ABSTRACT
The prevailing view of CD73 in cancer is that it is overexpressed in tumors and promotes cancer progression by dampening local T cell-mediated immune responses. We recently found that CD73 is down-regulated in poorly-differentiated and advanced stage endometrial carcinoma compared to normal endometrium and well-differentiated, early stage tumors. We revealed that CD73-generated adenosine induces a physiological response to protect epithelial integrity in well-differentiated, early stage endometrial carcinoma. The ability of CD73-generated adenosine to protect the barrier is not so different from its ability to induce immunosuppression and other physiological responses in cancerous tissues. In this commentary we examine the complexity of CD73 in cancer and suggest that a “one size fits all” approach to the role of CD73/adenosine in cancer is no longer warranted. Given that tumors often hijack normal cellular responses, we also provide consideration on how CD73s known role to protect barrier function may have implications in promoting tumor progression.
KEYWORDS: actin polymerization, adenosine, adenosine receptors, barrier function, cancer, CD73, cell 15 adhesions, endometrium, epithelial integrity, tumor progression
Introduction
Tissues are often subjected to stress such as hypoxia and inflammation. Epithelial cells have elaborate programs in place to minimize the damage to the barrier and to orchestrate recovery of cell-cell adhesions in the epithelium. Nearly two decades ago, the generation of extracellular adenosine by CD73 was discovered as being essential to protecting tissue barriers. CD73 or ecto-5′nucleotidase is a cell surface enzyme that catalyzes the dephosphorylation of extracellular 5′ adenosine monophosphate to adenosine. The release of adenine nucleotides, such as ATP, to the extracellular space increases with stress or tissue injury, occurring by cell lysis or non-lytic mechanisms. In concert with CD39, an ecto-nucleoside triphosphate diphosphohydrolase, CD73 elevates the concentration of extracellular adenosine (reviewed in Colgan et al.1). During episodes of inflammation, polymorphonuclear leukocyte (PMN) transendothelial migration can disrupt endothelial barrier function, giving rise to intravascular fluid extravasation and edema. Original studies by Lennon and colleagues demonstrated that CD73-generated adenosine limited damage-induced increases in endothelial barrier permeability and acted as a basic mechanism for resealing the vasculature during PMN transendothelial migration.2 The development of CD73 deficient mice significantly added to understanding the critical importance of CD73 to protecting the barrier. For the most part, CD73 deficient (Cd73−/−) mice are healthy and phenotypically normal in standard conditions. In response to hypoxia, however, the mice suffer from massive vascular leakage and pulmonary edema, due to the loss of endothelial barrier function.1 Central to its barrier protection is that CD73 is strongly induced by hypoxia-inducible factor (HIF).1 In models of increased intestinal permeability, such as inflammatory bowel diseases3,4 and exposure to bacterial toxins,5 CD73-generated adenosine is crucial to maintaining the epithelial barrier. Similar to endothelial cells, CD73-generated adenosine decreases paracellular permeability in intestinal epithelial cells and regulates cell adhesion molecules (CAM)s and CAM-associated proteins. We have shown that CD73-generated adenosine protects the endometrial epithelial barrier.6
CD73 also protects tissues by inducing local immunosuppression, angiogenesis, mucosal hydration, and ischemic preconditioning.1 These responses often occur by extracellular adenosine acting on one or more of 4 transmembrane G-protein coupled adenosine receptors, A1R, A2AR, A2BR, and A3R. Many studies have shown that CD73 is up-regulated in cancer and that the generation of adenosine by CD73 promotes tumor progression, particularly by dampening T cell-mediated immune responses.7,8 In contrast, we recently reported that CD73 is downregulated in poorly-differentiated and advanced stage endometrial carcinomas, and that in well-differentiated, early stage endometrial carcinoma CD73-generated adenosine induces a physiological response to protect epithelial integrity.6 In this commentary we examine the complexity of CD73 in cancer and how CD73s known role to protect barrier function may have implications in promoting tumor progression.
CD73 is upregulated in cancer and promotes tumor progression
The prevailing view of CD73 in cancer is that it is overexpressed in tumors and promotes cancer development and progression. CD73 is up-regulated in glioblastoma and carcinomas of the breast, colon, gallbladder, head and neck, pancreas, and ovary and often correlates with poor tumor differentiation, lymphatic involvement, advanced stage disease, and poor prognosis.7,9 CD73 is associated with poor prognosis and chemotherapy resistance in triple negative breast cancer (TNBC)10 and visceral metastases of breast cancer11 and melanoma.12 RNAi approaches and small molecule inhibitors of CD73 show CD73-generated adenosine to promote cancer cell proliferation, epithelial-to-mesenchymal transition, migration and invasion, and drug resistance. CD73 can promote tumor progression by non-catalytic actions, serving as an adhesion molecule for extracellular matrix components and mediating cancer cell migration on ECM via focal adhesion kinase. Overexpression of CD73 in cancer cells promotes subcutaneous tumor growth, and its down-regulation inhibits tumorgenicity. Cd73−/− mice show delayed tumor growth and are resistant to experimental metastasis in various murine tumor models. CD73 deficiency in mice also inhibits the development of chemically-induced fibrosarcomas and similarly suppresses tumorigenesis in transgenic mouse prostate models. Tumor cell chemotaxis and tumor angiogenesis are inhibited by CD73 deficiency as well. Targeting CD73 in vivo with anti-CD73 therapy decreases tumor levels of vascular endothelial growth factor (VEGF) and suppresses tumor angiogenesis. Similarly, anti-CD73 therapy has been shown to limit tumor growth and metastasis and improve chemotherapy sensitivity (reviewed in Antonioli et al.7 and Allard et al.9).
Much of the tumor promoting efforts of CD73 come from the potent action of extracellular adenosine to modulate immune cells, specifically suppressing anti-tumor T cell responses. CD73-generated adenosine encourages the escape of tumor cells from antitumor immune cells by inhibiting the activation, expansion, and homing of antitumor T cells, inducing T regulatory cell expansion and activity, inhibiting pro-inflammatory cytokine production and lytic activity by NK cells, converting macrophages from antitumor types (type I) to protumor types (type II), and skewing dendritic cell differentiation. Targeting CD73 alone or in combination with other immunotherapeutics, such as anti-CTLA-4 mAb and anti-PD1 mAb, reduces tumor growth (reviewed in Antonioli et al.7 and Allard et al.8).
CD73 is not always upregulated in cancer and is not always associated with poor prognostic features
We recently reported that CD73 is down-regulated in poorly-differentiated and advanced stage endometrial carcinomas and high CD73, seen in well-differentiated, early stage endometrial carcinoma, associates with better overall survival.6 Poorly-differentiated and advanced stage prostate,13 laryngeal,14 and high grade colon15 carcinomas also have been reported with downregulation of CD73. High CD73 expression associates with lower stage and grade, less adjacent carcinoma in situ, lower proliferation index, and is a predictor of better outcome in patients with nonmuscle-invasive bladder cancer.16 CD73 expression is associated with both good17 and poor7 prognosis in breast cancer. Down-regulation of CD73 in endometrial carcinoma occurs in the carcinoma cells.6 In prostate carcinoma, CD73 loss also occurs in the carcinoma cells,13 and it has been suggested that epigenetic silencing of CD73 in early stage tumors may abrogate differentiation and promote migration in a subset of melanomas.12 Additionally, in a minority of breast cancer cases, clinically aggressive disease develops in which the CD73 gene (NT5E) is methylated in the primary tumor.11 Methylation silencing of NT5E might promote enhanced migration in this subset of breast carcinomas through yet undefined mechanisms.11 We showed that CD73 is downregulated in ovarian high grade serous carcinoma (HGSC), a clinically aggressive malignancy, compared to normal ovary.6 Other studies have found high CD73 expression associates with poor prognosis in ovarian HGSC18 and that CD73 overexpression is associated with better prognosis, low stage, and better tumor differentiation in ovarian cancer.19
Tissue and cell specific differences of CD73 expression
CD73 is not uniformly expressed by all epithelial cells across all tissues. In the pancreas,20 breast,21 and the gastrointestinal tract,22 there is differential expression of CD73 across epithelial cell compartments. Notably, there is also nearly a 50-fold difference in CD73 activity and up to a 20-fold difference in AMP hydrolyzing activity not due to CD73 across different tissues, indicating tissue specificity.1 CD73 function can also differ in the same cell type from tissue to tissue. For example, CD73 regulates immune cell trafficking in vascular endothelium, but does not affect leukocyte movement across lymphatic endothelium.23 Within tumors, the tumor cells themselves can have different CD73 expression compared to adjacent stromal cells. For example, in rectal carcinoma, CD73 is differentially expressed in the tumor cells compared to stromal cells, with the combination of high tumor cell expression and low stromal expression having the strongest association with decreased survival.24 The combination of high CD73 in stromal cells and low CD73 in tumor cells is associated with better prognosis.24 Adenocarcinomas with high CD73 in both stromal and tumor cells have a comparatively good prognosis.24 The significance of the differential expression of CD73 between the cell compartments is not completely understood. Distinct roles of CD73 in hematopoietic and non-hematopoietic cells for tumor progression have been demonstrated.7,9
Although the endometrium and the breast are both regulated by estrogen signaling, CD73s role in the breast is likely distinct from its role in the endometrium. In normal breast, CD73 is mostly absent from acinar and ductal epithelial cells,21 but more frequently expressed by myoepithelial cells.21 CD73 is significantly up-regulated in TNBC as opposed to luminal or HER2+ subtypes.10 TNBC is an aggressive subtype characterized by an expression signature similar to that of basal/myoepithelial cells of the breast.25,26 MDA-MB-231 cells are a model for TNBC. MDA-MB-231 cells and TNBC express high CD73 and myoepithelial markers and have considerable stem cell-like/progenitor cell properties. Myoepithelial cells from normal human breast tissue exhibit a highly invasive capacity when grown at low density.26 We found that CD73 was significantly lower in The Cancer Genome Atlas immunoresponsive subset of endometrial carcinomas than the hormonal subset.6 Interestingly, both subtypes are comprised mostly of well-differentiated, early stage endometrial carcinomas.27 CD73 expression has been reported upregulated in BRAF mutant serous ovarian carcinomas compared to wild-type tumors and that serous carcinoma patients with BRAF or KRAS mutations have a better clinical outcome.28 CD73 expression is significantly elevated in cancer cell lines (n = 474) that have either KRAS and/or BRAF mutations, whereas CD73 is lower in cells with PTEN and/or TP53 mutations.29 In breast carcinomas, CD73 is negatively associated with estrogen receptor (ER) expression,9 positively associated with epidermal growth factor receptor (EGFR),7 and NT5E methylation is inversely associated with TP53 mutation.11 From these limited studies, it is clear that the molecular make-up of a tumor can have a profound influence on CD73 expression.
In cancer, like normal tissues, CD73 is just doing its job
Using cells that model well-differentiated, early stage endometrial carcinoma, we showed that CD73-generated adenosine induces a physiological response to protect epithelial integrity. Adenosine acting on the adenosine A1 receptor (A1R) induces actin polymerization, involving Rho GTPase, CDC42 and Neural Wiskott-Aldrich syndrome protein (N-WASP), members of the actin-related proteins, ARP2 and ARP3 actin polymerization complex.6 A1R increases cortical F-actin and the lengthening of cell-cell filopodia and increased E-cadherin and β-catenin at the membrane, leading to the re-forming of cell-cell adhesions in response to hypoxia.6 RNAi silencing and catalytic inhibition of CD73 increased the mobility and invasiveness of the cells, indicating that CD73 loss is important to tumor progression in endometrial carcinoma.6 The ability of CD73-generated adenosine to protect the barrier in well-differentiated, early stage tumors is not so different from the original idea proposed by Ohta and Sitkovsky that extracellular adenosine protects cancerous tissues by its physiological immunosuppressive actions. Their pivotal studies showed that: 1.) adenosine's activity on adenosine A2A receptor (A2AR) on immune cells is a critical axis that limits inflammatory responses, protecting normal tissues from an overly enthusiastic immune response,30 and 2.) similarly, that adenosine's activity on A2AR on immune cells served as a basis of immune escape in tumors, protecting cancers by inhibiting the infiltration of antitumor T cells.31
Other physiological responses of CD73, such as induction of angiogenesis, are also important in promoting cancer. CD73 promotes the production and release of VEGF, encourages endothelial cell proliferation and migration, and supports the formation of new tumor blood vessels in mice bearing melanoma tumors.7 The tumor microenvironment is reminiscent of the environment of acutely stressed, injured or diseased tissues. Both are overwhelmed by low oxygen, inflammation, and/or ischemia, leading to the increased release of adenine nucleotides to the cell surface and the generation of adenosine.7,8 The increase of extracellular adenosine in multiple tissues and disease, including cancer, is especially linked to hypoxia. Consistent with losing protection of the barrier in endometrial tumors, in our endometrial carcinoma cell models we found that the up-regulation of CD73 by hypoxia was uncoupled.6 Additionally, it is considered that CD73 expression/upregulation on tumor cells may help promote tumor progression by mimicking the role of CD73 in the normal migration of lymphocytes into the draining lymph nodes.32 High CD73 is associated with lymph node metastasis in prostate carcinomas.33 Considering its actions in both normal and cancerous tissues, it appears that CD73/adenosine is simply doing its job.
CD73s protection of the barrier: A dual role in endometrial carcinoma?
The role of CD73-generated adenosine to protect epithelial integrity by re-forming cell-cell adhesions in response to hypoxia in well-differentiated, early stage endometrial carcinoma is comparable to that of CD73 protecting the barrier in the gut. In the gut, CD73 has long been known to have a central role in maintaining intestinal epithelial integrity. Early studies in mice exposed to hypoxia revealed that oral delivery of the CD73 inhibitor, APCP increases intestinal permeability to inert tracers, such as FITC-labeled dextran. Several studies have subsequently described many beneficial situations that are central to the efforts of CD73/adenosine to maintain the intestinal barrier. Many have involved understanding the metabolic changes (e.g. oxygen, ATP, glucose) that occur with ongoing mucosal inflammation (including inflammatory hypoxia), the source of adenosine nucleotides (e.g., platelets, infiltrating PMN, and epithelial cells), interactions of immune cells with the intestinal epithelium (PMN transmigration and CD73s role in resealing the barrier), and HIF stabilization. Notably, HIF triggers the expression of several genes directly involved with adenosine metabolism and signaling (e.g., CD39, CD73, adenosine A2B receptor (A2BR)), which has proven key to enabling intestinal epithelial cells to function as an effective barrier. Similar to the endometrium, adenosine receptor signaling and cytoskeleton changes are the main elements involved in restitution of the barrier. In the gut, however, A2BR is the primary adenosine receptor involved (reviewed in Colgan et al.34).
In the gut, breakdown of the intestinal epithelial barrier contributes to the development of inflammatory bowel diseases (IBDs), including Crohn's disease and ulcerative colitis. IBDs are characterized by dysregulated innate and adaptive immune responses and increased epithelial barrier permeability. In trinitrobenzene sulfonate3 and dextran sulfate sodium4 colitis models, Cd73−/− mice have extensive destruction of colonic epithelial integrity, including epithelial crypt destruction and massive immune cell infiltration into the lamina propria. Thus, a major determinant of disease severity in IBDs is the loss of CD73s ability to protect the barrier. Similarly, in endometrial carcinoma cells (early stage disease model), pharmacological inhibition of CD73 (thus, the loss of epithelial integrity) increases in vitro migration and invasion.6 The intestinal epithelium closely interacts with underlying mucosal immune cells to control immune responses. Damage to the epithelial barrier leads to the production and release of inflammatory cytokines by immune and epithelial cells. CD73 deficiency in colitis models increases pro-inflammatory cytokines in colonic epithelia, including interferon-αA,3 interleukin-1β,4 and tumor necrosis factor-α.4
In the immunoresponsive TCGA subset of endometrial carcinomas, we found CD73 expression is significant lower than in the hormonal subset.6 The immunoresponsive subset is characterized by higher expression levels of immune response-related genes, including CD74, CD14, TNFRSF1B, and IL15RA, while the hormonal subset is characterized by higher expression of hormone receptors and estrogen-induced genes.27 Though genes of the immunoresponsive subset both activate and suppress immune responses, many have strong ties to pro-inflammatory responses. Thus, maintaining epithelial integrity (protecting the barrier) in low grade, non-invasive endometrial carcinoma may be a way to protect developing and growing tumors from their own induction of pro-inflammatory, anti-tumor immune responses caused by damage to the epithelial barrier. CD73 increasing cell-cell adhesions may therefore promote immunosuppression simply by acting as a physiological barrier (Fig. 1).6 For endometrial carcinoma, loss of barrier function is necessary for the subsequent migration, invasion, and metastasis.6 In later stages, endometrial tumors may not need the immune-escape induced by CD73s maintenance of the tissue barrier. Thus, CD73 may play very different roles (pro- and anti-tumor) in the same tumor type depending on tumor grade and stage.
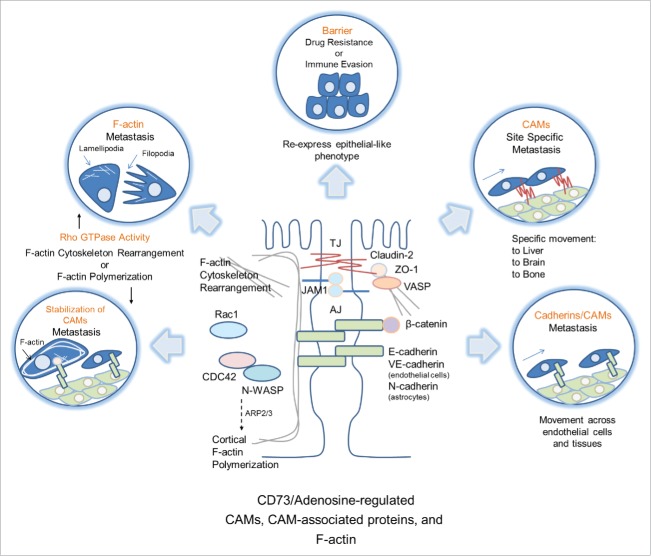
Figure 1.
Is CD73s protection of the barrier promoting immunosuppression in endometrial carcinoma? In both inflammatory bowel diseases and endometrial cancer, a major determinant of disease severity or progression is the loss of CD73s protection of the epithelial barrier. Loss or damage to the epithelial barrier often results in the production and release of pro-inflammatory mediators from immune and epithelial cells. In assessing CD73 expression in the TCGA data set of endometrial carcinoma we found CD73 was significantly lower in the immunoresponsive subset than the hormonal subset. Both subtypes are comprised mostly of well-differentiated, early stage endometrial carcinomas. The immunoresponsive subset is characterized by higher expression levels of immune response-related genes. Many of these genes are involved in pro-inflammatory responses. This raises the question of whether CD73s protection of the epithelial barrier may indirectly be promoting immunosuppression simply by acting as a physiological barrier.
CD73-mediated cell-cell adhesions promoting tumor progression?
Cancer cells often use an arsenal of approaches to dismantle cell-cell adhesions to encourage cell migration and invasion. Epithelial to mesenchymal transition (EMT) is a developmental program, hijacked by carcinoma cells, that induces transcriptional events to down-regulate intercellular adhesion genes. Cells transition from a cobblestone, cell-cell adhesive epithelial phenotype to a spindle-like, loosely or lacking adhesion, mesenchymal phenotype. With EMT it is common for cadherin expression to switch. Cadherins normally expressed in epithelial cells, such as E-cadherin, are down-regulated, while mesenchymal cadherins, including N- and Ob-cadherin, which promote tumor progression, are up-regulated. Other events, such as epigenetic changes and post-translational modifications of CAMs and CAM-associated proteins, also play a role in disrupting cell-cell adhesions to promote tumor progression. Though downregulation of CAMs (e.g., E-cadherin) and –associated proteins (e.g., β-catenin), enables cancer cell dissemination, cancer cells also up-regulate selective CAMs, including mesenchymal cadherins and claudins to promote mobility and invasiveness, vascular invasion, and tumor cell attachment to distant tissues (reviewed in Andrews et al.35). CD73s up-regulation of tumor promoting cell-cell adhesions may also provide another important mechanism, in addition to localized immune suppression, for high CD73 expression in cancers to promote tumor progression.
Recently, CD73 was shown to be a key regulator of TGF-β-induced EMT in gallbladder cancer cells.36 CD73 is upregulated in gallbladder carcinomas with poor differentiation, lymph node metastasis, and adjacent tissue invasion.36 Silencing CD73 in gallbladder carcinoma cells decreased vimentin and increased E-cadherin.36 High CD73 is associated with an EMT gene signature in ovarian HGSC.18 In ER-, highly tumorigenic breast cancer cells CD73 expression associates with absence of E-cadherin and increased expression of vimentin and N- and Ob-cadherin and the inverse (absence of N- and Ob-cadherin, increased E-cadherin) with ER+, less tumorigenic cells.37 In astrocytes, N-cadherin expression is increased by P2 and P1 (adenosine receptors) purinergic receptor activity.38 Interestingly, the location of N-cadherin to the cell surface membrane is independent of protein synthesis.38 In endometrial carcinoma cells we have also demonstrated the movement of E-cadherin and β-catenin to the membrane with no change in total protein.
N- and Ob-cadherin are up-regulated in several human cancers and are associated with invasiveness and poor prognosis. In breast cancer cells35 and melanoma cells,39 N-cadherin increases cancer cell dissemination by interacting with stromal and endothelial cells through homophillic binding of N-cadherin between these two cell types, enabling metastasis. Similarly, overexpression of Ob-cadherin (cadherin-11) in breast cancer cells increases the dissemination of the cells. Expression of cadherin-11 was shown to contribute to site specific metastasis, as these tumor cells were able to establish bone metastases, yet not lung metastases.40 This is facilitated by tumor cell expression of cadherin-11 interacting with cadherin-11 expressed on bone marrow stromal cells and osteoblastic cells, supporting the colonization of the tumor cells in bone.40 Visceral site and brain metastases are more common in primary melanomas lacking NT5E methylation, whereas primary melanomas with NT5E methylation metastasize mainly to lymph nodes or show limited metastatic potential.12 Similar is seen in breast carcinomas.11 Interestingly, in 3 of 4 paired breast carcinoma samples, NT5E was methylated in primary tumors and demethylated in CNS metastases, providing evidence for plasticity for CD73 expression in human cancers.11 In the limited number of endometrial carcinoma metastases we examined, an increase of CD73 relative to normal endometrium or grade 1 endometrioid endometrial carcinoma was not observed.6 The basis of CD73s regulation of site specific metastasis is unknown. N-cadherin expression follows similar site specificity, being significantly lower in melanoma-infiltrated lymph nodes than primary melanoma in the vertical growth phase and melanoma in the metastatic growth phase.41 Upregulation of zonula occludens protein-1 (ZO-1) in human melanomas is associated with N-cadherin, and in melanoma cells in vitro, ZO-1 co-immunoprecipitates with N-cadherin.42 Consistent with a role in steadying N-cadherin adhesion, silencing ZO-1 caused the cells to be less adherent and invasive.42 Adenosine receptor signaling phosphorylates vasodilator-stimulated phosphoprotein (VASP), an actin binding protein.43,44 Here, VASP can be co-immunoprecipitated with ZO-1 and associates with increased barrier function. VASP binding actin and interacting with tight junction proteins (ZO-1) is considered to stabilize adhesions, relaxing the actin cytoskeleton.43,44 Adenosine also induces actin reorganization and increases membrane VE-cadherin (cadherin-5), normally found on endothelial cells, to maintain blood vessel integrity.45 In breast carcinoma cells, cadherin-5 enhances the ability of the tumor cells to adhere to endothelial cells, encouraging the cancer cells to move easily in and out of the bloodstream, contributing to tumor cell dissemination.46 Other associations can be made. For example, in breast cancer liver metastases, claudin-2 is elevated in comparison to that of primary tumors and is indicated in directing site specific metastasis.47 Claudin-2 on the cancer cells forms adhesions with claudin-2 on hepatocytes.47 In colitis models, claudin-2 mRNA is significantly decreased in Cd73−/− mice.4
CD73/adenosine may also support CAMs for tumor progression by encouraging actin polymerization or the reorganization of the actin cytoskeleton for localizing and stabilizing tumor promoting CAMs at the membrane. Many studies link the pro-tumor, cell-cell adhesion effect of mesenchymal cadherins to Rho GTPase activity and changes in F-actin.35 We and others have shown that adenosine signaling can activate Rho GTPases and/or change the F-actin phenotype of cells.6,43,45,48 In a recent correlation and geneset enrichment analysis of gene expression across the Cancer Cell Line Encyclopedia panel (n = 967 cell lines), CD73 expression was positively correlated with pathways related to cell junctions and actin cytoskeleton organization and biogenesis in addition to extracellular matrix and regulation of cell migration.29 Accordingly, cancer cells may also hijack the ability of adenosine to regulate F-actin for forming structures (lamellipodia and/or filopodia) that promote mobility and invasion. Both RNAi silencing and pharmacologic blockade of the adenosine A2B receptor inhibited filopodia formation and invasive activity of breast cancer cells and reduced tumor metastasis to the lungs.49
Following metastasis, the ability of cancer cells to change back to a more epithelial-like phenotype is also important, as it allows for the formation of physiological barriers, which may represent a mechanism to decrease drug and immune cell access to tumors. Recently, miR-422a was shown to negatively regulate CD73 in larynx and oropharynx carcinoma cells.50 miR-422a is downregulated in stage III-IV loco-regional relapse (non-responders to radiation and chemotherapy) in oropharynx tumors compared to tumors without relapse (responders).50 Cells transfected with modified oligonucleotides inhibiting the expression of miR-422a (miRinhi) showed an increase in CD73 expression and activity.50 Notably, miRinhi cells showed intense labeling of F-actin at intercellular junctions.50 Cells treated with oligonucleotides that mimicked miR-422a had decreased expression of CD73 and disorganization of F-actin at the junctions.50 This is in agreement with our findings that CD73 increases cortical F-actin and membrane localization of CAMs in endometrial carcinoma cells for protecting epithelial integrity.6 miRinhi treated cells also have an increase in membranous E-cadherin.50 High CD73 appears unrelated to metastatic capacity in head and neck tumors, but it may be involved in early local recurrence.50 Thus, the increase in CD73 and its regulation of a more epithelial-like phenotype may be involved with therapy resistance.
Conclusions
The role of CD73-generated adenosine in cancer is complex. As discussed in this commentary adenosine may have very different roles in various human cancers. Therefore, a “one size fits all” approach to the mechanisms that adenosine regulates in cancer is not warranted. CD73s known ability to protect barrier function raises these questions when considering possible tumor promoting actions: 1.) Is CD73s protection of the barrier promoting immunosuppression? 2.) Does CD73 promote the expression, membrane localization, and/or stabilization of tumor promoting CAMs and CAM-associated proteins and/or changes in the F-actin phenotype in CD73 upregulated cancers (Fig. 2)? Phase I clinical trials with anti-CD73 mAb therapy are in progress with others set to begin soon. Defining inter-tumor differences will be important to understanding what specific cancer patients will more likely benefit from targeting CD73 clinically, which is essential for the success of developing CD73 as a cancer therapeutic and, more importantly, providing the best possible outcome for cancer patients.
Figure 2.
Possible tumor promoting roles of CD73-mediated cell-cell adhesions. Given that tumors often hijack normal cellular responses, it is possible that cancers with high CD73 use extracellular adenosine's ability to regulate CAMs, CAM-associated proteins, and F-actin for promoting tumor progression. CAMs may form heterotypic adhesions between tumor cells and stromal cells to promote tumor cell metastasis in general and direct metastatic cells to specific target organs. Additionally, activation of Rho GTPases and F-actin cytoskeleton reorganization or F-actin polymerization may promote the development of migration and invasion structures, such as lamellipodia and filopodia, and membrane localization and stabilization of tumor promoting CAMs. Following metastasis, cancer cells may also revert back to a more epithelial-like phenotype, which may encourage immune evasion and drug resistance in the metastasis target organ.
Abbreviations
- A1R
Adenosine A1 Receptor
- A2AR
Adenosine A2A Receptor
- A2BR
Adenosine A2B Receptor
- A3R
Adenosine A3 Receptor
- AJ
Adherens Junction
- AMP
Adenosine Monophosphate
- APCP
Adenosine 5′-(α,β-methylene) Diphosphate
- ARP2
Actin-related Protein 2
- ARP3
Actin-related Protein 3
- ATP
Adenosine Triphosphate
- BRAF
v-Raf Murine Sarcoma Viral Oncogene Homolog B
- CAM
Cell Adhesion Molecule
- CD14
Cluster of Differentiation 14
- CD39
Cluster of Differentiation 39 or Ectonucleoside Triphosphate Diphosphohydrolase-1
- CD73/NT5E
Cluster of Differentiation 73 or Ecto-5′nucleotidase
- CD74
Cluster of Differentiation 74
- CDC42
Cell Division Cycle 42
- CNS
Central Nervous System
- CTLA-4
Cytotoxic T-lymphocyte-associated Protein 4
- E-cadherin
Epithelial Cadherin or Cadherin-1
- EMT
Epithelial to Mesenchymal Transition
- ER
Estrogen Receptor
- F-actin
Filament Actin
- HER2
Human Epidermal Growth Factor Receptor 2
- HGSC
High Grade Serous Carcinoma
- HIF
Hypoxia-inducible Factor
- IBDs
Inflammatory Bowel Diseases
- IL15RA
Interleukin 15 Receptor, Alpha
- JAM1
Junctional Adhesion Molecule-1
- KRAS
V-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homolog
- MDA-MB-231
MD Anderson-Metastatic Breast-231
- miRinhi
Modified Oligonucleotides Inhibiting the Expression of miR-422a
- N-cadherin
Neural Cadherin or Cadherin-2
- N-WASP
Neural Wiskott-Aldrich Syndrome (N-WASP) Protein
- NK
Natural Killer
- Ob-cadherin
Osteoblast Cadherin or Cadherin-11
- PD-1
Programmed Death-1
- PMN
Polymorphonuclear Leukocyte
- PTEN
Phosphatase and Tensin Homolog
- RAC1
Ras-related C3 Botulinum Toxin Substrate 1 or Rho Family, Small GTP Binding Protein Rac1
- RNAi
RNA Interference
- TCGA
The Cancer Genome Atlas
- TGF-β
Transforming Growth Factor Beta
- TJ
Tight Junction
- TNBC
Triple Negative Breast Cancer
- TNFRSF1B
Tumor Necrosis Factor Receptor Superfamily Member 1B
- TP53
Tumor Protein 53
- VASP
Vasodilator-stimulated Phosphoprotein
- VE-cadherin
Vascular Endothelial Cadherin or Cadherin-5
- VEGF
Vascular Endothelial Growth Factor
- ZO-1
Zonula Occludens Protein-1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Due to space limitations, many relevant original research and review articles cannot be cited.
Funding
NIH/NCI P50CA098258 (RRB) and 5P50CA098258-10 (JLB), the Lupe C. Garcia Fellowship in Cancer Research (JLB), and the Interdisciplinary Translational Education and Research Training Program Fellowship (JLB) in the Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center.
References
- [1].Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2006; 2:351-60; PMID:18404475; http://dx.doi.org/ 10.1007/s11302-005-5302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med 1998; 188:1433-43; PMID:9782120; http://dx.doi.org/ 10.1084/jem.188.8.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol 2008; 180:4246-55; PMID:18322237; http://dx.doi.org/ 10.4049/jimmunol.180.6.4246 [DOI] [PubMed] [Google Scholar]
- [4].Bynoe MS, Waickman AT, Mahamed DA, Mueller C, Mills JH, Czopik A. CD73 is critical for the resolution of murine colonic inflammation. J Biomed Biotechnol 2012; 2012:260983; PMID:23118501; http://dx.doi.org/ 10.1155/2012/260983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schenck LP, Hirota SA, Hirota CL, Boasquevisque P, Tulk SE, Li Y, Wadhwani A, Doktorchik CT, Macnaughton WK, Beck PL, et al.. Attenuation of Clostridium difficile toxin-induced damage to epithelial barrier by ecto-5′-nucleotidase (CD73) and adenosine receptor signaling. Neurogastroenterol Motili 2013; 25:e441-53; http://dx.doi.org/ 10.1111/nmo.12139 [DOI] [PubMed] [Google Scholar]
- [6].Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K Jr., Broaddus RR. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J Clin Invest 2016; 126:220-38; PMID:26642367; http://dx.doi.org/ 10.1172/JCI79380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Hasko G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2016; 2:95-109; PMID:27014745; http://dx.doi.org/ 10.1016/j.trecan.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol 2016; 29:7-16; PMID:27209048; http://dx.doi.org/ 10.1016/j.coph.2016.04.001 [DOI] [PubMed] [Google Scholar]
- [9].Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets 2014; 18:863-81; PMID:24798880; http://dx.doi.org/ 10.1517/14728222.2014.915315 [DOI] [PubMed] [Google Scholar]
- [10].Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013; 110:11091-6; PMID:23776241; http://dx.doi.org/ 10.1073/pnas.1222251110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lo Nigro C, Monteverde M, Lee S, Lattanzio L, Vivenza D, Comino A, Syed N, McHugh A, Wang H, Proby C, et al.. NT5E CpG island methylation is a favourable breast cancer biomarker. Br J Cancer 2012; 107:75-83; PMID:22653144; http://dx.doi.org/ 10.1038/bjc.2012.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang H, Lee S, Nigro CL, Lattanzio L, Merlano M, Monteverde M, Matin R, Purdie K, Mladkova N, Bergamaschi D, et al.. NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. British J Cancer 2012; 106:1446-52; http://dx.doi.org/ 10.1038/bjc.2012.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rackley RR, Lewis TJ, Preston EM, Delmoro CM, Bradley EL Jr., Resnick MI, Pretlow TP, Pretlow TG. 5′-nucleotidase activity in prostatic carcinoma and benign prostatic hyperplasia. Cancer Res 1989; 49:3702-7; PMID:2471588 [PubMed] [Google Scholar]
- [14].Durak I, Isik AC, Canbolat O, Akyol O, Kavutcu M. Adenosine deaminase, 5′ nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in cancerous and noncancerous human laryngeal tissues. Free Radic Biol Med 1993; 15:681-4; PMID:8138195; http://dx.doi.org/ 10.1016/0891-5849(93)90174-S [DOI] [PubMed] [Google Scholar]
- [15].Eroglu A, Canbolat O, Demirci S, Kocaoglu H, Eryavuz Y, Akgul H. Activities of adenosine deaminase and 5′-nucleotidase in cancerous and noncancerous human colorectal tissues. Med Oncol 2000; 17:319-24; PMID:11114712; http://dx.doi.org/ 10.1007/BF02782198 [DOI] [PubMed] [Google Scholar]
- [16].Wettstein MS, Buser L, Hermanns T, Roudnicky F, Eberli D, Baumeister P, Sulser T, Wild P, Poyet C. CD73 Predicts Favorable Prognosis in Patients with Nonmuscle-Invasive Urothelial Bladder Cancer. Dis Markers 2015; 2015:785461; PMID:26543299; http://dx.doi.org/ 10.1155/2015/785461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Supernat A, Markiewicz A, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J, Sejda A, Szade J, Czapiewski P, Biernat W, Zaczek A. CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol 2012; 20:103-7; PMID:22553809; http://dx.doi.org/ 10.1097/PAI.0b013e3182311d82 [DOI] [PubMed] [Google Scholar]
- [18].Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T, et al.. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer research 2015; 75:4494-503; PMID:26363007; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-3569 [DOI] [PubMed] [Google Scholar]
- [19].Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS. Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol 2012; 23:274-81; PMID:23094131; http://dx.doi.org/ 10.3802/jgo.2012.23.4.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Flocke K, Lesch G, Elsasser HP, Bosslet K, Mannherz HG. Monoclonal antibodies against 5′-nucleotidase from a human pancreatic tumor cell line: their characterization and inhibitory capacity on tumor cell adhesion to fibronectin substratum. Eur J Cell Biol 1992; 58:62-70; PMID:1644065 [PubMed] [Google Scholar]
- [21].Kruger KH, Thompson LF, Kaufmann M, Moller P. Expression of ecto-5′-nucleotidase (CD73) in normal mammary gland and in breast carcinoma. Br J Cancer 1991; 63:114-8; PMID:1989648; http://dx.doi.org/ 10.1038/bjc.1991.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Investigat 1997; 99:2588-601; http://dx.doi.org/ 10.1172/JCI119447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Algars A, Karikoski M, Yegutkin GG, Stoitzner P, Niemela J, Salmi M, Jalkanen S. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood 2011; 117:4387-93; PMID:21346249; http://dx.doi.org/ 10.1182/blood-2010-11-321646 [DOI] [PubMed] [Google Scholar]
- [24].Zhang B, Song B, Wang X, Chang XS, Pang T, Zhang X, Yin K, Fang GE. The expression and clinical significance of CD73 molecule in human rectal adenocarcinoma. Tumour Biol 2015; 36:5459-66; PMID:25677906; http://dx.doi.org/ 10.1007/s13277-015-3212-x [DOI] [PubMed] [Google Scholar]
- [25].Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al.. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98:10869-74; PMID:11553815; http://dx.doi.org/ 10.1073/pnas.191367098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gordon LA, Mulligan KT, Maxwell-Jones H, Adams M, Walker RA, Jones JL. Breast cell invasive potential relates to the myoepithelial phenotype. Inter J Cancer 2003; 106:8-16; http://dx.doi.org/ 10.1002/ijc.11172 [DOI] [PubMed] [Google Scholar]
- [27].Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al.. Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497:67-73; PMID:23636398; http://dx.doi.org/ 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, Malpica A, Wolf JK, Lu KH, Gershenson DM. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol 2010; 177:1611-7; PMID:20802181; http://dx.doi.org/ 10.2353/ajpath.2010.100212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nevedomskaya E, Perryman R, Solanki S, Syed N, Mayboroda OA, Keun HC. A Systems oncology approach identifies NT5E as a key metabolic regulator in tumor cells and modulator of platinum sensitivity. J Proteome Res 2016; 15:280-90; PMID:26629888; http://dx.doi.org/ 10.1021/acs.jproteome.5b00793 [DOI] [PubMed] [Google Scholar]
- [30].Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001; 414:916-20; PMID:11780065; http://dx.doi.org/ 10.1038/414916a [DOI] [PubMed] [Google Scholar]
- [31].Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al.. A2A adenosine receptor protects tumors from antitumor T cells. Proc Nat Acad Sci U S A 2006; 103:13132-7; http://dx.doi.org/ 10.1073/pnas.0605251103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Salmi M, Jalkanen S. Homing-associated molecules CD73 and VAP-1 as targets to prevent harmful inflammations and cancer spread. FEBS letters 2011; 585:1543-50; PMID:21515268; http://dx.doi.org/ 10.1016/j.febslet.2011.04.033 [DOI] [PubMed] [Google Scholar]
- [33].Yang Q, Du J, Zu L. Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol Oncol Res 2013; 19:811-4; PMID:23653114; http://dx.doi.org/ 10.1007/s12253-013-9648-7 [DOI] [PubMed] [Google Scholar]
- [34].Colgan SP, Curtis VF, Lanis JM, Glover LE. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers 2015; 3:e970936; PMID:25838978; http://dx.doi.org/ 10.4161/21688362.2014.970936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Andrews JL, Kim AC, Hens JR. The role and function of cadherins in the mammary gland. Breast Cancer Res 2012; 14:203; PMID:22315958; http://dx.doi.org/ 10.1186/bcr3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xiong L, Wen Y, Miao X, Yang Z. NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res 2014; 355:365-74; PMID:24310606; http://dx.doi.org/ 10.1007/s00441-013-1752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ostapkowicz A, Inai K, Smith L, Kreda S, Spychala J. Lipid rafts remodeling in estrogen receptor-negative breast cancer is reversed by histone deacetylase inhibitor. Mol Cancer Ther 2006; 5:238-45; PMID:16505096; http://dx.doi.org/ 10.1158/1535-7163.MCT-05-0226 [DOI] [PubMed] [Google Scholar]
- [38].Tran MD, Wanner IB, Neary JT. Purinergic receptor signaling regulates N-cadherin expression in primary astrocyte cultures. J Neurochem 2008; 105:272-86; PMID:18182057; http://dx.doi.org/ 10.1111/j.1471-4159.2008.05214.x [DOI] [PubMed] [Google Scholar]
- [39].Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 2001; 61:3819-25; PMID:11325858 [PubMed] [Google Scholar]
- [40].Tamura D, Hiraga T, Myoui A, Yoshikawa H, Yoneda T. Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int J Oncol 2008; 33:17-24; PMID:18575746 [PubMed] [Google Scholar]
- [41].Watson-Hurst K, Becker D. The role of N-cadherin, MCAM and beta3 integrin in melanoma progression, proliferation, migration and invasion. Cancer Biol Ther 2006; 5:1375-82; PMID:16969099; http://dx.doi.org/ 10.4161/cbt.5.10.3241 [DOI] [PubMed] [Google Scholar]
- [42].Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol 2005; 166:1541-54; PMID:15855653; http://dx.doi.org/ 10.1016/S0002-9440(10)62370-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 2002; 16:583-5; PMID:11919161 [DOI] [PubMed] [Google Scholar]
- [44].Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol 2002; 282:C1235-45; PMID:11997237; http://dx.doi.org/ 10.1152/ajpcell.00288.2001 [DOI] [PubMed] [Google Scholar]
- [45].Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vascular Pharmacol 2010; 52:199-206; http://dx.doi.org/ 10.1016/j.vph.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Labelle M, Schnittler HJ, Aust DE, Friedrich K, Baretton G, Vestweber D, Breier G. Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor beta signaling. Cancer Res 2008; 68:1388-97; PMID:18316602; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2706 [DOI] [PubMed] [Google Scholar]
- [47].Tabaries S, Dupuy F, Dong Z, Monast A, Annis MG, Spicer J, Ferri LE, Omeroglu A, Basik M, Amir E, et al.. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Mol Cell Biol 2012; 32:2979-91; PMID:22645303; http://dx.doi.org/ 10.1128/MCB.00299-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lu Q, Harrington EO, Newton J, Casserly B, Radin G, Warburton R, Zhou Y, Blackburn MR, Rounds S. Adenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancement. Am J Physiol Lung Cell Mol Physiol 2010; 298:L755-67; PMID:20228181; http://dx.doi.org/ 10.1152/ajplung.00330.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Desmet CJ, Gallenne T, Prieur A, Reyal F, Visser NL, Wittner BS, Smit MA, Geiger TR, Laoukili J, Iskit S, et al.. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci U S A 2013; 110:5139-44; PMID:23483055; http://dx.doi.org/ 10.1073/pnas.1222085110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bonnin N, Armandy E, Carras J, Ferrandon S, Battiston-Montagne P, Aubry M, Guihard S, Meyronet D, Foy JP, Saintigny P, et al.. MiR-422a promotes loco-regional recurrence by targeting NT5E/CD73 in head and neck squamous cell carcinoma. Oncotarget 2016; 7(28): 44023-44038; PMID:27281619 [DOI] [PMC free article] [PubMed] [Google Scholar]