. doi: 10.1186/1750-1172-8-1. http://dx.doi.org/10.1186/1750-1172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanders RJA, Waterham HR, Leroy BP. Refsum disease. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] University of Washington; Seattle, Seattle (WA): Mar 20, 2006. (1993–2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1353/, Updated 2015 Jun 11) [Google Scholar]
- 30.Wierzbicki AS. Peroxisomal disorders affecting phytanic acid alpha-oxidation: a review. Biochem Soc Trans. 2007;35(Pt 5):881–886. doi: 10.1042/BST0350881. http://dx.doi.org/10.1042/BST0350881. [DOI] [PubMed] [Google Scholar]
- 31.Ferdinandusse S, Kostopoulos P, Denis S, Rusch H, Overmars H, Dillmann U, Reith W, Haas D, Wanders RJ, Duran M, Marziniak M. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukencephalopathy with dystonia and motor neuropathy. Am J Hum Genet. 2006;78(6):1046–1052. doi: 10.1086/503921. http://dx.doi.org/10.1086/503921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignarri A, Vinciguerra C, Giorgio A, Ferdinandusse S, Waterham H, Wanders R, Bertini E, Dotti MT, Federico A. Zellweger spectrum disorder with mild phenotype caused by PEX2 gene mutations. JIMD Rep. 2012;6:43–46. doi: 10.1007/8904_2011_102. http://dx.doi.org/10.1007/8904_2011_102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohba C, Osaka H, Iai M, Yamashita S, Suzuki Y, Aida N, Shimozawa N, Takamura A, Doi H, Tomita-Katsumoto A, Nishiyama K, Tsurusaki Y, Nakashima M, Miyake N, Eto Y, Tanaka F, Matsumoto N, Saitsu H. Diagnostic utility of whole exome sequencing in patients showing cerebellar and/or vermis atrophy in childhood. Neurogenetics. 2013;14(3–4):225–232. doi: 10.1007/s10048-013-0375-8. http://dx.doi.org/10.1007/s10048-013-0375-8. [DOI] [PubMed] [Google Scholar]
- 34.Schabhuttl M, Wieland T, Senderek J, Baets J, Timmerman V, De Jonghe P, Reilly MM, Stieglbauer K, Laich E, Windhager R, Erwa W, Trajanoski S, Strom TM, Auer-Grumbach M. Whole-exome sequencing in patients with inherited neuropathies: outcome and challenges. J Neurol. 2014;261(5):970–982. doi: 10.1007/s00415-014-7289-8. http://dx.doi.org/10.1007/s00415-014-7289-8. [DOI] [PubMed] [Google Scholar]
- 35.Zeharia A, Ebberink MS, Wanders RJ, Waterham HR, Gutman A, Nissenkorn A, Korman SH. A novel PEX12 mutation identified as the cause of a peroxisomal biogenesis disorder with mild clinical phenotype, mild biochemical abnormalities in fibroblasts and a mosaic catalase immunofluorescence pattern, even at 40 degrees C. J Hum Genet. 2007;52(7):599–606. doi: 10.1007/s10038-007-0157-y. http://dx.doi.org/10.1007/s10038-007-0157-y. [DOI] [PubMed] [Google Scholar]
- 36.Levesque S, Morin C, Guay SP, Villeneuve J, Marquis P, Yik WY, Jiralerspong S, Bouchard L, Steinberg S, Hacia JG, Dewar K, Braverman NE. A founder mutation in the PEX6 gene is responsible for increased incidence of Zellweger syndrome in a French Canadian population. BMC Med Genet. 2012;13:72. doi: 10.1186/1471-2350-13-72. http://dx.doi.org/10.1186/1471-2350-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Wang L, Wei X, Zhu Q, Yang Y, Lan Z, Qu N, Chu Y, Wang Y, Yang S, Liang Y, Wang W, Yi X. Analysis of a Chinese pedigree with Zellweger syndrome reveals a novel PEX1 mutation by next-generation sequencing. Clin Chim Acta. 2013;417:57–61. doi: 10.1016/j.cca.2012.12.005. http://dx.doi.org/10.1016/j.cca.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Buchert R, Tawamie H, Smith C, Uebe S, Innes AM, Al Hallak B, Ekici AB, Sticht H, Schwarze B, Lamont RE, Parboosingh JS, Bernier FP, Abou Jamra R. A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am J Hum Genet. 2014;95(5):602–610. doi: 10.1016/j.ajhg.2014.10.003. http://dx.doi.org/10.1016/j.ajhg.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosewich H, Ohlenbusch A, Gartner J. Genetic and clinical aspects of Zellweger spectrum patients with PEX1 mutations. J Med Genet. 2005;42(9):e58. doi: 10.1136/jmg.2005.033324. http://dx.doi.org/10.1136/jmg.2005.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walter C, Gootjes J, Mooijer PA, Portsteffen H, Klein C, Waterham HR, Barth PG, Epplen JT, Kunau WH, Wanders RJ, Dodt G. Disorders of peroxisome biogenesis due to mutations in PEX1: phenotypes and PEX1 protein levels. Am J Hum Genet. 2001;69(1):35–48. doi: 10.1086/321265. http://dx.doi.org/10.1086/321265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubbard WC, Moser AB, Liu AC, Jones RO, Steinberg SJ, Lorey F, Panny SR, Vogt RF, Jr, Macaya D, Turgeon CT, Tortorelli S, Raymond GV. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC–MS/MS) method. Mol Genet Metab. 2009;97(3):212–220. doi: 10.1016/j.ymgme.2009.03.010. http://dx.doi.org/10.1016/j.ymgme.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Vogel BH, Bradley SE, Adams DJ, D’Aco K, Erbe RW, Fong C, Iglesias A, Kronn D, Levy P, Morrissey M, Orsini J, Parton P, Pellegrino J, Saavedra-Matiz CA, Shur N, Wasserstein M, Raymond GV, Caggana M. Newborn screening for X-linked adrenoleukodystrophy in New York State: diagnostic protocol, surveillance protocol and treatment guidelines. Mol Genet Metab. 2015;114(4):599–603. doi: 10.1016/j.ymgme.2015.02.002. http://dx.doi.org/10.1016/j.ymgme.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Raymond GV, Jones RO, Moser AB. Newborn screening for adrenoleukodystrophy: implications for therapy. Mol Diagn Ther. 2007;11(6):381–384. doi: 10.1007/BF03256261. [DOI] [PubMed] [Google Scholar]
- 44.Al-Sayed M, Al-Hassan S, Rashed M, Qeba M, Coskun S. Preimplantation genetic diagnosis for Zellweger syndrome. Fertil Steril. 2007;87(6):1468. doi: 10.1016/j.fertnstert.2006.09.014. http://dx.doi.org/10.1016/j.fertnstert.2006.09.014 (e1-3) [DOI] [PubMed] [Google Scholar]
- 45.Lee PR, Raymond GV. Child neurology: Zellweger syndrome. Neurology. 2013;80(20):e207–e210. doi: 10.1212/WNL.0b013e3182929f8e. http://dx.doi.org/10.1212/WNL.0b013e3182929f8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkovich AJ, Peck WW. MR of Zellweger syndrome. AJNR Am J Neuroradiol. 1997;18(6):1163–1170. [PMC free article] [PubMed] [Google Scholar]
- 47.Van Duyn MA, Moser AE, Brown FR, III, Sacktor N, Liu A, Moser HW. The design of a diet restricted in saturated very long-chain fatty acids: therapeutic application in adrenoleukodystrophy. Am J Clin Nutr. 1984;40(2):277–284. doi: 10.1093/ajcn/40.2.277. [DOI] [PubMed] [Google Scholar]
- 48.Brown FR, III, Van Duyn MA, Moser AB, Schulman JD, Rizzo WB, Snyder RD, Murphy JV, Kamoshita S, Migeon CJ, Moser HW. Adrenoleukodystrophy: effects of dietary restriction of very long chain fatty acids and of administration of carnitine and clofibrate on clinical status and plasma fatty acids. Johns Hopkins Med J. 1982;151(4):164–172. [PubMed] [Google Scholar]
- 49.Moser AB, Borel J, Odone A, Naidu S, Cornblath D, Sanders DB, Moser HW. A new dietary therapy for adrenoleukodystrophy: biochemical and preliminary clinical results in 36 patients. Ann Neurol. 1987;21(3):240–249. doi: 10.1002/ana.410210305. http://dx.doi.org/10.1002/ana.410210305. [DOI] [PubMed] [Google Scholar]
- 50.Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaque I, Jakobezak C, Lemaitre A, Boureau F, Wolf C, et al. A two-year trial of oleic and erucic acids (“Lorenzo’s oil”) as treatment for adrenomyeloneuropathy. N Engl J Med. 1993;329(11):745–752. doi: 10.1056/NEJM199309093291101. http://dx.doi.org/10.1056/NEJM199309093291101. [DOI] [PubMed] [Google Scholar]
- 51.Rizzo WB, Leshner RT, Odone A, Dammann AL, Craft DA, Jensen ME, Jennings SS, Davis S, Jaitly R, Sgro JA. Dietary erucic acid therapy for X-linked adrenoleukodystrophy. Neurology. 1989;39(11):1415–1422. doi: 10.1212/wnl.39.11.1415. [DOI] [PubMed] [Google Scholar]
- 52.Uziel G, Bertini E, Bardelli P, Rimoldi M, Gambetti M. Experience on therapy of adrenoleukodystrophy and adrenomyeloneuropathy. Dev Neurosci. 1991;13(4–5):274–279. doi: 10.1159/000112173. [DOI] [PubMed] [Google Scholar]
- 53.Moser AE, Singh I, Brown FR, III, Solish GI, Kelley RI, Benke PJ, Moser HW. The cerebrohepatorenal (Zellweger) syndrome. Increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984;310(18):1141–1146. doi: 10.1056/NEJM198405033101802. http://dx.doi.org/10.1056/NEJM198405033101802. [DOI] [PubMed] [Google Scholar]
- 54.Watkins PA, Moser AB, Toomer CB, Steinberg SJ, Moser HW, Karaman MW, Ramaswamy K, Siegmund KD, Lee DR, Ely JJ, Ryder OA, Hacia JG. Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functions. BMC Physiol. 2010;10:19. doi: 10.1186/1472-6793-10-19. http://dx.doi.org/10.1186/1472-6793-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egge H, Murawski U, Gyorgy P, Zilliken F. Minor constituents of human milk (I) identification of cyclohexaneundecanoic acid and phytanic acid in human milk fat by a combination gas chromatograph-mass spectrometer. FEBS Lett. 1969;2(4):255–258. doi: 10.1016/0014-5793(69)80035-9. [DOI] [PubMed] [Google Scholar]
- 56.Millar JH. Refsum disease—the effect of diet. Ulster Med J. 1985;54(1):41–45. [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg D, Mize CE, Herndon JH, Jr, Fales HM, Engel WK, Vroom FQ. Phytanic acid in patients with Refsum’s syndrome and response to dietary treatment. Arch Intern Med. 1970;125(1):75–87. [PubMed] [Google Scholar]
- 58.Takemoto Y, Suzuki Y, Horibe R, Shimozawa N, Wanders RJ, Kondo N. Gas chromatography/mass spectrometry analysis of very long chain fatty acids, docosahexaenoic acid, phytanic acid and plasmalogen for the screening of peroxisomal disorders. Brain Dev. 2003;25(7):481–487. doi: 10.1016/s0387-7604(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 59.Martinez M. Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Res. 1992;583(1–2):171–182. doi: 10.1016/s0006-8993(10)80021-6. [DOI] [PubMed] [Google Scholar]
- 60.Paker AM, Sunness JS, Brereton NH, Speedie LJ, Albanna L, Dharmaraj S, Moser AB, Jones RO, Raymond GV. Docosahexaenoic acid therapy in peroxisomal diseases: results of a double-blind, randomized trial. Neurology. 2010;75(9):826–830. doi: 10.1212/WNL.0b013e3181f07061. http://dx.doi.org/10.1212/WNL.0b013e3181f07061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clayton PT. Inborn errors of bile acid metabolism. J Inherit Metab Dis. 1991;14(4):478–496. doi: 10.1007/BF01797919. [DOI] [PubMed] [Google Scholar]
- 62.Lawson AM, Madigan MJ, Shortland D, Clayton PT. Rapid diagnosis of Zellweger syndrome and infantile Refsum’s disease by fast atom bombardment–mass spectrometry of urine bile salts. Clin Chim Acta. 1986;161(2):221–231. doi: 10.1016/0009-8981(86)90215-9. [DOI] [PubMed] [Google Scholar]
- 63.Setchell KD, Bragetti P, Zimmer-Nechemias L, Daugherty C, Pelli MA, Vaccaro R, Gentili G, Distrutti E, Dozzini G, Morelli A, et al. Oral bile acid treatment and the patient with Zellweger syndrome. Hepatology. 1992;15(2):198–207. doi: 10.1002/hep.1840150206. [DOI] [PubMed] [Google Scholar]
- 64.Maeda K, Kimura A, Yamato Y, Nittono H, Takei H, Sato T, Mitsubuchi H, Murai T, Kurosawa T. Oral bile Acid treatment in two Japanese patients with Zellweger syndrome. J Pediatr Gastroenterol Nutr. 2002;35(2):227–230. doi: 10.1097/00005176-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 65.United States Food and Drug Administration. FDA approves Cholbam to treat rare bile acid synthesis disorders [Press Release] 2015 Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm438572.htm.
- 66.Theil AC, Schutgens RB, Wanders RJ, Heymans HS. Clinical recognition of patients affected by a peroxisomal disorder: a retrospective study in 40 patients. Eur J Pediatr. 1992;151(2):117–120. doi: 10.1007/BF01958955. [DOI] [PubMed] [Google Scholar]
- 67.Broomfield SJ, Bruce IA, Henderson L, Ramsden RT, Green KM. Cochlear implantation in children with syndromic deafness. Int J Pediatr Otorhinolaryngol. 2013;77(8):1312–1316. doi: 10.1016/j.ijporl.2013.05.022. http://dx.doi.org/10.1016/j.ijporl.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Wilson GN, Holmes RG, Custer J, Lipkowitz JL, Stover J, Datta N, Hajra A. Zellweger syndrome: diagnostic assays, syndrome delineation, and potential therapy. Am J Med Genet. 1986;24(1):69–82. doi: 10.1002/ajmg.1320240109. http://dx.doi.org/10.1002/ajmg.1320240109. [DOI] [PubMed] [Google Scholar]
- 69.Hittner HM, Kretzer FL, Mehta RS. Zellweger syndrome. Lenticular opacities indicating carrier status and lens abnormalities characteristic of homozygotes. Arch Ophthalmol. 1981;99(11):1977–1982. doi: 10.1001/archopht.1981.03930020853008. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi Y, Suzuki Y, Kumazaki K, Tanabe Y, Akaboshi S, Miura K, Shimozawa N, Kondo N, Nishiguchi T, Terada K, Orii T. Epilepsy in peroxisomal diseases. Epilepsia. 1997;38(2):182–188. doi: 10.1111/j.1528-1157.1997.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 71.Rush ET, Goodwin JL, Braverman NE, Rizzo WB. Low bone mineral density is a common feature of Zellweger spectrum disorders. Mol Genet Metab. 2015 doi: 10.1016/j.ymgme.2015.11.009. http://dx.doi.org/10.1016/j.ymgme.2015.11.009. [DOI] [PubMed]
- 72.Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res. 2014;29(2):467–478. doi: 10.1002/jbmr.2036. http://dx.doi.org/10.1002/jbmr.2036. [DOI] [PubMed] [Google Scholar]
- 73.Acharya BS, Ritwik P, Velasquez GM, Fenton SJ. Medical-dental findings and management of a child with infantile Refsum disease: a case report. Spec Care Dentist. 2012;32(3):112–117. doi: 10.1111/j.1754-4505.2012.00248.x. http://dx.doi.org/10.1111/j.1754-4505.2012.00248.x. [DOI] [PubMed] [Google Scholar]
- 74.Lertsirivorakul J, Wongswadiwat M, Treesuwan P. Oral manifestations and dental management of a child with Zellweger syndrome. Spec Care Dentist. 2014;34(1):46–50. doi: 10.1111/scd.12003. http://dx.doi.org/10.1111/scd.12003. [DOI] [PubMed] [Google Scholar]
- 75.Tran D, Greenhill W, Wilson S. Infantile refsum disease with enamel defects: a case report. Pediatr Dent. 2011;33(3):266–270. [PubMed] [Google Scholar]
- 76.Berendse K, Engelen M, Linthorst GE, van Trotsenburg AS, Poll-The BT. High prevalence of primary adrenal insufficiency in Zellweger spectrum disorders. Orphanet J Rare Dis. 2014;9:133. doi: 10.1186/s13023-014-0133-5. http://dx.doi.org/10.1186/s13023-014-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Woerden CS, Groothoff JW, Wijburg FA, Duran M, Wanders RJ, Barth PG, Poll-The BT. High incidence of hyperoxaluria in generalized peroxisomal disorders. Mol Genet Metab. 2006;88(4):346–350. doi: 10.1016/j.ymgme.2006.03.004. http://dx.doi.org/10.1016/j.ymgme.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhang R, Chen L, Jiralerspong S, Snowden A, Steinberg S, Braverman N. Recovery of PEX1-Gly843Asp peroxisome dysfunction by small-molecule compounds. Proc Natl Acad Sci U S A. 2010;107(12):5569–5574. doi: 10.1073/pnas.0914960107. http://dx.doi.org/10.1073/pnas.0914960107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang XM, Yik WY, Zhang P, Lu W, Huang N, Kim BR, Shibata D, Zitting M, Chow RH, Moser AB, Steinberg SJ, Hacia JG. Induced pluripotent stem cell models of Zellweger spectrum disorder show impaired peroxisome assembly and cell type-specific lipid abnormalities. Stem Cell Res Ther. 2015;6:158. doi: 10.1186/s13287-015-0149-3. http://dx.doi.org/10.1186/s13287-015-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baes M, Van Veldhoven PP. Mouse models for peroxisome biogenesis defects and beta-oxidation enzyme deficiencies. Biochim Biophys Acta. 2012;1822(9):1489–1500. doi: 10.1016/j.bbadis.2012.03.003. http://dx.doi.org/10.1016/j.bbadis.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Hiebler S, Masuda T, Hacia JG, Moser AB, Faust PL, Liu A, Chowdhury N, Huang N, Lauer A, Bennett J, Watkins PA, Zack DJ, Braverman NE, Raymond GV, Steinberg SJ. The Pex1-G844D mouse: a model for mild human Zellweger spectrum disorder. Mol Genet Metab. 2014;111(4):522–532. doi: 10.1016/j.ymgme.2014.01.008. http://dx.doi.org/10.1016/j.ymgme.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Veldhoven PP, Baes M. Peroxisome deficient invertebrate and vertebrate animal models. Front Physiol. 2013;4:335. doi: 10.3389/fphys.2013.00335. http://dx.doi.org/10.3389/fphys.2013.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berendse K, Ebberink MS, Ijlst L, Poll-The BT, Wanders RJ, Waterham HR. Arginine improves peroxisome functioning in cells from patients with a mild peroxisome biogenesis disorder. Orphanet J Rare Dis. 2013;8:138. doi: 10.1186/1750-1172-8-138. http://dx.doi.org/10.1186/1750-1172-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pierce EA, Bennett J. The status of RPE65 gene therapy trials: safety and efficacy. Cold Spring Harb Perspect Med. 2015;5(9):a017285. doi: 10.1101/cshperspect.a017285. http://dx.doi.org/10.1101/cshperspect.a017285. [DOI] [PMC free article] [PubMed] [Google Scholar]