Abstract
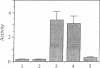
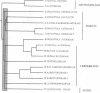
Primary sequences of cholinesterases and related proteins have been systematically compared. The cholinesterase-like domain of these proteins, about 500 amino acids, may fulfill a catalytic and a structural function. We identified an aspartic acid residue that is conserved among esterases and lipases (Asp-397 in Torpedo acetylcholinesterase) but that had not been considered to be involved in the catalytic mechanism. Site-directed mutagenesis demonstrated that this residue is necessary for activity. Analysis of evolutionary relationships shows that the noncatalytic members of the family do not constitute a separate subgroup, suggesting that loss of catalytic activity occurred independently on several occasions, probably from bifunctional molecules. Cholinesterases may thus be involved in cell-cell interactions in addition to the hydrolysis of acetylcholine. This would explain their specific expression in well-defined territories during embryogenesis before the formation of cholinergic synapses and their presence in noncholinergic tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthalay Y., Hipeau-Jacquotte R., de la Escalera S., Jiménez F., Piovant M. Drosophila neurotactin mediates heterophilic cell adhesion. EMBO J. 1990 Nov;9(11):3603–3609. doi: 10.1002/j.1460-2075.1990.tb07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies L., Biegelmann E., Döring V., Gerisch G., Krafft-Czepa H., Noegel A. A., Schleicher M., Humbel B. M. Membrane-enclosed crystals in Dictyostelium discoideum cells, consisting of developmentally regulated proteins with sequence similarities to known esterases. J Cell Biol. 1990 Mar;110(3):669–679. doi: 10.1083/jcb.110.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon S., Méflah K., Musset F., Grassi J., Massoulié J. An immunoglobulin M monoclonal antibody, recognizing a subset of acetylcholinesterase molecules from electric organs of Electrophorus and Torpedo, belongs to the HNK-1 anti-carbohydrate family. J Neurochem. 1987 Dec;49(6):1720–1731. doi: 10.1111/j.1471-4159.1987.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Brady L., Brzozowski A. M., Derewenda Z. S., Dodson E., Dodson G., Tolley S., Turkenburg J. P., Christiansen L., Huge-Jensen B., Norskov L. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature. 1990 Feb 22;343(6260):767–770. doi: 10.1038/343767a0. [DOI] [PubMed] [Google Scholar]
- Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988 Aug 11;334(6182):528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- Collet C., Nielsen K. M., Russell R. J., Karl M., Oakeshott J. G., Richmond R. C. Molecular analysis of duplicated esterase genes in Drosophila melanogaster. Mol Biol Evol. 1990 Jan;7(1):9–28. doi: 10.1093/oxfordjournals.molbev.a040582. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Largman C., Rutter W. J. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987 Aug 21;237(4817):909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- Di Lauro R., Obici S., Condliffe D., Ursini V. M., Musti A., Moscatelli C., Avvedimento V. E. The sequence of 967 amino acids at the carboxyl-end of rat thyroglobulin. Location and surroundings of two thyroxine-forming sites. Eur J Biochem. 1985 Apr 1;148(1):7–11. doi: 10.1111/j.1432-1033.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Doctor B. P., Chapman T. C., Christner C. E., Deal C. D., De La Hoz D. M., Gentry M. K., Ogert R. A., Rush R. S., Smyth K. K., Wolfe A. D. Complete amino acid sequence of fetal bovine serum acetylcholinesterase and its comparison in various regions with other cholinesterases. FEBS Lett. 1990 Jun 18;266(1-2):123–127. doi: 10.1016/0014-5793(90)81522-p. [DOI] [PubMed] [Google Scholar]
- Doctor B. P., Smyth K. K., Gentry M. K., Ashani Y., Christner C. E., de la Hoz D. M., Ogert R. A., Smith S. W. Structural and immunochemical properties of fetal bovine serum acetylcholinesterase. Prog Clin Biol Res. 1989;289:305–316. [PubMed] [Google Scholar]
- Drews U. Cholinesterase in embryonic development. Prog Histochem Cytochem. 1975;7(3):1–52. [PubMed] [Google Scholar]
- Fournier D., Bride J. M., Karch F., Bergé J. B. Acetylcholinesterase from Drosophila melanogaster. Identification of two subunits encoded by the same gene. FEBS Lett. 1988 Oct 10;238(2):333–337. doi: 10.1016/0014-5793(88)80507-6. [DOI] [PubMed] [Google Scholar]
- Gibney G., Camp S., Dionne M., MacPhee-Quigley K., Taylor P. Mutagenesis of essential functional residues in acetylcholinesterase. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7546–7550. doi: 10.1073/pnas.87.19.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Marshall T. L., Rosenberry T. L. Drosophila acetylcholinesterase: demonstration of a glycoinositol phospholipid anchor and an endogenous proteolytic cleavage. Biochemistry. 1988 Aug 23;27(17):6453–6457. doi: 10.1021/bi00417a038. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Malcolm C. A. The acetylcholinesterase gene of Anopheles stephensi. Cell Mol Neurobiol. 1991 Feb;11(1):131–141. doi: 10.1007/BF00712805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5' leader. EMBO J. 1986 Nov;5(11):2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hanzlik T. N., Abdel-Aal Y. A., Harshman L. G., Hammock B. D. Isolation and sequencing of cDNA clones coding for juvenile hormone esterase from Heliothis virescens. Evidence for a catalytic mechanism for the serine carboxylesterases different from that of the serine proteases. J Biol Chem. 1989 Jul 25;264(21):12419–12425. [PubMed] [Google Scholar]
- Keilhauer G., Faissner A., Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature. 1985 Aug 22;316(6030):728–730. doi: 10.1038/316728a0. [DOI] [PubMed] [Google Scholar]
- Korza G., Ozols J. Complete covalent structure of 60-kDa esterase isolated from 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced rabbit liver microsomes. J Biol Chem. 1988 Mar 5;263(7):3486–3495. [PubMed] [Google Scholar]
- Krejci E., Coussen F., Duval N., Chatel J. M., Legay C., Puype M., Vandekerckhove J., Cartaud J., Bon S., Massoulié J. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 1991 May;10(5):1285–1293. doi: 10.1002/j.1460-2075.1991.tb08070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyger E. M., Wiegand R. C., Lange L. G. Cloning of the bovine pancreatic cholesterol esterase/lysophospholipase. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1302–1309. doi: 10.1016/0006-291x(89)91811-1. [DOI] [PubMed] [Google Scholar]
- Layer P. G. Cholinesterases during development of the avian nervous system. Cell Mol Neurobiol. 1991 Feb;11(1):7–33. doi: 10.1007/BF00712798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- Long R. M., Satoh H., Martin B. M., Kimura S., Gonzalez F. J., Pohl L. R. Rat liver carboxylesterase: cDNA cloning, sequencing, and evidence for a multigene family. Biochem Biophys Res Commun. 1988 Oct 31;156(2):866–873. doi: 10.1016/s0006-291x(88)80924-0. [DOI] [PubMed] [Google Scholar]
- MacPhee-Quigley K., Vedvick T. S., Taylor P., Taylor S. S. Profile of the disulfide bonds in acetylcholinesterase. J Biol Chem. 1986 Oct 15;261(29):13565–13570. [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987 Jun 15;165(3):491–498. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- Maulet Y., Camp S., Gibney G., Rachinsky T. L., Ekström T. J., Taylor P. Single gene encodes glycophospholipid-anchored and asymmetric acetylcholinesterase forms: alternative coding exons contain inverted repeat sequences. Neuron. 1990 Feb;4(2):289–301. doi: 10.1016/0896-6273(90)90103-m. [DOI] [PubMed] [Google Scholar]
- McTiernan C., Adkins S., Chatonnet A., Vaughan T. A., Bartels C. F., Kott M., Rosenberry T. L., La Du B. N., Lockridge O. Brain cDNA clone for human cholinesterase. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6682–6686. doi: 10.1073/pnas.84.19.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Mori N., Itoh N., Salvaterra P. M. Evolutionary origin of cholinergic macromolecules and thyroglobulin. Proc Natl Acad Sci U S A. 1987 May;84(9):2813–2817. doi: 10.1073/pnas.84.9.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouches C., Pauplin Y., Agarwal M., Lemieux L., Herzog M., Abadon M., Beyssat-Arnaouty V., Hyrien O., de Saint Vincent B. R., Georghiou G. P. Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2574–2578. doi: 10.1073/pnas.87.7.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeshott J. G., Collet C., Phillis R. W., Nielsen K. M., Russell R. J., Chambers G. K., Ross V., Richmond R. C. Molecular cloning and characterization of esterase-6, a serine hydrolase of Drosophila. Proc Natl Acad Sci U S A. 1987 May;84(10):3359–3363. doi: 10.1073/pnas.84.10.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson P. F., Fessler L. I., Nelson R. E., Sterne R. E., Campbell A. G., Fessler J. H. Glutactin, a novel Drosophila basement membrane-related glycoprotein with sequence similarity to serine esterases. EMBO J. 1990 Apr;9(4):1219–1227. doi: 10.1002/j.1460-2075.1990.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J. Isolation, properties, and the complete amino acid sequence of a second form of 60-kDa glycoprotein esterase. Orientation of the 60-kDa proteins in the microsomal membrane. J Biol Chem. 1989 Jul 25;264(21):12533–12545. [PubMed] [Google Scholar]
- Prody C. A., Zevin-Sonkin D., Gnatt A., Goldberg O., Soreq H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3555–3559. doi: 10.1073/pnas.84.11.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachinsky T. L., Camp S., Li Y., Ekström T. J., Newton M., Taylor P. Molecular cloning of mouse acetylcholinesterase: tissue distribution of alternatively spliced mRNA species. Neuron. 1990 Sep;5(3):317–327. doi: 10.1016/0896-6273(90)90168-f. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Acetylcholinesterase. Adv Enzymol Relat Areas Mol Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- Rubino S., Mann S. K., Hori R. T., Pinko C., Firtel R. A. Molecular analysis of a developmentally regulated gene required for Dictyostelium aggregation. Dev Biol. 1989 Jan;131(1):27–36. doi: 10.1016/s0012-1606(89)80035-1. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Sugihara A., Tominaga Y., Iizumi T., Tsunasawa S. cDNA molecular cloning of Geotrichum candidum lipase. J Biochem. 1989 Sep;106(3):383–388. doi: 10.1093/oxfordjournals.jbchem.a122862. [DOI] [PubMed] [Google Scholar]
- Sikorav J. L., Duval N., Anselmet A., Bon S., Krejci E., Legay C., Osterlund M., Reimund B., Massoulié J. Complex alternative splicing of acetylcholinesterase transcripts in Torpedo electric organ; primary structure of the precursor of the glycolipid-anchored dimeric form. EMBO J. 1988 Oct;7(10):2983–2993. doi: 10.1002/j.1460-2075.1988.tb03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J. L., Krejci E., Massoulié J. cDNA sequences of Torpedo marmorata acetylcholinesterase: primary structure of the precursor of a catalytic subunit; existence of multiple 5'-untranslated regions. EMBO J. 1987 Jul;6(7):1865–1873. doi: 10.1002/j.1460-2075.1987.tb02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang S., Standing T., Fletterick R. J., Stroud R. M., Finer-Moore J., Xuong N. H., Hamlin R., Rutter W. J., Craik C. S. The three-dimensional structure of Asn102 mutant of trypsin: role of Asp102 in serine protease catalysis. Science. 1987 Aug 21;237(4817):905–909. doi: 10.1126/science.3112942. [DOI] [PubMed] [Google Scholar]
- Swillens S., Ludgate M., Mercken L., Dumont J. E., Vassart G. Analysis of sequence and structure homologies between thyroglobulin and acetylcholinesterase: possible functional and clinical significance. Biochem Biophys Res Commun. 1986 May 29;137(1):142–148. doi: 10.1016/0006-291x(86)91187-3. [DOI] [PubMed] [Google Scholar]
- de la Escalera S., Bockamp E. O., Moya F., Piovant M., Jiménez F. Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 1990 Nov;9(11):3593–3601. doi: 10.1002/j.1460-2075.1990.tb07570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]