Abstract
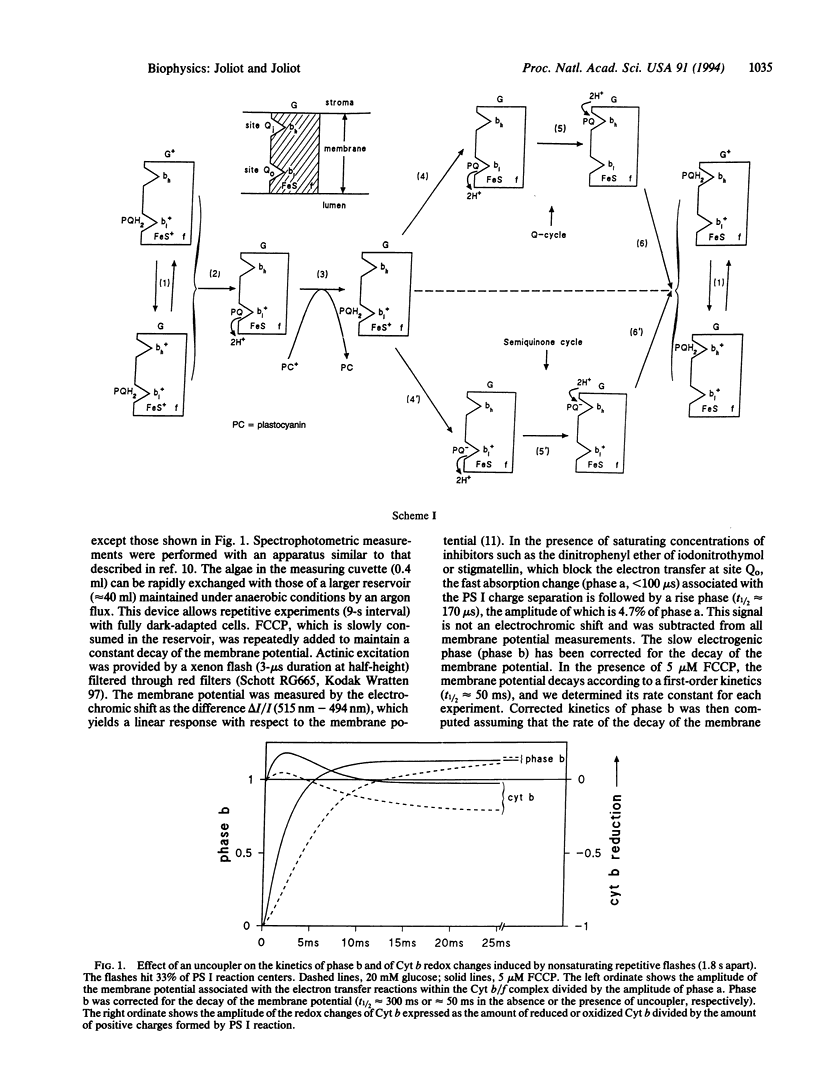
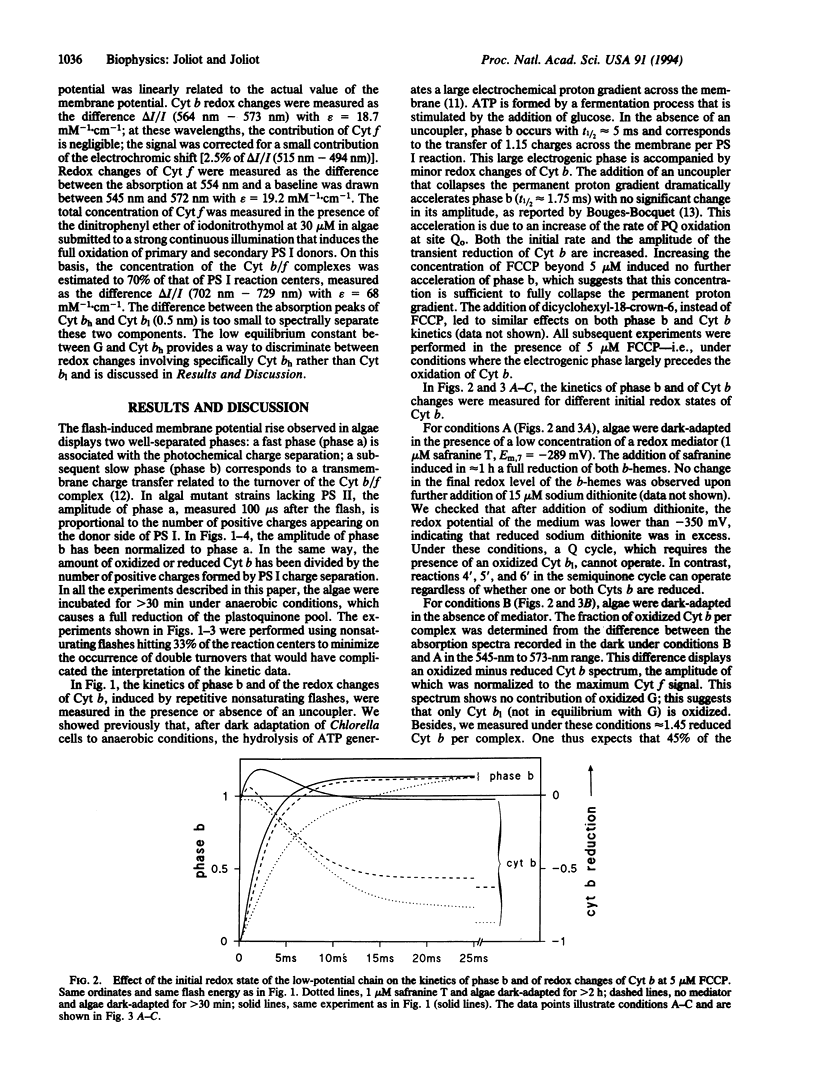
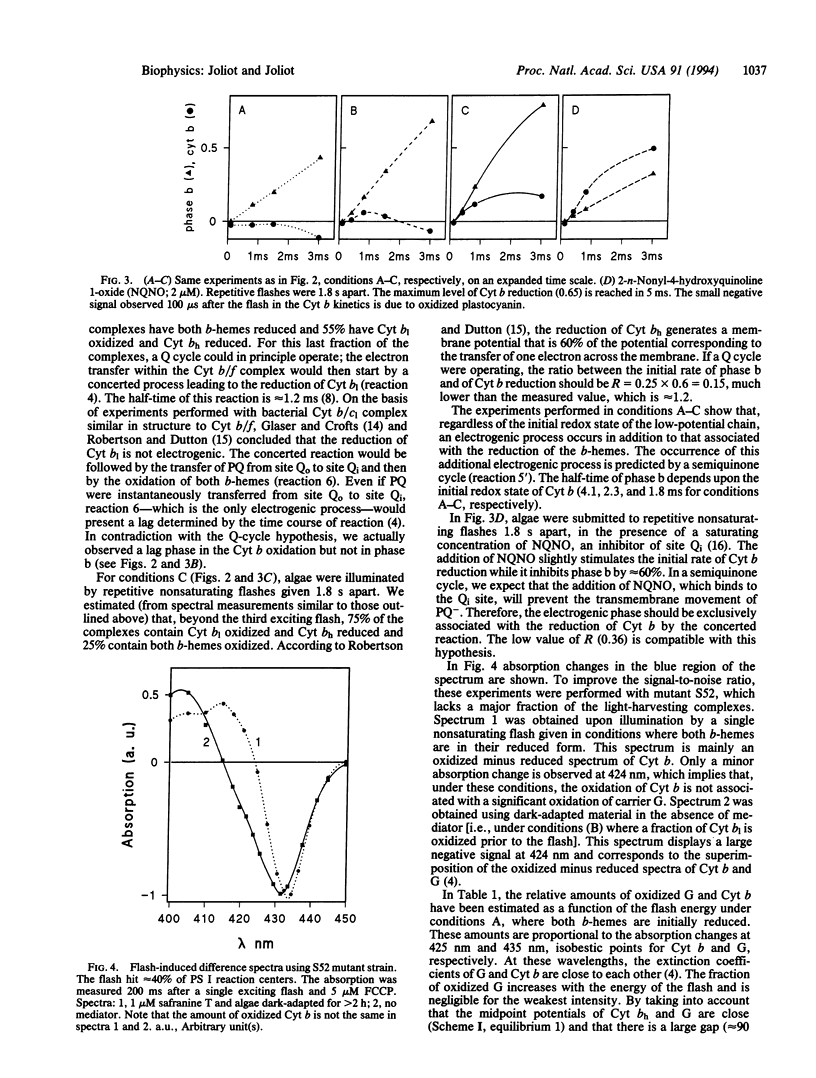
The most widely accepted mechanism of electron and proton transfer within the cytochrome (Cyt) b/f complex derives from the Q-cycle hypothesis originally proposed for the mitochondrial Cyt b/c1 complex by Mitchell [Mitchell, P. (1975) FEBS Lett. 57, 135-137]. In chloroplasts, the Cyt b/f complex catalyzes the oxidation of a plastoquinol at a site, Qo (the plastoquinol binding site), close to the inner aqueous phase and the reduction of a quinone at a site, Qi (the plastoquinone binding site), close to the stromal side of the membrane. In an alternative model, the semiquinone cycle [Wikström, M. & Krab, K. (1986) J. Bioenerg. Biomembr. 18, 181-193], a charged semiquinone formed at site Qo is transferred to site Qi where it is reduced into quinol. Flash-induced kinetics of the redox changes of Cyt b and of the formation of a transmembrane potential have been measured in Chlorella sorokiniana cells incubated in reducing conditions that induce a full reduction of the plastoquinone pool. The experiments were performed in the presence of an uncoupler that collapses the permanent electrochemical proton gradient and thus accelerates the rate of the electrogenic processes. The results show that the electrogenic reaction driven by the Cyt b/f complex precedes the processes of reduction or oxidation of the b-hemes. This electrogenic process is probably due to a transmembrane movement of a charged semiquinone, in agreement with the semiquinone-cycle hypothesis. This mechanism may represent an adaptation to reducing conditions when no oxidized quinone is available at the Qi site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouges-Bocquet B. Factors regulating the slow electrogenic phase in green algae and higher plants. Biochim Biophys Acta. 1981 Apr 13;635(2):327–340. doi: 10.1016/0005-2728(81)90031-1. [DOI] [PubMed] [Google Scholar]
- Hope A. B. The chloroplast cytochrome bf complex: a critical focus on function. Biochim Biophys Acta. 1993 Jun 10;1143(1):1–22. doi: 10.1016/0005-2728(93)90210-7. [DOI] [PubMed] [Google Scholar]
- Joliot P., Delosme R. Flash-induced 519 nm absorption change in green algae. Biochim Biophys Acta. 1974 Aug 23;357(2):267–284. doi: 10.1016/0005-2728(74)90066-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975 Nov 15;59(2):137–139. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Dutton P. L. The nature and magnitude of the charge-separation reactions of ubiquinol cytochrome c2 oxidoreductase. Biochim Biophys Acta. 1988 Oct 5;935(3):273–291. doi: 10.1016/0005-2728(88)90223-x. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. The semiquinone cycle. A hypothesis of electron transfer and proton translocation in cytochrome bc-type complexes. J Bioenerg Biomembr. 1986 Jun;18(3):181–193. doi: 10.1007/BF00743463. [DOI] [PubMed] [Google Scholar]


