Abstract
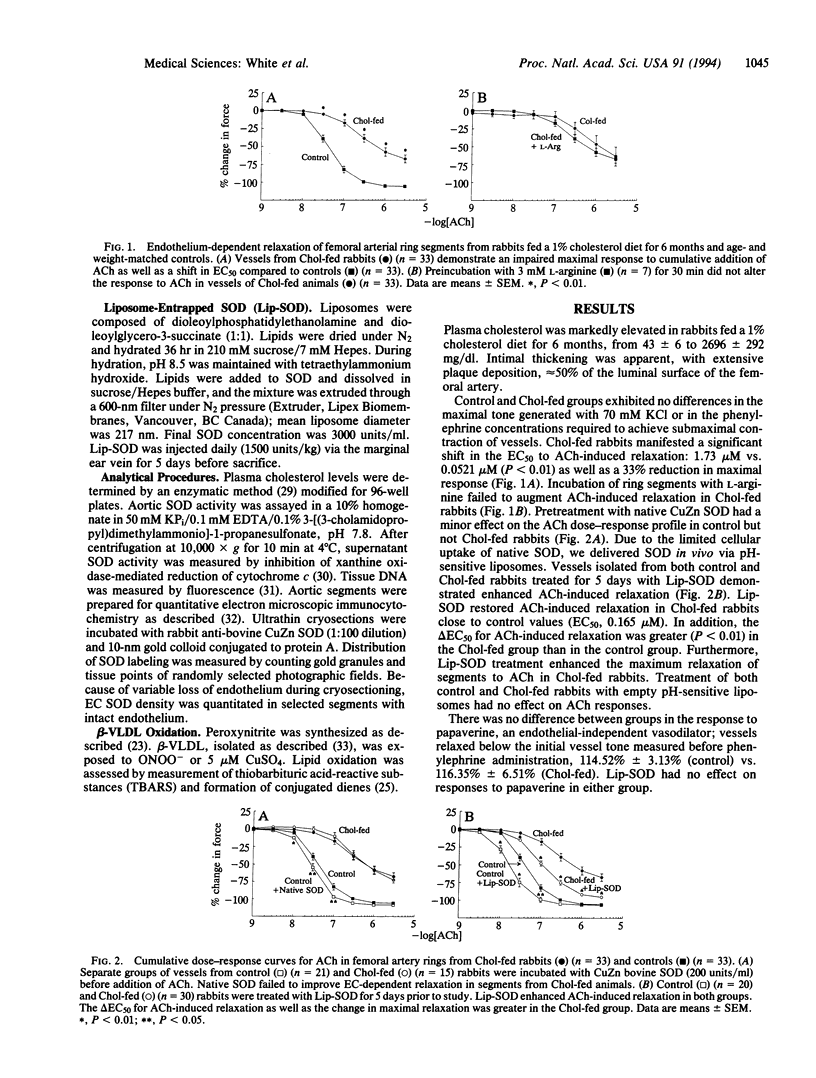
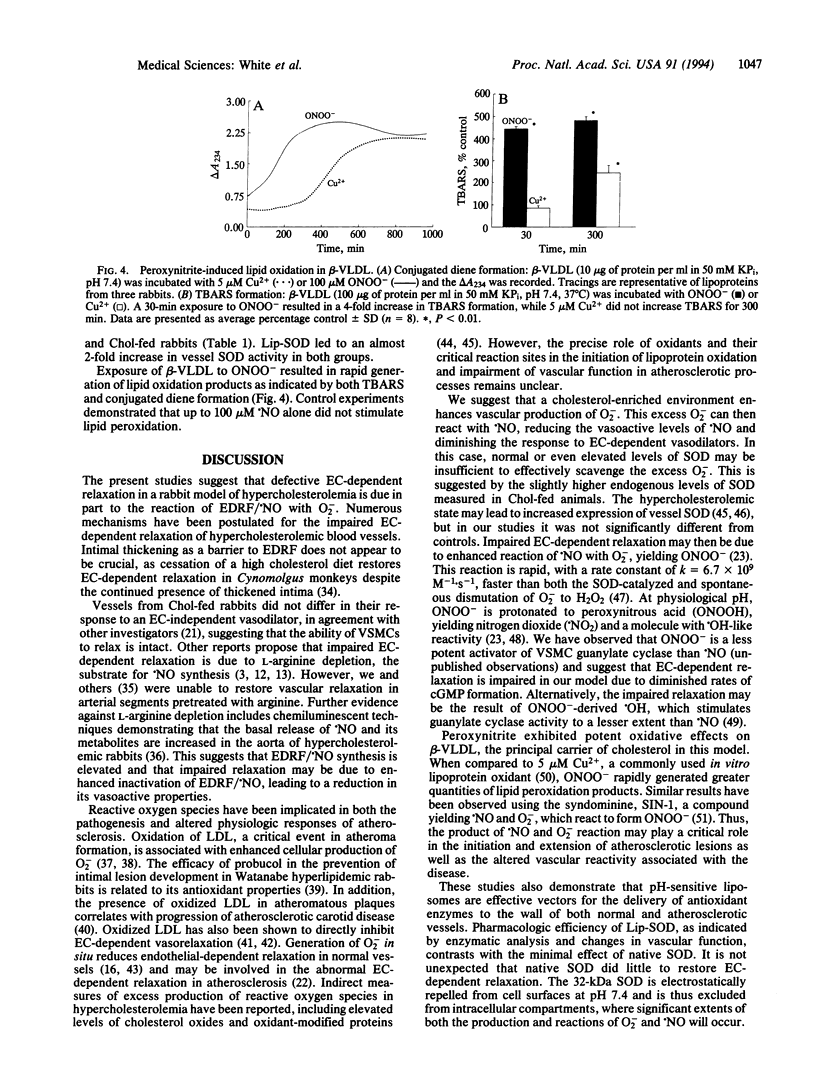
The role of reactive oxygen species in the vascular pathology associated with atherosclerosis was examined by testing the hypothesis that impaired vascular reactivity results from the reaction of nitric oxide (.NO) with superoxide (O2-), yielding the oxidant peroxynitrite (ONOO-). Contractility studies were performed on femoral arteries from rabbits fed a cholesterol-supplemented diet. Cholesterol feeding shifted the EC50 for acetylcholine (ACh)-induced relaxation and impaired the maximal response to ACh. We used pH-sensitive liposomes to deliver CuZn superoxide dismutase (SOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1) to critical sites of .NO reaction with O2-. Intravenously injected liposomes (3000 units of SOD per ml) augmented ACh-induced relaxation in the cholesterol-fed group to a greater extent than in controls. Quantitative immunocytochemistry demonstrated enhanced distribution of SOD in both endothelial and vascular smooth muscle cells as well as in the extracellular matrix. SOD activity in vessel homogenates of liposome-treated rabbits was also increased. Incubation of beta very low density lipoprotein with ONOO- resulted in the rapid formation of conjugated dienes and thiobarbituric acid-reactive substances. Our results suggest that the reaction of O2- with .NO is involved in the development of atherosclerotic disease by yielding a potent mediator of lipoprotein oxidation, as well as by limiting .NO stimulation of vascular smooth muscle guanylate cyclase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson T., Brandt U., Marklund S. L., Sjöqvist P. O. Vascular bound recombinant extracellular superoxide dismutase type C protects against the detrimental effects of superoxide radicals on endothelium-dependent arterial relaxation. Circ Res. 1992 Feb;70(2):264–271. doi: 10.1161/01.res.70.2.264. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell S. P., Lewis M. J., Henderson A. H. Effect of lipid feeding on endothelium dependent relaxation in rabbit aortic preparations. Cardiovasc Res. 1987 Jan;21(1):34–38. doi: 10.1093/cvr/21.1.34. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Zitnay K. M., Haudenschild C. C., Cunningham L. D. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988 Nov;63(5):903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Andon N. A., Girerd X. J., Hirsch A. T., Creager M. A. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation. 1991 Mar;83(3):1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Oury T., Rabouille C., Slot J. W., Chang L. Y. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley-Usmar V. M., Hogg N., O'Leary V. J., Wilson M. T., Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1992;17(1):9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- Del Boccio G., Lapenna D., Porreca E., Pennelli A., Savini F., Feliciani P., Ricci G., Cuccurullo F. Aortic antioxidant defence mechanisms: time-related changes in cholesterol-fed rabbits. Atherosclerosis. 1990 Mar;81(2):127–135. doi: 10.1016/0021-9150(90)90019-f. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mügge A., Alheid U., Haverich A., Frölich J. C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988 Feb;62(2):185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- Gianturco S. H., Bradley W. A., Gotto A. M., Jr, Morrisett J. D., Peavy D. L. Hypertriglyceridemic very low density lipoproteins induce triglyceride synthesis and accumulation in mouse peritoneal macrophages. J Clin Invest. 1982 Jul;70(1):168–178. doi: 10.1172/JCI110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Armstrong M. L., Freiman P. C., Heistad D. D. Restoration of endothelium-dependent relaxation by dietary treatment of atherosclerosis. J Clin Invest. 1987 Dec;80(6):1808–1811. doi: 10.1172/JCI113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Connor J., Wang C. Y. pH-sensitive immunoliposomes. Methods Enzymol. 1987;149:88–99. doi: 10.1016/0076-6879(87)49046-0. [DOI] [PubMed] [Google Scholar]
- Huie R. E., Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Plane F., Bruckdorfer K. R. Native and oxidized low-density lipoproteins have different inhibitory effects on endothelium-derived relaxing factor in the rabbit aorta. Br J Pharmacol. 1990 May;100(1):21–26. doi: 10.1111/j.1476-5381.1990.tb12045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakody L., Senaratne M., Thomson A., Kappagoda T. Endothelium-dependent relaxation in experimental atherosclerosis in the rabbit. Circ Res. 1987 Feb;60(2):251–264. doi: 10.1161/01.res.60.2.251. [DOI] [PubMed] [Google Scholar]
- Ku D. D. Mechanism of thrombin-induced endothelium-dependent coronary vasodilation in dogs: role of its proteolytic enzymatic activity. J Cardiovasc Pharmacol. 1986 Jan-Feb;8(1):29–36. doi: 10.1097/00005344-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Armstrong M. L., Harrison D. G., Piegors D. J., Heistad D. D. Vascular responses to leukocyte products in atherosclerotic primates. Circ Res. 1989 Oct;65(4):1078–1086. doi: 10.1161/01.res.65.4.1078. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Minor R. L., Jr, Myers P. R., Guerra R., Jr, Bates J. N., Harrison D. G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990 Dec;86(6):2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: a physiological regulator of guanosine 3',5'-monophosphate formation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Harrison D. G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol. 1991 Feb;260(2 Pt 1):C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Mügge A., Harrison D. G. L-arginine does not restore endothelial dysfunction in atherosclerotic rabbit aorta in vitro. Blood Vessels. 1991;28(5):354–357. doi: 10.1159/000158881. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Panasenko O. M., Vol'nova T. V., Azizova O. A., Vladimirov Y. A. Free radical modification of lipoproteins and cholesterol accumulation in cells upon atherosclerosis. Free Radic Biol Med. 1991;10(2):137–148. doi: 10.1016/0891-5849(91)90007-p. [DOI] [PubMed] [Google Scholar]
- Plane F., Kerr P., Bruckdorfer K. R., Jacobs M. Inhibition of endothelium-dependent relaxation by oxidized low-density lipoproteins. Biochem Soc Trans. 1990 Dec;18(6):1177–1178. doi: 10.1042/bst0181177. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Radi R., Cosgrove T. P., Beckman J. S., Freeman B. A. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993 Feb 15;290(Pt 1):51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. Vascular effects of oxygen-derived free radicals. Free Radic Biol Med. 1988;4(2):107–120. doi: 10.1016/0891-5849(88)90071-8. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Vanhoutte P. M. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ Res. 1991 Jan;68(1):209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- Sharma R. C., Crawford D. W., Kramsch D. M., Sevanian A., Jiao Q. Immunolocalization of native antioxidant scavenger enzymes in early hypertensive and atherosclerotic arteries. Role of oxygen free radicals. Arterioscler Thromb. 1992 Apr;12(4):403–415. doi: 10.1161/01.atv.12.4.403. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Kim P., Vanhoutte P. M. Endothelium-dependent relaxation to aggregating platelets in isolated basilar arteries of control and hypercholesterolemic pigs. Circ Res. 1988 Sep;63(3):604–612. doi: 10.1161/01.res.63.3.604. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988 Mar 4;959(1):20–30. doi: 10.1016/0005-2760(88)90145-2. [DOI] [PubMed] [Google Scholar]
- Tagawa H., Tomoike H., Nakamura M. Putative mechanisms of the impairment of endothelium-dependent relaxation of the aorta with atheromatous plaque in heritable hyperlipidemic rabbits. Circ Res. 1991 Feb;68(2):330–337. doi: 10.1161/01.res.68.2.330. [DOI] [PubMed] [Google Scholar]
- Warnick G. R. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]