Abstract
Abstinent alcohol-dependent individuals experience an enduring sensitivity to cue-induced craving and relapse to drinking. There is considerable evidence indicating that structures within the midbrain and extended amygdala are involved in this process. Individually, the ventral tegmental area (VTA) and the bed nucleus of the stria terminalis (BNST) have been shown to modulate cue-induced ethanol-seeking behavior. It is hypothesized that cue-induced seeking is communicated through a direct projection from the BNST to VTA. In the current experiments, an intersectional viral strategy was used in DBA/2J mice to selectively target and inhibit BNST projections to the VTA during a test of ethanol conditioned place preference (CPP). Inhibitory designer receptors exclusively activated by designer drugs (hM4Di DREADDs) were expressed in VTA-projecting BNST (BNST-VTA) cells by infusing a retrograde herpes-simplex virus encoding cre recombinase (HSV-Cre) into VTA and a cre-inducible adeno-associated virus encoding hM4Di (AAV-DIO-hM4Di) into BNST. Before testing the expression of preference, clozapine-N-oxide (CNO) was peripherally administered to activate hM4Di receptors and selectively inhibit these cells. Ethanol CPP expression was blocked by CNO-mediated inhibition of BNST-VTA cells. A follow-up study revealed this effect was specific to CNO activation of hM4Di as saline- and CNO-treated mice infused with a control vector (HSV-GFP) in place of HSV-Cre showed significant CPP. These findings establish a role for a direct BNST input to VTA in cue-induced ethanol-seeking behavior.
1. Introduction
It is thought that mesocorticolimbic dopamine (DA) transmission directs reward-related behaviors and is a prime neural signal driving drug seeking and relapse. Originating predominantly from within the ventral tegmental area (VTA), this DA signal has been the focus of many studies. The bulk of these studies have centered on neural circuits originating within the VTA and projecting to outside targets such as the nucleus accumbens (reviewed in Ikemoto, 2007). However, attention to VTA afferents and their influence in generating diverse motivational states is growing.
Evidence is emerging that one source of input to VTA, the extended amygdala, is highly involved in regulating VTA-mediated states of reward and aversion. Specifically, the bed nucleus of the stria terminalis (BNST) of the extended amygdala sends strong projections to VTA (Dong & Swanson, 2004; 2006a; 2006b; Kudo et al., 2012) that potently innervate DA cells (Georges & Aston-Jones, 2001; 2002) and inhibit gamma-aminobutyric acid (GABA) cells (Jennings et al., 2013; Kudo et al., 2014). Behavioral evidence further implicates the BNST in drug seeking induced by exposure to stress- and drug-associated cues. Inactivation of this region has been shown to impair drug- and cue-primed reinstatement of heroin seeking (Rogers, Ghee, & See, 2008) as well as stress- and cue-induced reinstatement of cocaine seeking (Buffalari & See, 2011), and cocaine conditioned place preference (CPP) expression (Sartor & Aston-Jones, 2012). The BNST appears to be also involved in ethanol seeking. Not only is this structure activated by ethanol-associated cues (Dayas, Liu, Simms, & Weiss, 2007; Hill, Ryabinin, & Cunningham, 2007; Zhao et al., 2006), but its direct inhibition impairs the expression of an ethanol place preference (Pina, Young, Ryabinin, & Cunningham, 2015).
It is unclear however whether the BNST’s involvement in cue-induced drug seeking is driven by its input to the VTA. Previous studies attempted to address this question using a combination of tract tracing and c-Fos staining or intracranial pharmacological manipulations. For example, retrogradely labeled BNST projections to the VTA showed enhanced c-Fos immunoreactivity following cue-induced cocaine seeking and cocaine CPP expression (Mahler & Aston-Jones, 2012; Sartor & Aston-Jones, 2012). Pharmacological disconnection procedures have also shown that a BNST-VTA projection is involved in cocaine CPP expression (Sartor & Aston-Jones, 2012) and stress-induced cocaine seeking (Vranjkovic, Gasser, Gerndt, Baker, & Mantsch, 2014).
Though informative, these studies involved methodologies that do not allow direct circuit-selective manipulation during seeking behavior. Recent advances in viral-mediated gene transfer have provided an unprecedented opportunity for not just cell-type selective targeting but also circuit-selective targeting. Most notably, an intersectional strategy involving infusion of two viruses – a retrograde vector encoding cre recombinase and a cre-dependent adeno-associated virus (AAV) vector – into two distinct yet directly connected nuclei has provided a means for discrete circuit modulation. This approach has been successfully employed in rats using a cre-encoding canine adenovirus-2 (CAV-2) to express designer receptors exclusively activated by designer drugs (DREADD) in projection neurons to and from the VTA (Boender et al., 2014; Nair, Strand, & Neumaier, 2013). This approach enables the direct manipulation of VTA circuit activity during the performance of behavioral tasks. A similar strategy has also been applied in mice using an HSV vector to express channelrhodopsin-2 in distinct populations of VTA efferents (Fenno et al., 2014; Stamatakis et al., 2013).
In the present experiments, we investigated the role of a direct neuronal projection from the BNST to VTA in seeking behavior induced by an ethanol-associated cue. We employed a convergent dual-virus strategy to selectively manipulate BNST-VTA cells in mice during ethanol-seeking behavior. An HSV encoding cre recombinase (HSV-Cre) was combined with a cre-inducible AAV encoding the inhibitory hM4Di DREADD (AAV-DIO-hM4Di). In this manner, hM4Di expression was restricted to BNST-VTA projection neurons, enabling us to inhibit their activity during ethanol seeking modeled in a CPP procedure. We hypothesized that inactivation of BNST-VTA cells during the CPP test would disrupt ethanol place preference expression, thus suggesting that ethanol seeking is conveyed through a direct BNST projection to VTA.
2. Materials and Methods
2.1. Animals
Male DBA/2J mice (n=116; The Jackson Laboratory, Sacramento, CA) were 7 weeks old at arrival. Mice were housed 4/cage in a colony room maintained at 21±1°C set to a 12:12 light-dark cycle (lights on at 07:00 am). Home cage access to food and water was provided ad libitum throughout all experiments. All procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 2011) and were approved by Oregon Health & Science University’s Institutional Animal Care and Use Committee and Institutional Biosafety Committee.
2.2. Drugs
Ethanol (20% v/v in 0.9% saline) was administered intraperitoneally (IP) at a dose of 2 g/kg (12.5 mL/kg).
To stimulate hM4Di receptors, clozapine-N-oxide (CNO; Tocris Bioscience, Ellisville, MO) dissolved in 0.9% saline was administered at a dose of 10 mg/kg (10 mL/kg, IP) 30 min before the CPP test. In the absence of DREADD expression, this dose of CNO produces no physiological or behavioral response in rodents (Mahler et al., 2014; Ray et al., 2011; 2013; Vazey & Aston-Jones, 2014). In addition, we have shown that in the absence of hM4Di, CNO (10 or 20 mg/kg) does not affect ethanol CPP expression or locomotor activity in DBA/2J mice (Pina et al., 2015).
2.3. Stereotaxic surgery
Mice were anesthetized with isoflurane (4% in O2 for induction) and secured in a stereotaxic frame (Kopf Instruments, Tujunga, CA). Anesthesia was maintained (1–3% isoflurane in O2) for the duration of the procedure. To minimize post-operative discomfort, meloxicam (0.2 mg/kg; 10 mL/kg) was subcutaneously delivered immediately before and 24 h after surgery. Stereotaxic coordinates for VTA and BNST were derived from a standard atlas of the mouse brain (Paxinos & Franklin, 2001) and both regions were targeted during the same surgery. For VTA, injectors were aimed at the more medial aspect of this region (from bregma: posterior (AP) −3.2, lateral (ML) ±0.5, ventral (DV) −4.69). To avoid the lateral ventricles, the BNST was approached at a 20° coronal angle. Starting coordinates for BNST were as follows: AP+0.26, ML±0.8, DV-4.07 from bregma. Entry holes were drilled in the skull ±2.3 mm lateral and +0.26 mm rostral to bregma. The head was then tilted 20° left or right on a coronal axis and an injector was lowered 4.33 mm from the top of the skull on each side. Vectors were delivered using 32-ga stainless steel injectors (26-ga encasing) attached via polyethylene tubing (PE-20) to 1 µl Hamilton syringes. Infusions were delivered by syringe pump (Harvard Apparatus, Plymouth Meeting, PA) at a rate of 20 nL/min and injectors were left in place for 5 min after infusions. A post-surgical delay of 8 weeks was used. This delay was based on the following: (1) VTA to BNST retrograde transport and full transgene expression were achieved within 2 weeks of HSV-GFP delivery (Supplementary Fig. 1); (2) Axonal transport of most AAV serotypes occurs within 4–9 weeks (Castle, Gershenson, Giles, Holzbaur, & Wolfe, 2014; Salegio et al., 2012; Smith, Bucci, Luikart, & Mahler, 2016); and (3) our previous work has demonstrated robust AAV-mediated hM4Di expression in soma and axons of BNST neurons within 6 weeks (Pina et al., 2015).
2.4. Viral-mediated gene transfer
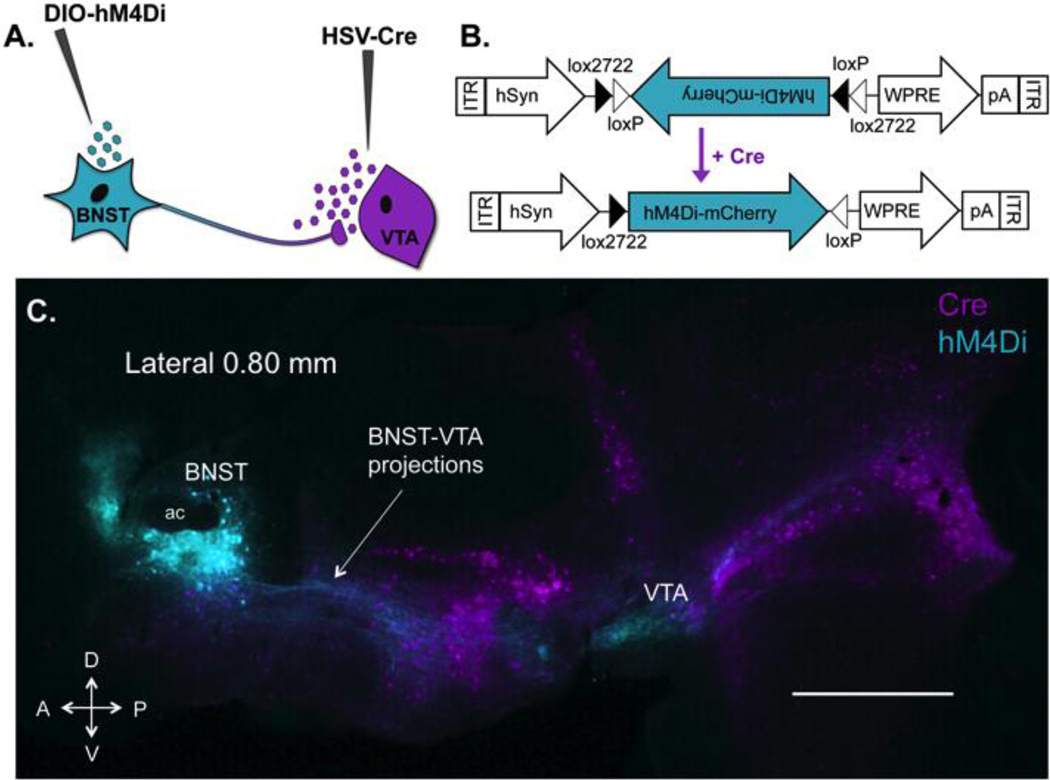
To inhibit the BNST-VTA circuit, hM4Di DREADDs (Armbruster, Li, Pausch, Herlitze, & Roth, 2007) were selectively expressed in BNST-VTA cells. Transgene expression in this discrete subset of BNST neurons was achieved using a retrograde intersectional approach that combines two viral vectors (Fig. 1). First, a retrograde HSV-Cre vector was infused into the VTA to drive long-term expression of cre-recombinase in VTA-projecting cells. Next, cre-inducible AAV-DIO-hM4Di was delivered into the BNST to selectively express hM4Di in VTA-projecting BNST cells.
Figure 1. Expression of hM4Di receptors in VTA-projecting BNST neurons using a retrograde intersectional strategy.
(A) A dual-viral approach was used to drive expression of inhibitory designer receptors (hM4Di) in a distinct yet intermixed subpopulation of BNST neurons that project to VTA. A long-term retrograde HSV encoding cre recombinase was delivered into the VTA and a cre-dependent AAV-hM4Di was delivered into the BNST. (B) hM4Di-mCherry AAV vector design employing the double-floxed inverted open reading frame (DIO) strategy. Two pairs of heterotypic, antiparallel loxP-type recombination sites achieve cre-mediated hM4Di inversion and expression under the control of a human synapsin (hSyn) promoter. WPRE, woodchuck hepatitis virus post-transcriptional regulatory element; ITR, inverted terminal repeat, pA, human growth hormone polyadenylation site (C) Sagittal section showing intersection of retrogradely transported HSV-cre (VTA-projecting cre+ cells are pseudocolored magenta) and AAV expressing cre-dependent hM4Di (hM4Di+ BNST-VTA cells are pseudocolored cyan) 8 weeks after vector infusions. Robust expression of hM4Di is visible in soma (in BNST) and fibers (to VTA) of BNST-VTA neurons. HSV-cre transfected neurons projecting to VTA are visible throughout the brain, with the exception of VTA as retrograde HSV does not infect cell bodies at the site of injection. Note the absence of hM4Di in all HSV-Cre transfected cells outside the BNST. This illustrates that hM4Di is localized to VTA projecting neurons within the BNST only. D, dorsal; V, ventral; A, anterior; P, posterior; scale bar, 1 mm.
2.4.1. Herpes simplex virus (HSV) vector
HSV vectors were purchased from Massachusetts Institute of Technology’s Viral Gene Transfer Core (Cambridge, MA) and infused bilaterally into VTA (200 nL/side). HSV encoding for cre recombinase (HSV-Cre; hEF1α-EGFP-IRES-Cre, > 3×10e8 TU/mL) was used in Exp. 1, whereas a control HSV carrying an enhanced green fluorescence protein-encoding gene (HSV-GFP; hEF1α-EGFP, > 3×10e8 TU/mL) was used in Exp. 2. The control HSV-GFP vector served as a (1) retrograde tracer to determine the post-infusion delay required for maximal transgene expression in BNST (Supplementary Fig. 1), and a (2) control vector to test for nonspecific effects of surgery and transgene expression on behavior (Exp. 2).
2.4.2. Adeno-associated virus (AAV) vector
In all experiments, AAV8-hSyn-DIO-hM4D(Gi)-mCherry (AAV-DIO-hM4Di; 5–7×10e12 vg/mL, University of North Carolina Vector Core, Chapel Hill, NC) was infused bilaterally into BNST (200 nL/side).
2.5. Immunohistochemistry
Mice were anesthetized with isoflurane and transcardially perfused with ice cold 1X phosphate buffered saline (PBS; pH 7.4), then 4% paraformaldehyde (PFA) in 1X PBS. Brains were removed and immersed overnight in 4% PFA, then cryoprotected in 20% sucrose in PBS containing 0.1% NaN3 (to inhibit bacterial growth) for 24–48 h, followed by 30% sucrose/0.1% NaN3/PBS for 24–48 h. Using a cryostat, coronal sections (30 µm) were taken from the rostral end of BNST to the caudal end of VTA (from +0.62 to −4.0 mm from bregma). From each cohort, mice from CNO and vehicle groups (n=1, each) were sagittally sectioned (60 µm) to view the rostral-caudal extent of hM4Di+ BNST projections to VTA. Slices were stored in 24-well plates containing 0.1% NaN3/PBS until free-floating sections were processed for immunofluorescence (IF) to detect GFP/YFP (HSV-GFP and HSV-Cre) and mCherry-tagged hM4Di protein. Sections were washed in PBS, then immersed in sodium borohydride (NaBH4, 1% w/v in 1X PBS) for 30 min to minimize fixative-induced autofluorescence (Beisker, Dolbeare, & Gray, 1987). Tissue was rinsed in tris-buffered saline (TBS) to remove NaBH4 before being permeabilized and blocked in 5% normal donkey serum (NDS)/0.3% Triton X-100/TBS for 45 min. Sections were incubated overnight with gentle agitation at 4°C in 5% NDS/0.3% Triton X-100/TBS containing a goat polyclonal antibody to GFP/YPF and a rabbit polyclonal antibody to mCherry (1:2000 each; Abcam, Cambridge, MA). Next, sections were rinsed in TBS and incubated for 2 h in 5% NDS/0.3% Triton X-100/TBS containing Alexa Fluor 488-conjugated donkey anti-goat IgG and Alexa Fluor 594-conjugated donkey anti-rabbit IgG (1:400 each; Abcam). After final washes, sections were rinsed in PBS, mounted on gelatinized slides, coverslipped and sealed in ProLong Diamond with DAPI (Life Technologies, Eugene, OR). Tissue was imaged on a Leica DM4000 B microscope. Slices from the BNST to VTA of each mouse were analyzed to determine the location and extent of transgene expression. For presentation, channels were merged and images cropped and contrast adjusted in Fiji (NIH).
2.6. Apparatus
Detailed descriptions of the CPP apparatus have been published (Cunningham, Gremel, & Groblewski, 2006). In brief, animals were conditioned in 12 identical chambers, each housed in light- and sound-attenuating enclosures. Locomotor activity and position within the apparatus were detected by infrared phototransistors lining each chamber. Interchangeable tactile floor halves (grid or hole) that are equally preferred by drug-naïve DBA/2J mice (Cunningham, Ferree, & Howard, 2003) were used as conditioned stimuli (CS).
2.7. CPP Procedure
General methods of our ethanol CPP procedure have been described in detail (Cunningham et al., 2006; Pina et al., 2015). Mice were randomly assigned to one of two drug treatment groups, CNO or vehicle. An unbiased procedure was used that involved three phases: habituation (one 5-min session), conditioning (four 5-min sessions) and preference test (one 30-min session).
2.7.1. Habituation
Habituation was conducted between the hours of 12 – 2 pm and consisted of one 5-min session, where mice were injected with saline and immediately placed inside the apparatus on a white paper floor.
2.7.2. Conditioning
After assignment to drug treatment groups (CNO or vehicle), mice were subdivided into conditioning subgroups (Grid+ or Grid−). In the Grid+ subgroup, ethanol was paired with the grid floor (CS+) and saline was paired with the hole floor (CS−), whereas in the Grid− subgroup, ethanol was paired with the hole floor (CS+) and saline was paired with the grid floor (CS−). During conditioning, a clear acrylic divider was placed in the center of the apparatus to separate the floor cues. The position of each floor type was counterbalanced (i.e., left vs. right) within each conditioning subgroup. A two-trial-per-day procedure was used, where saline (CS−) trials were administered in the morning from 10 am – 12 pm and ethanol (CS+) trials were administered in the afternoon from 2 to 4 pm. Mice received two 5-min conditioning trials of each type (CS+ and CS−) over a 2-day period before the preference test.
2.7.3. Place preference test
A preference test was performed 24 h after the last CS+ conditioning session. Testing took place between the hours of 12 – 2 pm. The acrylic divider was removed before the test and mice were given free access to the entire chamber consisting of both grid and hole floors for 30 min. No ethanol was administered on the test day; instead, a saline injection was given in place of ethanol. In order to activate hM4Di receptors, an IP injection of CNO (10 mg/kg) was delivered 30 min before the preference test.
2.8. Experimental Design
2.8.1. Exp. 1 – Effect of BNST-VTA inhibition on ethanol CPP expression
In Exp. 1 (n = 70), the involvement of BNST-VTA cells in ethanol CPP expression was assessed. Designer hM4Di receptors were selectively expressed in all mice by delivering HSV-Cre into VTA and AAV-DIO-hM4Di into BNST. To activate hM4Di receptors and thereby inhibit BNST-VTA cells, CNO was administered before the CPP test.
2.8.2. Exp. 2 – Effect of control transgene expression on ethanol CPP
In Exp. 2 (n = 46), we tested for hM4Di expression in the absence of Cre (i.e., viral leakage) and the effect of CNO and control transgene expression on ethanol CPP. Hence, this control experiment was designed to 1) confirm that expression of hM4Di was dependent on the presence of cre and therefore confined to BNST-VTA cells, and 2) control for the non-specific effects of surgery, transgene expression, and CNO on ethanol CPP expression. All mice were infused with HSV-GFP (no cre) in VTA and AAV-DIO-hM4Di in BNST. As in Exp. 1, CNO was administered before the CPP test.
2.9. General Statistical Analysis
2.9.1. Preference tests
The main dependent variable for preference test analyses was time spent on the grid floor. Place preference was denoted when a significant difference in grid time between Grid+ and Grid− subgroups was found (Cunningham et al., 2003; 2006). Preference data were analyzed by two-way ANOVA (drug×conditioning), where drug indicates test pre-treatment (CNO vs. vehicle) and conditioning refers to conditioning subgroup (Grid+ vs. Grid−). To assess the impact of BNST-VTA inactivation over the course of the test, data were expressed as percent time on the ethanol-paired floor (CS+) by collapsing across conditioning subgroups (Grid+ and Grid−), then averaged across 5-min intervals and analyzed by two-way mixed-factorial ANOVA (drug × time). Test activity was analyzed by one-way ANOVA (drug).
2.9.2. Conditioning activity
Conditioning activity (counts/min) was collapsed over sessions by trial type (CS+ and CS−) and analyzed by two-way mixed-factorial ANOVA (drug×trial type).
3. Results
3.1. Exp. 1 – Effect of BNST-VTA inhibition on ethanol CPP expression
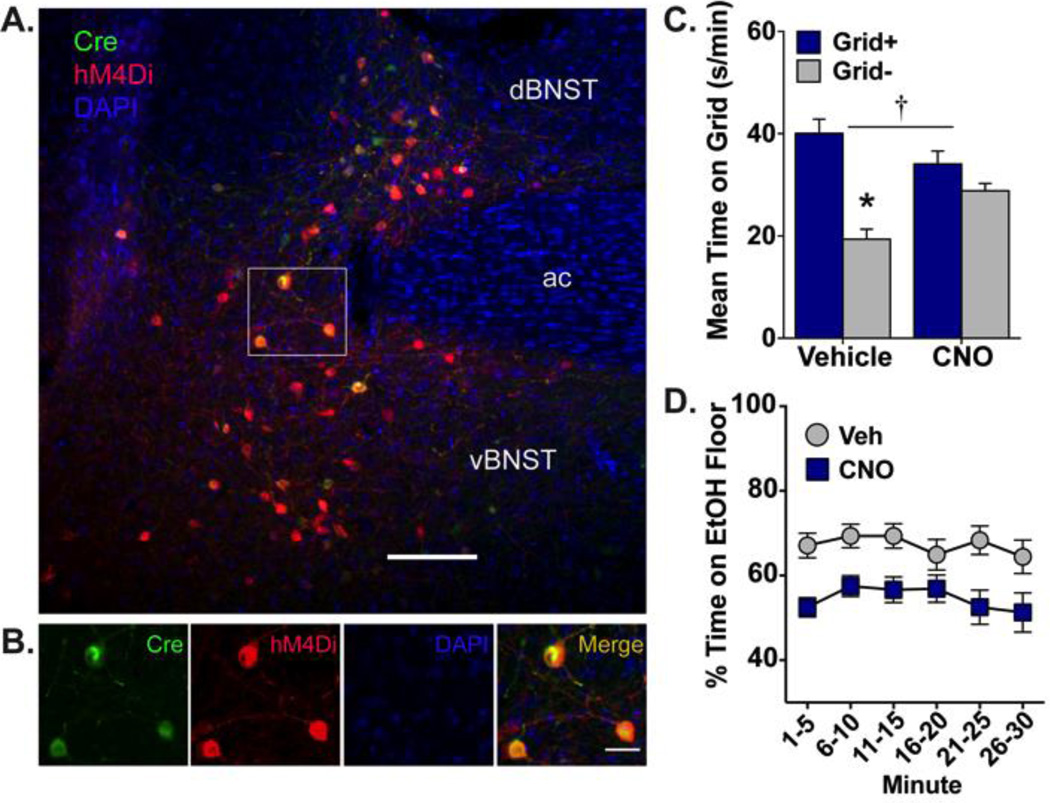
The involvement of a BNST projection to VTA in ethanol place preference expression was assessed in Exp. 1. Using an intersectional viral approach, hM4Di receptors were selectively expressed in BNST-VTA cells and activated during the expression test by CNO. Figures 1C and 1A–B illustrate the intersection of AAV-DIO-hM4Di (cyan; injected into BNST) and HSV-Cre (magenta; injected into VTA). Robust expression of hM4Di was visible in BNST 8 weeks after vector infusions. Mice were excluded from analyses for surgical issues (n=3) or hM4Di expression that was absent (bilateral n=1; unilateral n=7) or outside BNST (n=1), resulting in a final sample size of n=58. As shown in Fig. 2C, inactivation of the BNST-VTA cells (via CNO-induced hM4Di activation) blocked ethanol CPP expression [interaction: F(1,54)=11.48, p≤0.001; conditioning main effect: F(1,54)=32.45, p<0.001; no drug main effect]. Post-hoc analyses revealed a significant difference in time spent on the grid floor between conditioning subgroups (Grid+ and Grid−) in the vehicle-treated group only (p<0.001, Bonferonni-corrected). When assessed over time (Fig. 2D), CNO reduced the percent time spent on the ethanol-paired floor compared to vehicle across the duration of the test [main effect of drug: F(1,52)=11.44, p≤0.001; no time main effect or interaction].
Figure 2. Ethanol-induced CPP expression is blocked by activation of hM4Di receptors selectively expressed in BNST-VTA cells.
(A) Heterologous expression of cre-dependent hM4Di (red; visualized by IF detection of mCherry) is observed in VTA-projecting neurons (green; visualized by IF detection of EYFP) of dorsal and ventral BNST. Nuclei are counterstained blue with DAPI; ac, anterior commissure; scale bar, 200 µm. (B) High magnification image of region outlined in white box above illustrating HSV-mediated cre expression (green), DIO-hM4Di expression (red), and their overlap (orange-yellow), with nuclei stained blue. Scale bar, 50 µm. (C) Mean (+SEM) time spent on the grid floor (in s/min) during 30-min preference test. VTA-projecting BNST neurons were inhibited via CNO (10 mg/kg)-mediated stimulation of hM4Di. Inhibition of BNST-VTA signaling blocked the expression of ethanol-induced CPP. † p≤0.001 interaction between drug and conditioning subgroup (Grid+ vs. Grid−); * p<0.001 between conditioning subgroups; n=13–15/subgroup. (D) Mean percent time (±SEM) spent on the ethanol-paired floor in 5-min intervals across the 30-min preference test. BNST-VTA inhibition reduced place preference in a consistent manner across the test.
Table 1 includes mean activity counts per min (±SEM) during the preference test and conditioning. CNO-mediated hM4Di activation in BNST-VTA cells did not affect locomotor activity during the preference test (no main effect of drug). During conditioning (before CNO treatment), animals exhibited robust ethanol-stimulated locomotor activity and there were no group differences on saline or ethanol trials [main effect of trial type (CS+ vs. CS−): F(1,56)=509.29, p<0.001; no group main effect or interaction].
Table 1. Locomotor activity.
Mean Activity Counts per Minute (± SEM) during conditioning and preference test
| Group | CS+ Trials | CS− Trials | Preference Test | |
|---|---|---|---|---|
| Experiment 1 | ||||
| BNST-VTA hM4Di | Vehicle | 98.0 ± 4.5 | 37.4 ± 1.2 | 37.7 ± 1.3 |
| CNO | 100.2 ± 4.3 | 35.8 ± 1.7 | 37.0 ± 1.1 | |
| Experiment 2 | ||||
| BNST-VTA GFP | Vehicle | 107.1 ± 5.0 | 38.9 ± 2.0 | 39.9 ± 1.2 |
| CNO | 110.9 ± 4.2 | 40.5 ± 1.4 | 39.1 ± 2.0 |
3.2. Exp. 2 – effect of GFP expression on ethanol CPP
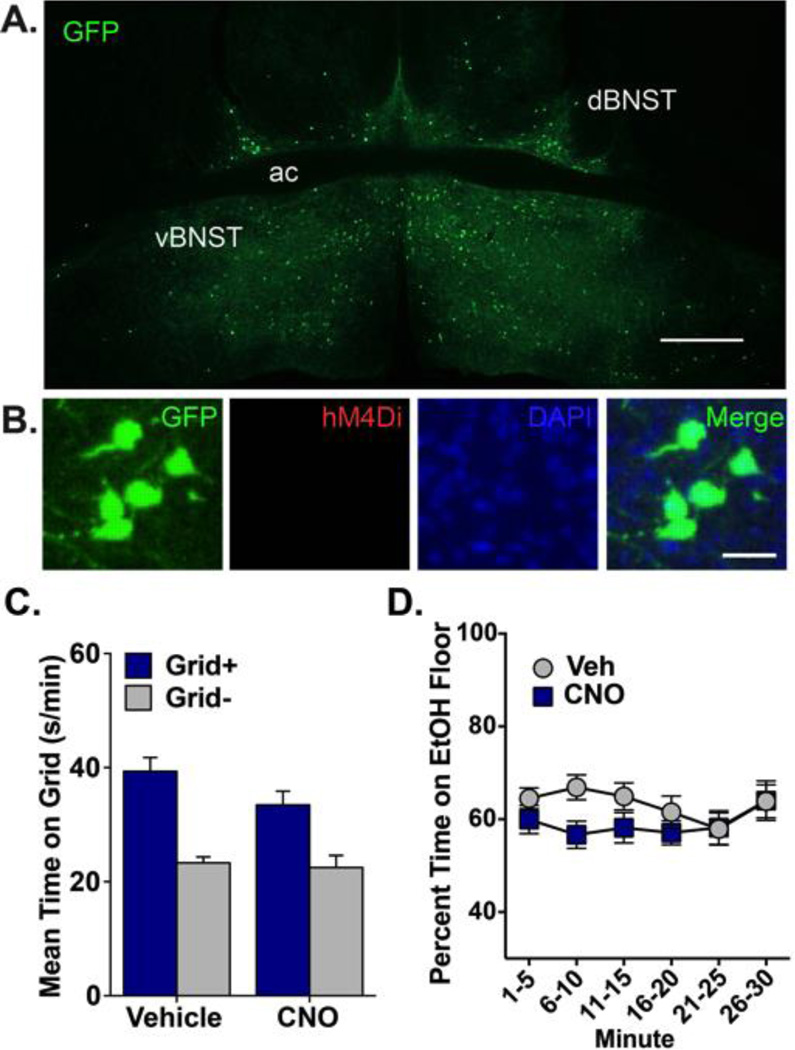
To control for non-specific effects of surgery, transgene expression, and CNO on ethanol CPP, mice were tested in the absence of hM4Di. A cre-inducible AAV carrying hM4Di was infused into BNST and a control vector lacking cre (HSV-GFP) was infused into VTA. Given the absence of cre, no hM4Di expression was observed after 8 weeks of incubation, indicating that no transgene leakage occurred with this strategy (Fig. 3A and B). Moreover, CNO did not affect ethanol CPP in the absence of hM4Di (Fig. 3C and D) [main effect of conditioning: F(1,42)=43.13, p<0.001; no drug main effect or interaction]. No significant differences in percent time spent on the ethanol floor were found between CNO- and vehicle-treated groups across the duration of the test, as indicated by non-significant effects of drug, time, and drug×time. Neither test nor conditioning activity differed between CNO and vehicle groups. As expected, conditioning activity was higher on ethanol trials than on saline trials [main effect of trial type: F(1,44)=503.04, p<0.001].
Figure 3. Ethanol CPP expression is not disrupted by CNO in mice expressing GFP in VTA-projecting BNST cells.
(A) Expression of GFP in dBNST and vBNST 8 weeks after infusion of HSV-GFP into VTA and DIO-hM4Di into BNST. GFP+ cells (green) indicate VTA-projecting neurons. No hM4Di expression was visible in BNST. ac, anterior commissure; scale bar, 500 µm. (B) High magnification image from BNST of GFP (green; visualized by IF detection of GFP), hM4Di (absent; visualized by IF detection of mCherry), nuclei (DAPI) and all channels merged. Note that hM4Di is not expressed in the absence of cre; scale bar, 50 µm. (C) Mean (+SEM) time spent on the grid floor (in s/min) during 30-min preference test. CNO did not disrupt ethanol CPP expression in mice expressing GFP in BNST-VTA cells; n=11–12 / conditioning subgroup (Grid+, Grid). (D) There was no significant difference in percent time spent on the ethanol-paired floor between groups when analyzed in 5-min intervals across the 30-min preference test.
4. Discussion
The present experiments assessed the involvement of a direct neuronal projection from the BNST to the VTA in ethanol CPP expression. An intersectional viral strategy was used to selectively target inhibitory DREADDs (hM4Di) to BNST-VTA cells. This involved infusions of a long-term retrograde HSV vector encoding cre recombinase (HSV-Cre) into VTA and a cre-inducible vector encoding for hM4Di (AAV-DIO-hM4Di) into BNST. This approach allowed for direct modulation of BNST-VTA cells during ethanol-seeking behavior via CNO-mediated hM4Di activation.
In Exp. 1, hM4Di was expressed in BNST-VTA cells and activated with CNO prior to the preference test, leading to BNST-VTA inhibition. Place preference was blocked in CNO-treated animals, indicating that activation of a direct BNST projection to VTA is necessary for ethanol CPP expression. In Exp. 2, we controlled for nonspecific effects of surgery, transgene expression, and CNO administration on ethanol CPP expression. Procedures were identical to those in Exp. 1 except that a control HSV-GFP vector was used in place of HSV-Cre. This resulted in GFP expression in VTA-projecting cells and a marked absence of hM4Di in BNST. Mice lacking hM4Di exhibited normal levels of ethanol CPP that did not significantly differ between CNO- or vehicle-treated groups. These results indicate that disruptions in CPP observed in Exp. 1 were due to CNO-mediated hM4Di inhibition of BNST-VTA cells. Together, our findings demonstrate that ethanol CPP is expressed through a serial projection from the BNST to VTA.
To our knowledge, this is the first experiment to demonstrate that a BNST to VTA projection is involved in ethanol CPP. This finding is consistent with other studies that have shown a role for the BNST and BNST-VTA neural circuit in relapse to drug seeking. For instance, earlier work has indicated that the BNST is activated by cocaine- and ethanol-associated stimuli (Hill et al., 2007; Mahler & Aston-Jones, 2012; Zhao et al., 2006). BNST inactivation has also reduced cue-induced cocaine-, heroin-, and ethanol-seeking behavior (Buffalari & See, 2011; Pina et al., 2015; Rogers et al., 2008; Sartor & Aston-Jones, 2012). Whereas these studies broadly implicate the BNST in general, additional evidence has suggested this region modulates positive motivational states through a direct projection to the VTA. Of note, BNST inputs to VTA from dorsal (dBNST) and ventral (vBNST) subdivisions are known to potently innervate DA cells (Georges & Aston-Jones, 2001; 2002; Jalabert, Aston-Jones, Herzog, Manzoni, & Georges, 2009). This innervation triggers VTA DA cell burst firing, which is a putative mechanism of motivated behavior (Adamantidis et al., 2011; Schultz, 1986; Wanat, Willuhn, Clark, & Phillips, 2009). More directly, BNST afferents of the VTA are activated by cocaine-cue exposure and pharmacological disconnection of this circuit has been shown to block cocaine CPP (Sartor & Aston-Jones, 2012). Other evidence indicates that a BNST-VTA pathway is explicitly engaged following ethanol exposure and withdrawal. For instance, simultaneous manipulation of GABAA receptors in BNST and D2 receptors in VTA disrupted ethanol seeking in preferring (P) rats (Eiler, Seyoum, Foster, Mailey, & June, 2003) and chronic ethanol intake and withdrawal enhanced excitatory input onto VTA-projecting BNST cells (Silberman, Matthews, & Winder, 2013). Our findings support earlier work and further implicate a role of the BNST-VTA circuit in seeking behavior engaged by ethanol-associated cue exposure. Combined with work demonstrating ethanol intake- and withdrawal-related enhancements in excitatory input on BNST-VTA cells, our results suggest that cue-exposure may engage an already-upregulated circuit further strengthening ethanol-motivated behaviors.
Much of the work demonstrating BNST modulation of DA cells proposes that excitatory, likely glutamatergic, projections from the BNST directly innervate VTA DA neurons (Jalabert et al., 2009). Indeed, the BNST is known to send monosynaptic inputs to VTA DA cells (Watabe-Uchida, Zhu, Ogawa, Vamanrao, & Uchida, 2012), which renders DA activity tractable to modulation by the BNST. However, more recent work indicates that BNST efferents preferentially innervate non-DAergic (putatively GABAergic) VTA cells (Jennings et al., 2013). Overall, 70–90% of BNST cells are GABAergic (Le Gal LaSalle, Paxinos, & Ben-Ari, 1978; Sun & Cassell, 1993). Moreover, of the three distinct types of BNST-VTA cells (GAD+/VGlut−, VGlut2+/GAD−, VGlut3+/GAD+), 90% are GAD+/VGlut−, i.e., GABAergic/non-glutamatergic (Kudo et al., 2012). Thus, it is likely that VTA DA cell activation may result from BNST GABA innervation of VTA GABA cells, the net result of which is DA disinhibition. Notably, Jennings et al. (2013) have shown that BNST glutamatergic innervation of VTA generates an aversive state, which is the diametric opposite of the positive motivational state produced by BNST GABA to VTA. In addition, a direct corticotropin releasing factor (CRF) projection from BNST to VTA has also been identified (Rodaros, Caruana, Amir, & Stewart, 2007; Vranjkovic et al., 2014) and is likely contained in GABAergic neurons (Dabrowska, Hazra, Guo, DeWitt, & Rainnie, 2013). Notably, a BNST CRF projection to VTA was recently shown to regulate binge-like consumption of ethanol (Rinker et al., 2016).
Thus, while the two-virus strategy employed here establishes a role for a BNST projection to VTA in ethanol seeking, the neurochemical nature of this projection remains unclear. Although intersectional approaches that target genetically defined cells within neural circuits exist, they require the use of transgenic animals (Fenno et al., 2014; Stamatakis et al., 2013). Considering the difficulty of establishing ethanol CPP in some mouse strains (Cunningham, 1995; 2014), the use of cre transgenic lines was not presently feasible. Follow-up studies are needed to identify the exact cell populations within the BNST and VTA that are responsible for ethanol CPP expression.
In addition to cell-specific contributions, another important consideration is the involvement of distinct BNST subdivisions in ethanol CPP. While there are discrepancies in the boundaries and total number of nuclei that comprise the BNST (Ju & Swanson, 1989; Moga, Saper, & Gray, 1989), studies have ascribed distinct roles to several divisions. For example, in the dBNST, the oval (ovBNST) and anterodorsal (adBNST) nuclei control varying anxiogenic behaviors (Kim et al., 2013). The vBNST has been implicated in maternal behavior (Numan & Numan, 1997), cocaine CPP expression (Sartor & Aston-Jones, 2012), and heroin-primed reinstatement (Rogers et al., 2008), whereas the medial posterior (mpBNST) subdivision is involved in both heroin- and cue-primed reinstatement (Rogers et al., 2008). Given the scope of these previous studies, the connectivity and terminal sites of each of these subdivisions were not experimentally addressed. Likewise, the roles of subdivision-specific projections to VTA in behavior and emotional states have not been as well described. The studies that exist have reported involvement of vBNST-VTA CRF in footshock-induced reinstatement of cocaine seeking (Vranjkovic et al., 2014), vBNST-VTA GABA in reward, and vBNST-VTA glutamate in aversion and anxiety (Jennings et al., 2013). Overall, these studies suggest that specific subdivisions, in addition to distinct cell types within each, may differentially contribute to motivation, emotion, and behavior.
Considering the small scale of the mouse brain and spread of hM4Di expression through dBNST and vBNST divisions, our data offer little evidence of a subdivision-specific mechanism. Nevertheless, some information was provided because of the differential expression obtained in the various BNST subdivisions. The retrograde HSV vector used here produced transgene expression in a broad range of BNST nuclei, spanning across dorsoventral and rostrocaudal gradients (Fig. S2) Overall, HSV-mediated transgene expression was highest in medial portions of anterior and posterior vBNST and dBNST. Expression was notably absent in the ovBNST and weaker in the juxtacapsular (juBNST) nucleus (Supplementary Fig. 2). Given the weak to absent cre/GFP (and therefore hM4Di) expression in these nuclei, it can be concluded that the ovBNST and likely juBNST were not responsible for the observed disruption in ethanol CPP. Moreover, it is likely that the anteromedial BNST (amBNST) provided the greatest contribution given its high levels of cre/GFP (and therefore hM4Di) expression. Finally, findings of absent or weak cre/GFP expression should not necessarily be interpreted as evidence of a lack of direct VTA projection. For instance, though we did not find VTA-projecting cells in ovBNST, others have shown this projection exists (Rodaros et al., 2007). Therefore, our results may simply reflect a lack of transgene expression in all neurons.
Whereas other studies have used a retrograde HSV vector and an AAV-DIO construct to achieve circuit-specific expression of opsins (Fenno et al., 2014; Stamatakis et al., 2013), our experiments are among those to demonstrate circuit-selective DREADD expression using this viral combination (see also Gremel et al., 2016). Several previous studies have utilized a similar retrograde intersectional approach to obtain DREADD expression in projection neurons. In these studies, CAV2-Cre was combined with AAV-DIO constructs to drive DREADD expression in projection neurons of the rat (Boender et al., 2014; Nair et al., 2013). In these studies, no opsin or DREADD expression in the absence of recombinase proteins has been reported. In our experiments, we found no evidence of hM4Di expression outside of cre-positive cells or in cre-positive cells outside of the BNST (Supplementary Fig. 3). This finding is important as it demonstrates the absence of transgene leakage and confirms hM4Di expression was confined to VTA-projecting BNST cells. Overall, our work further demonstrates the utility of retrograde intersectional approaches and shows that HSV can be used in mice for robust long-term transgene expression and combined with AAV-DIO constructs to selectively express DREADDs in serially connected brain nuclei. This approach enables circuit accessibility and highly selective modulation of distinct yet intermixed populations of projection neurons.
4. Conclusion
Here, we show successful circuit-selective expression of hM4Di using a unique vector combination. With this approach, VTA-projecting BNST neurons were inhibited by CNO-mediated hM4Di activation. Inhibition of BNST-VTA cells disrupted the expression of ethanol place preference. Expression of hM4Di alone did not affect ethanol CPP, as vehicle-treated mice showed significant place preference. As previously shown, in the absence of hM4Di, CNO did not impact ethanol CPP expression (Pina et al., 2015). Here, we further demonstrate that in the presence of a control GFP transgene, CNO did not affect ethanol CPP. These findings demonstrate that ethanol CPP is expressed through a direct BNST projection to VTA. In addition to supporting studies showing BNST and BNST-VTA involvement in cue-induced drug seeking, these experiments demonstrate that the BNST and BNST-VTA circuit are important neural substrates of ethanol seeking indexed by CPP.
Supplementary Material
Highlights.
-
-
hM4Di was expressed in BNST-VTA cells via a retrograde intersectional viral approach
-
-
hM4Di-mediated inhibition of BNST-VTA cells blocked ethanol CPP expression
-
-
Control GFP expression and CNO alone did not affect ethanol CPP expression
-
-
Findings indicate BNST-VTA cells regulate the expression of ethanol-cue seeking
Acknowledgments
Funding
This research was supported by the National Institutes of Health under award R01AA007702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A Scholar Award from the ARCS Foundation Portland Chapter, Graduate Scholarship from the OHSU-Vertex Partnership, Neurobiology of Disease Fellowship from the OHSU Brain Institute, Dissertation Research Award from the American Psychological Association, Graduate Research Grant from Psi Chi, and N.L Tartar Trust Fellowship from the OHSU Foundation provided additional support (MMP). Experiments reported here comply with the current laws of the United States.
We owe much gratitude to Dr. Christina Gremel for her original suggestions regarding the intersectional strategy and vectors, Dr. Sunila Nair for her advice and support in the implementation of an intersectional approach, and Lindsey Schuette for her help with tissue sectioning. We also thank Dr. Andrey Ryabinin for his comments on previous versions of this manuscript.
Footnotes
Disclosures
The authors declare no conflict of interest.
References
- Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31(30):10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. http://doi.org/10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. http://doi.org/10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisker W, Dolbeare F, Gray JW. An improved immunocytochemical procedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry. 1987;8(2):235–239. doi: 10.1002/cyto.990080218. http://doi.org/10.1002/cyto.990080218. [DOI] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MCM, van der Plasse G, Adan RAH. Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS ONE. 2014;9(4):e95392. doi: 10.1371/journal.pone.0095392. http://doi.org/10.1371/journal.pone.0095392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213(1):19–27. doi: 10.1007/s00213-010-2008-3. http://doi.org/10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Gershenson ZT, Giles AR, Holzbaur ELF, Wolfe JH. Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Human Gene Therapy. 2014;25(8):705–720. doi: 10.1089/hum.2013.189. http://doi.org/10.1089/hum.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology. 1995;120(1):28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behavioral Neuroscience. 2014;128(4):430–445. doi: 10.1037/a0036459. http://doi.org/10.1037/a0036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y. http://doi.org/10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. http://doi.org/10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo J-D, DeWitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00156. http://doi.org/10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct Patterns of Neural Activation Associated with Ethanol Seeking: Effects of Naltrexone. Biological Psychiatry. 2007;61(8):979–989. doi: 10.1016/j.biopsych.2006.07.034. http://doi.org/10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. The Journal of Comparative Neurology. 2004;471(4):396–433. doi: 10.1002/cne.20002. http://doi.org/10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. The Journal of Comparative Neurology. 2006a;494(1):142–178. doi: 10.1002/cne.20788. http://doi.org/10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. The Journal of Comparative Neurology. 2006b;494(1):75–107. doi: 10.1002/cne.20790. http://doi.org/10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler WJA, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48(1):45–56. doi: 10.1002/syn.10181. http://doi.org/10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nature Methods. 2014;11(7):763–772. doi: 10.1038/nmeth.2996. http://doi.org/10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones GS. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2001;21(16):RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones GS. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2002;22(12):5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve RL, Ramakrishnan C, et al. Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron. 2016;90(6):1312–1324. doi: 10.1016/j.neuron.2016.04.043. http://doi.org/10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KG, Ryabinin AE, Cunningham CL. FOS expression induced by an ethanol-paired conditioned stimulus. Pharmacology, Biochemistry, and Behavior. 2007;87(2):208–221. doi: 10.1016/j.pbb.2007.04.017. http://doi.org/10.1016/j.pbb.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. http://doi.org/10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Aston-Jones GS, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(8):1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. http://doi.org/10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. http://doi.org/10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. The Journal of Comparative Neurology. 1989;280(4):587–602. doi: 10.1002/cne.902800409. http://doi.org/10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–223. doi: 10.1038/nature12018. http://doi.org/10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Konno K, Uchigashima M, Yanagawa Y, Sora I, Minami M, Watanabe M. GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis. The European Journal of Neuroscience. 2014 doi: 10.1111/ejn.12503. http://doi.org/10.1111/ejn.12503. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, et al. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2012;32(50):18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. http://doi.org/10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal LaSalle G, Paxinos G, Ben-Ari Y. Neurochemical mapping of GABAergic systems in the amygdaloid complex and bed nucleus of the stria terminalis. Brain Research. 1978;155(2):397–403. doi: 10.1016/0006-8993(78)91037-5. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2012;32(38):13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. http://doi.org/10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience. 2014;17(4):577–585. doi: 10.1038/nn.3664. http://doi.org/10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. The Journal of Comparative Neurology. 1989;283(3):315–332. doi: 10.1002/cne.902830302. http://doi.org/10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Nair SG, Strand NS, Neumaier JF. DREADDing the lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain Research. 2013;1511:93–101. doi: 10.1016/j.brainres.2012.10.011. http://doi.org/10.1016/j.brainres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. Journal of Neuroendocrinology. 1997;9(5):369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press. 2001:1–350. [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, Cunningham CL. The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology. 2015;99:627–638. doi: 10.1016/j.neuropharm.2015.08.033. http://doi.org/10.1016/j.neuropharm.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science (New York, N.Y.) 2011;333(6042):637–642. doi: 10.1126/science.1205295. http://doi.org/10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Soriano LP, Nattie EE, Dymecki SM. Egr2-neurons control the adult respiratory response to hypercapnia. Brain Research. 2013;1511:115–125. doi: 10.1016/j.brainres.2012.12.017. http://doi.org/10.1016/j.brainres.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, et al. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge EthanolIntake. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.029. http://doi.org/10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. http://doi.org/10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–588. doi: 10.1016/j.neuroscience.2007.10.012. http://doi.org/10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salegio EA, Samaranch L, Kells AP, Mittermeyer G, Sebastian WS, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Therapy. 2012;20(3):348–352. doi: 10.1038/gt.2012.27. http://doi.org/10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. The European Journal of Neuroscience. 2012;36(11):3549–3558. doi: 10.1111/j.1460-9568.2012.08277.x. http://doi.org/10.1111/j.1460-9568.2012.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. Journal of Neurophysiology. 1986;56(5):1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2013;33(3):950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. http://doi.org/10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDS: Use and application in behavioral neuroscience. Behavioral Neuroscience. 2016;130(2):137–155. doi: 10.1037/bne0000135. http://doi.org/10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80(4):1039–1053. doi: 10.1016/j.neuron.2013.08.023. http://doi.org/10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. The Journal of Comparative Neurology. 1993;330(3):381–404. doi: 10.1002/cne.903300308. http://doi.org/10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones GS. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(10):3859–3864. doi: 10.1073/pnas.1310025111. http://doi.org/10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2014;34(37):12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. http://doi.org/10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PEM. Phasic dopamine release in appetitive behaviors and drug addiction. Current Drug Abuse Reviews. 2009;2(2):195–213. doi: 10.2174/1874473710902020195. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2877500&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. http://doi.org/10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MAS, Martin-Fardon R, Weiss F. Activation of Group II Metabotropic Glutamate Receptors Attenuates Both Stress and Cue-Induced Ethanol-Seeking and Modulates c-fos Expression in the Hippocampus and Amygdala. Journal of Neuroscience. 2006;26(39):9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. http://doi.org/10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.