Abstract
The increased risks conferred by inflammatory bowel disease (IBD) to the development of colorectal cancer (CRC) gave rise to the term “colitis-associated cancer” and the concept that inflammation promotes colon tumorigenesis. A condition more common than IBD is low-grade inflammation, which correlates with altered gut microbiota composition and metabolic syndrome, both present in many cases of CRC. Recent findings suggest that low-grade inflammation in the intestine is promoted by consumption of dietary emulsifiers, a ubiquitous component of processed foods which alter the composition of gut microbiota. Here, we demonstrate in a pre-clinical model of colitis-induced CRC that regular consumption of dietary emulsifiers carboxymethylcellulose or polysorbate-80 exacerbated tumor development. Enhanced tumor development was associated with an altered microbiota metagenome characterized by elevated levels of lipopolysaccharide and flagellin. We found that emulsifier-induced alterations in the microbiome were necessary and sufficient to drive alterations in major proliferation and apoptosis signaling pathways thought to govern tumor development. Overall, our findings support the concept that perturbations in host-microbiota interactions that cause low-grade gut inflammation can promote colon carcinogenesis.
Keywords: Emulsifiers, inflammation, microbiota, colonic carcinogenesis
Introduction
Colorectal cancer (CRC) is among the most common human malignancies (1) and has been firmly linked to chronic intestinal inflammation, giving rise to the term “colitis-associated cancer” (CAC) (2,3). The development of CAC in patients suffering from IBD is one of the best characterized examples of an association between intestinal inflammation and carcinogenesis (4–7). Among patients with ulcerative colitis (UC), the risk of colon cancer has been found to be as high as 2% at 10 years, 8% at 20 years, and 18% at 30 years after initial diagnosis (4). In contrast, the lifetime risk of sporadic colorectal cancer in the United States is only 5% (8).
Gut microbiota is the collective term for the large diverse community of microorganisms that inhabits the intestine. Gut microbiota play an important role in health, particularly in promoting immune system development and aiding metabolism. Alterations in microbiota composition, often referred to as dysbiosis, is thought to play a central role in the pathogenesis of numerous intestinal disorders including inflammatory bowel disease (IBD) (9), and is associated with CRC (10). However, whether microbial dysbiosis observed in CRC patients is a consequence of the pathology or is causal remains unclear. An altered microbiota can play a role in promoting CAC, not only through induction of inflammation, but also through the production of toxins that create a favorable niche for tumor cells (11). Indeed, commensal organisms can have an enormous impact on tumorigenesis through the production of tumor-promoting genotoxins that can induce chromosomal instability (12). For example, certain strains of Escherichia coli harboring the Pks island, involved in the synthesis of the colibactin toxin, are frequently associated with human colorectal tumors. These strains inhibit DNA mismatch repair proteins, and were also reported to have carcinogenic effects in mice (11,13–16). Besides, treatment of mice with antibiotics confers some degree of protection against CAC, supporting the pivotal role of the gut microbiota during tumorigenesis (17). Moreover, azoxymethane (AOM)-treated germ-free IL10−/− mice failed to develop colitis and colorectal tumors, indicating that the presence of colitogenic bacteria is essential for the development of CAC (18).
While gut inflammation is classically defined histopathologically, specifically by the presence of immune cell infiltrates, it is now appreciated that a much more common form of inflammation is “low-grade intestinal inflammation”, which is defined by elevated systemic expression of pro-inflammatory cytokines in the absence of the classical aggregates of immune cell infiltrates. Alterations in host/microbiota relationship have been associated with and can promote low-grade intestinal inflammation (19). Moreover, it is increasingly appreciated that low-grade chronic inflammation in the gut can promote metabolic disorders such as type II diabetes, atherosclerosis, and obesity, which is itself associated with increased incidence of colon cancer (20,21).
Emulsifiers are detergent-like molecules that are incorporated into most processed foods to improve texture and stability, and we recently demonstrated that emulsifiers disrupted mucus-bacterial interactions, inducing intestinal inflammation (22). In this recent study, we investigated the effect of two commonly used emulsifiers, namely carboxymethylcellulose (CMC) and polysorbate-80 (P80) on the host’s intestine. CMC was previously described to promote overgrowth and inflammation of small intestine in genetically susceptible mice (23), and P80 is able to increase bacterial translocation across epithelia in vitro (24,25).
These two emulsifiers are indigestible and mainly excreted in the feces (26–29), and we recently found that both CMC and P80 promoted microbiota encroachment and increased levels of pro-inflammatory flagellin and lipopolysaccharide (LPS), which correlated with a change in microbiota composition and intestinal inflammation. Such alterations promoted colitis in mice genetically predisposed to this disorder, and induced low-grade inflammation and metabolic syndrome in WT mice. Importantly, such effects were dependent upon the presence of the microbiota (no phenotype observed in germ-free mice), and fecal microbiota transplant from emulsifier-treated mice to germ-free mice, transferred some features of intestinal inflammation and metabolic syndrome (22).
In the present study, we hypothesized that emulsifiers could be involved in CRC development through the promotion of low-grade intestinal inflammation and alterations of the intestinal microbiota. To test this hypothesis, we used the well-established murine model of CAC using the carcinogen AOM, followed by two cycles of dextran sulfate sodium (DSS) in mice subjected to chronic exposures of two commonly used emulsifiers, CMC and P80. We herein report that dietary emulsifying agents created and maintained a pro-inflammatory environment in the colon, associated with alterations of the proliferation/apoptosis balance that resulted in exacerbated carcinogenesis. These changes were associated with, a dependent upon, alterations in microbiota composition and diversity that created a favorable niche for tumorigeneisis. These findings support the concept that a perturbed host-microbiota interaction resulting in alterations of the intestinal homeostasis can promote colonic carcinogenesis.
Methods
Materials
Sodium carboxymethylcellulose (CMC, average MW ~250,000, degree of substitution = 0.7) and Polysorbate-80 (P80) were purchased from Sigma (Sigma, St. Louis, MO).
Mice
Four-week old male C57BL/6 WT mice were used in this study. All mice were bred and housed at Georgia State University, Atlanta, Georgia, USA under institutionally-approved protocols (IACUC # A14033). Mice were housed in specific pathogen-free conditions and fed ad libitum with regular chow diet. Animals used in figures 1–6 and S1–S10 were housed in Helicobacter positive room, and animals used in figure 7 and S11 were housed in Helicobacter negative room.
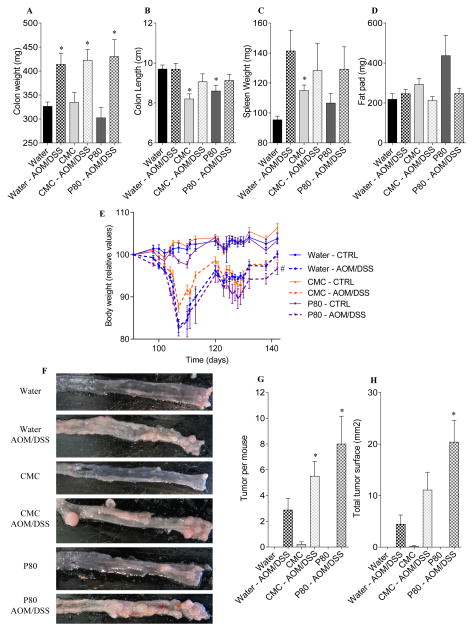
Figure 1. Dietary emulsifiers promote colitis-associated cancer.
WT mice were exposed to drinking water containing CMC or P80 (1.0%) for 13 weeks. Mice were then injected intraperitoneally with AOM (10 mg/kg body weight), maintained for 7 days, and then subjected to a two-cycle DSS treatment (each cycle consisted of 7 days of 2.5% DSS and 14 days of H2O). (A) colon weights, (B) colon lengths, (C) spleen weights, (D) fat-pad mass, (E) Body weight over time, (F) representative colon samples from each experimental group at the end of the AOM/DSS protocol, (G) number of tumor per mouse, (H) total tumor surface determined using a dissecting microscope fitted with an ocular micrometer. Data are the means +/− S.E.M. (n=5–8). Significance was determined using t-test (* indicates p<0.05) or two-way group ANOVA corrected for multiple comparisons with a Bonferroni test (# indicates statistical significance).
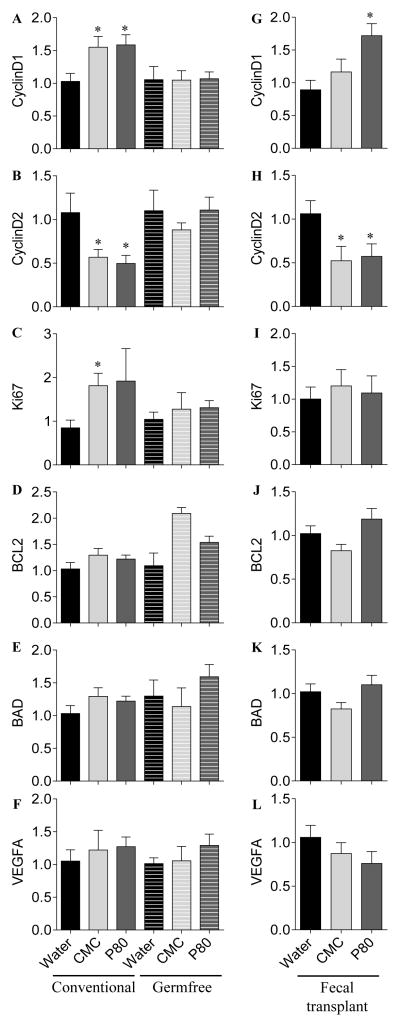
Figure 6. Dietary emulsifiers alter epithelial cell proliferation and apoptosis in a microbiota-dependent manner.
Conventional and germfree Swiss-Webster WT mice were exposed to drinking water containing CMC or P80 (1.0%) for 13 weeks. Intestinal microbiota from conventional Swiss-Webster WT mice exposed to drinking water containing CMC or P80 (1.0%) for 13 weeks were transplanted to germfree Swiss-Webster WT mice. Analysis of (A and G) CyclinD1, (B and H) CyclinD2, (C and I) Ki67, (D and J) BCL2, (E and K) BAD and (F and L) VEGFA mRNA expression by q-RT-PCR in the colon following emulsifier treatment under germfree conditions (1st column, A–F) and following microbiota transplantation (2nd column, G–L).
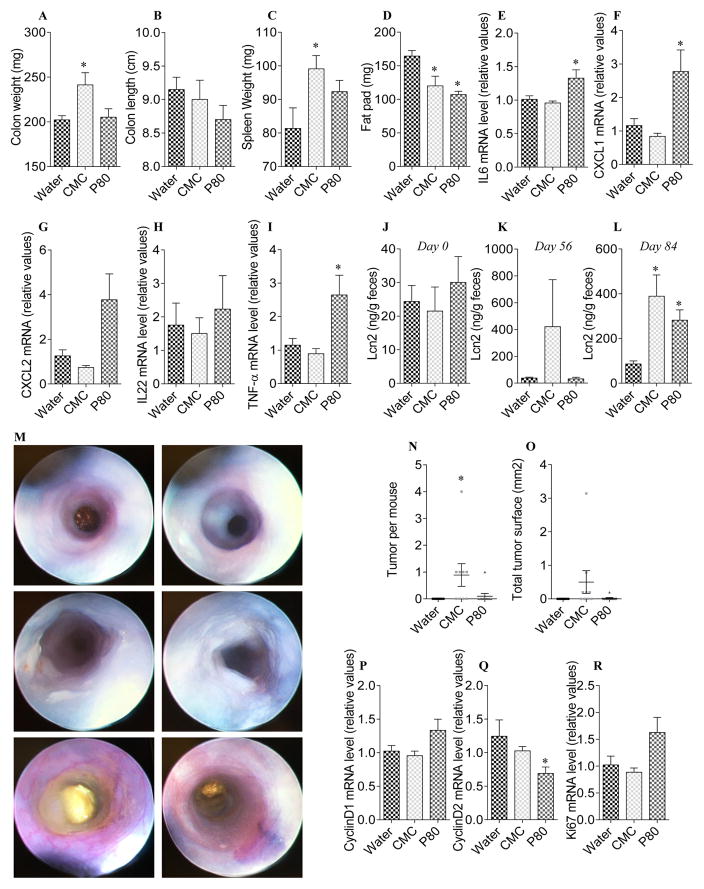
Figure 7. Dietary emulsifiers promote intestinal inflammation and carcinogenesis in the absence of DSS.
WT mice were exposed to drinking water containing CMC or P80 (1.0%) for 12 weeks. Mice were injected intraperitoneally with AOM (10 mg/kg body weight) weekly for a total of 7 injections. (A) Colon weights, (B) colon lengths, (C) spleen weights and (D) fat-pad mass. (E–I) Analysis of (E) IL-6, (F) CXCL1, (G) CXCL2, (H) IL-22 and (I) TNF-α mRNA expression by q-RT-PCR in colons of emulsifier-AOM treated mice. (J–L) Fecal Lcn2 concentration at day 0 (J), 56 (K) and 84 (L). (M) Representative colonoscopy from each experimental group at the end of the protocol. (N) Number of tumor per mouse and (O) total tumor surface determined using a dissecting microscope fitted with an ocular micrometer. (P–R) Analysis of (P) CyclinD1, (Q) CyclinD2 and (R) Ki67 mRNA expression by q-RT-PCR in colons of emulsifier-AOM treated mice. Data are the means +/− S.E.M. (n=10). Significance was determined using t-test (* indicates p<0.05).
Emulsifier agent treatment
Mice were exposed to CMC or P80 diluted in the drinking water (1.0%). The same water (reverse-osmosis treated Atlanta city water) was used for the water-treated (control) group. These solutions were changed every week. Body weights were measured every week and expressed as percentages of the initial body weight (day 0 defined as 100%) in order to study emulsifier effect on body weight gain. Fresh feces were collected every week for downstream analysis.
Colitis-associated cancer model
Colitis-associated cancer (CAC) was induced as previously described with some modifications (30). As schematized in Figure S1, after 13 weeks (91 days) of emulsifier administration, mice were intraperitoneally injected with AOM (10 mg/kg body weight) (Sigma-Aldrich, St. Louis, MO) diluted in PBS and maintained on regular chow diet and water or emulsifier-supplemented water for 5 days. Mice were then subjected to two cycles of DSS treatment (MP Biomedicals, Solon, OH, USA), in which each cycle consisted of 2.5% DSS for 7 days followed by a 14-day recovery period with regular water or emulsifier-supplemented water. Body weights were measured every week and expressed as percentages of the initial body weight (day 91 = post emulsifier defined as 100%) in order to study AOM-DSS protocol effect on body weight gain. After treatment, mice were fasted for 5h at which time blood was collected by retrobulbar intraorbital capillary plexus. Hemolysis-free serum was generated by centrifugation of blood using serum separator tubes (Becton Dickinson, Franklin Lakes, NJ). After colitis-associated cancer protocol, mice were euthanized, and colon length, colon weight, spleen weight and adipose weight were measure. Organs and blood were collected for downstream analysis. Colonic tumors were counted and surface measured using a dissecting microscope. The total area of tumors for each colon was determined.
As schematized in Figure S11, emulsifier-treated animals were also weekly treated with AOM (10 mg/kg body weight) diluted in PBS. At the end of the experiment, mice were fasted for 15h, colonoscopy procedure was performed (Karl Storz Endoskope, El Segundo, CA) and mice were euthanized and organs collected as previously described.
Germ-free experiments
Germ-free Swiss Webster mice were kept under germfree conditions in a Park Bioservices isolator in our germ-free facility. CMC and P80 were diluted to 1% in water and then autoclaved for germ-free purpose. The same water was used for the water-treated (control) group. Conventional age-matched and sex-matched Swiss Webster mice were used in parallel. After 3 months of emulsifier agent treatment, terminal analyses were performed.
Microbiota transplantation
Cecal contents from Swiss Webster detergent-treated mice were suspended in 30%glycerol diluted in PBS (1.0ml) and stocked at −80°C until analysis. Germ-free Swiss Webster mice (4 weeks old) were removed from the isolator and were orally administered 200 μl of fecal suspension made using glycerol stocks. Transplanted mice were monitored for 3 months before terminal analysis.
Colonic myeloperoxidase (MPO) assay, quantification of fecal Lcn-2 and serum CXCL-1 and IL-6 by ELISA
See supplemental methods section.
Fecal flagellin and lipopolysaccharide load quantification
We quantified flagellin and lipopolysaccharide (LPS) as previously described (31) using human embryonic kidney (HEK)-Blue-mTLR5 and HEK-BluemTLR4 cells, respectively (Invivogen, San Diego, California, USA). We resuspended fecal material in PBS to a final concentration of 100 mg/mL and homogenized using a Mini-Beadbeater-24 without the addition of beads to avoid bacteria disruption. Supernatants were serially diluted and applied to mammalian cells. Purified E. coli flagellin and LPS (Sigma, St Louis, Missouri, USA) were used for standard curve determination. After 24 h of stimulation, we applied cell culture supernatant to QUANTI-Blue medium (Invivogen, San Diego, California, USA) and measured alkaline phosphatase activity at 620 nm after 30 min.
RNA Extraction, Real-Time RT-PCR and bacterial quantification by qPCR
See supplemental methods section and table S1.
Fecal microbiota analysis by 16S rRNA gene sequencing using Illumina technology and metagenome prediction
16S rRNA gene sequencing was performed as previously described (22), with data deposited in the European Nucleotide Archive under accession number PRJEB8035. For details, please see supplementary methods.
Ki67 immunohistochemistry
Mouse proximal colon devoid of tumor were fixed in 10%-buffered formalin for 24 h at room temperature and subsequently embedded in paraffin. Tissues were sectioned at 5-μm thickness and deparaffinized. Sections were incubated in sodium citrate buffer and cooked in a pressure cooker for 10 minutes for antigen retrieval. Sections were then blocked with 5% goat serum in TBS followed by one hour incubation with anti-Ki67 (1:100, Vector Laboratories, Burlingame, CA) at 37º C. After washing with TBS, sections were treated with biotinylated secondary antibodies for 30 minutes at 37°C, and color development was performed using the Vectastain ABC kit (Vector Laboratories). Sections were then counterstained with hematoxylin, dehydrated, and coverslipped. Ki67-positive cells were counted per crypt.
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL)
To quantitate the number of apoptotic cells in colonic epithelial cells, mouse proximal colon devoid of tumor were fixed in 10%-buffered formalin for 24 h at room temperature, embedded in paraffin, sectioned at 5-μm thickness, deparaffinized and stained for apoptotic nuclei according to the manufacturer’s instructions using the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN). TUNEL-positive cells overlapping with DAPI nuclear staining were counted per crypt.
Statistical Analysis
Data are presented as means ± SEM. Significance was determined using t-tests, with each treatment group compared with the control group. Two-way group ANOVA corrected for multiple comparisons using Bonferroni post-test was used for body weight over time and alpha diversity analysis (GraphPadPrismsoftware, version 6.01). * and # indicate statistically significant differences.
Results
Dietary emulsifying agents induce low-grade intestinal inflammation associated with metabolic syndrome
Multiple mouse litters were equally split at weaning into three groups that received either water, carboxymethylcellulose (CMC) or polysorbate-80 (P80) in drinking water (1.0% w/v or v/v, respectively) for 13 weeks, as previously reported (22) (Figure S1). In accord with our previous work, emulsifier consumption resulted in features of chronic low-grade intestinal inflammation, including shortened colons and splenomegaly (Figure S2). Fecal lipocalin-2 (Lcn2), which is a sensitive and broadly dynamic marker of intestinal inflammation in mice (32), was used to quantify the intestinal inflammation, and showed that emulsifier-treated mice exhibited elevated fecal Lcn2 levels after 9 weeks of dietary emulsifier consumption (day 63) (Figure S2A–C), confirming the induction of low-grade inflammation. As expected, both CMC and P80 induced a modest but statistically significant increase in body mass (Figure S2D). Emulsifier’s treatment also impaired glycaemic control as assessed by fasting blood glucose concentration (Figure S2E), and was associated with an increase of food consumption (Figure S2F), confirming our previous observation that emulsifiers induce low-grade intestinal inflammation and impair glucose metabolism (22).
Emulsifier consumption exacerbates carcinogenesis in a colitis-associated cancer model
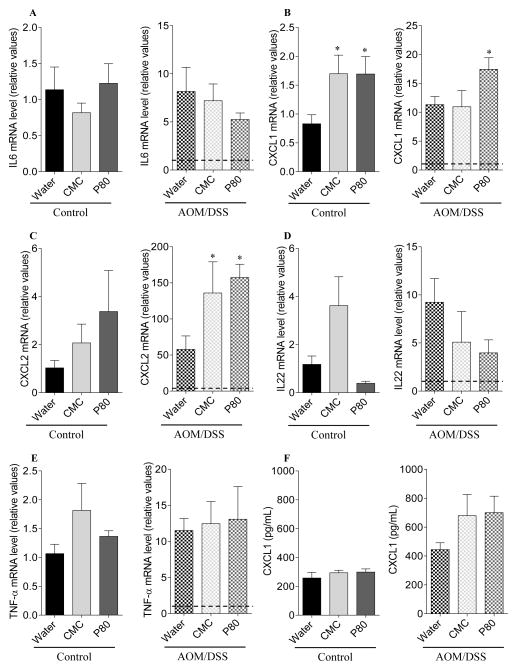
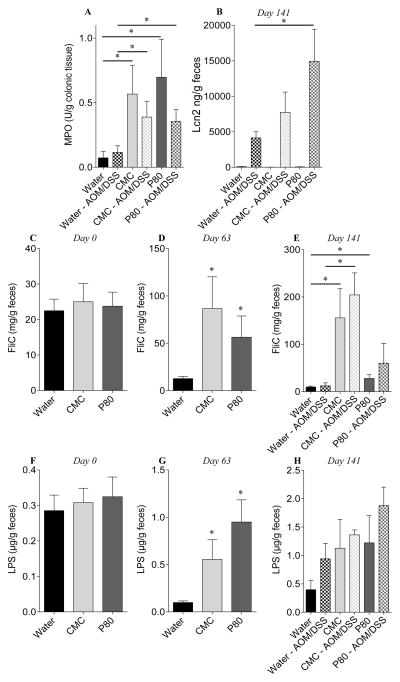
In an effort to investigate whether low-grade intestinal inflammation induced by emulsifier consumption might predispose to the development of colitis-associated carcinogenesis, mice that had consumed emulsifiers for 90 days were subsequently administered AOM and DSS while maintaining emulsifier consumption except during DSS administration (Figure S1). All groups of AOM/DSS-treated mice displayed acute weight loss during DSS treatment and, when euthanized, exhibited gross features of inflammation, including increased colon and spleen weights (Figure 1A–E). While emulsifier treatment, by itself, induced some indicators of inflammation such as colon shortening and mild splenomegaly, the extent of inflammatory changes induced by AOM/DSS treatment (i.e. fold change induced by AOM/DSS treatment) was not greater in mice that had consumed emulsifiers. However, emulsifier consumption increased tumor development in response to AOM/DSS compared with AOM/DSS control animals, as assessed by number and size of tumors (Figure 1F–H). Histological examination revealed the presence of larger adenomas and increased areas of inflammatory cell infiltration in emulsifier/AOM/DSS-treated animals compared to the water/AOM/DSS group (Figure S3). Histologically, no gross difference between water-treated and emulsifier-treated mice was observed in the absence of AOM/DSS (Figure S3). We next examined the expression of pro-inflammatory cytokines by q-RT-PCR using mRNA extracted from whole distal colon devoid of tumor. mRNAs were thus isolated from a mixture of epithelial cells, immune cells and other colonic cells, allowing the analysis of global molecular alterations of the colon. Emulsifier consumption by itself resulted in increased expression of the pro-inflammatory cytokine CXCL1, confirming that emulsifiers induce the development of low-grade intestinal inflammation (Figure 2A–E). AOM/DSS significantly increased the expression levels of all the tested cytokines/chemokines (IL-6, CXCL1, CXCL2, IL-22 and TNF-α) compared to the corresponding control groups (Figure 2A–E), as were the levels of circulating CXCL1 (Figure 2F). After AOM/DSS-induced carcinogenesis, both CXCL1 and CXCL2 were significantly increased in emulsifier-treated group compared to water-treated group (Figure 2B and C), supporting the previous observation that AOM/DSS-induced tumors were infiltrated by inflammatory cells. Importantly, we noted that mice receiving P80 exhibited the highest tumor and a greater increase of the CXCL1 and CXCL2 compared to CMC (Figure 2B and C). As a further readout of the inflammatory state of the intestine, we next examined colonic myeloperoxidase (MPO) activity and fecal Lcn2 level following AOM/DSS treatment (figure 3A–B). These analyses confirmed the observation that emulsifier’s consumption per se was sufficient to drive intestinal inflammation, as revealed by a moderate but nonetheless significant increase in MPO in emulsifier-only treated groups compared to water-only treated group (Figure 3A). Furthermore, emulsifiers led to an exacerbation of the intestinal inflammation observed after AOM/DSS, as revealed by both MPO and Lcn2 measurements at day 141 (Figure 3A–B).
Figure 2. Dietary emulsifiers promote intestinal inflammation and carcinogenesis.
Analysis of (A) IL-6, (B) CXCL1, (C) CXCL2, (D) IL-22 and (E) TNF-α mRNA expression by q-RT-PCR in the colon following emulsifier treatment and following the induction of colonic neoplasia. (F) Analysis of serum CXCL1 level by ELISA following emulsifier treatment and following the induction of colonic neoplasia. Data are the means +/− S.E.M. (n=5–8). Significance was determined using t-test (* indicates p<0.05).
Figure 3. Dietary emulsifiers favor a pro-inflammatory microbiota.
(A) Colonic myeloperoxidase (MPO) levels, (B) fecal Lcn2 concentration at day 141, and (C–H) bioactive levels of fecal flagellin (FliC, C–E) and LPS (F–H) assayed with TLR5 and TLR4 reporter cells, respectively, at day 0 (C, F), day 63 (D, G) and day 126 (E, H). Data are the means +/− S.E.M. (n=5–8). Significance was determined using t-test (* indicates p<0.05).
Dietary emulsifying agents alter the intestinal microbiota composition, leading to a pro-inflammatory intestinal environment
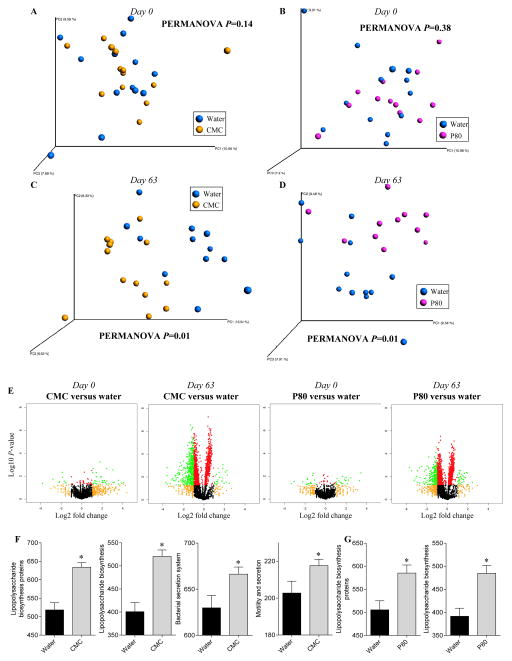
We next considered the possibility that emulsifier-induced alterations of the gut microbiota might underlie its tumor promoting effects. Microbiota composition analysis were previously performed on CMC and P80 treated animals and revealed a strong clustering following treatment irrespective of cage grouping, thus confirming our observation that emulsifiers alter microbiota composition (22). Based on previous reports that some pathovars of Escherichia coli can produce the pro-carcinogenic toxin colibactin (11,14,33), we aimed to elucidate whether such pathovars were playing a role in the observed emulsifier-induced exacerbation of tumorigenesis. The quantification of γ-Proteobacteria, Enterobacteriaceae, Escherichia coli, or ClbB encoding gene (colibactin polyketide synthesis system) revealed that none of these phylotypes were significantly altered following emulsifier consumption (day 0, day 21 and day 63) or after tumorigenesis induction (day 141), suggesting that colibactin-producing bacteria were not involved in the aforementioned emulsifier-induced exacerbation of tumorigenesis (Figure S4). We next performed a more-in-depth analysis of the intestinal microbiota after emulsifier treatment, and found that both CMC and P80 led to a significant reduction of microbiota diversity after 9 weeks (day 63) of treatment (Figure S5A–B), as well as profound alteration of the bacterial community at the phylum, class and order levels (Figure S5C–E). Such alterations were characterized by an increase in Bacteroidales and a decrease in Clostridiales orders upon CMC or P80 consumption (Figure S5C–E). LEfSe (LDA Effect Size), used to identify the most differentially abundant taxons and OTUs between water and emulsifier-treated groups, revealed a decrease of numerous Firmicutes members, such as Lactobacillus, upon emulsifier consumption, together with an increase of Bacteroidetes members (Figure S6).
We next wanted to investigate whether such alterations of the microbiota were associated with any modification of its inherent ability to induce pro-inflammatory gene expression in the host. Hence, we measured the capacity of feces from control and emulsifier-treated mice to activate pro-inflammatory gene expression via the lipopolysaccharide (LPS) and flagellin receptors Toll-like receptor 4 (TLR4) and Toll-like receptor 5 (TLR5), respectively. As previously reported, at day 63, exposure to emulsifiers increased levels of bioactive fecal LPS and flagellin (Figure 3C–H). Elevated fecal flagellin and LPS were not associated with an elevation in the total fecal bacterial load (Figure S7), indicating that this increase in microbiota pro-inflammatory potential was independent of bacterial load and, rather likely a consequence of altered species composition. The induction of tumorigenesis by AOM/DSS was found to further increase the pro-inflammatory potential of the microbiota, in both water and emulsifier-treated groups (Figure 3E and H). The analysis of the predicted metagenome indicated the presence of an altered metagenome in emulsifier-treated animals compared to water-only treated animals (Figures 4, S8 and S9) (34). Using principal coordinate analysis, a strong and distinct clustering (P=0.01) was observed between metagenomes of water and CMC or P80-treated groups at day 63 (Figure 4C and D), while predicted metagenomes of all groups were similar at day 0 (Figures 4A and B). This was further confirmed using Volcano plots of KEGG pathways abundance, demonstrating a drastic alteration in microbiota metagenomes following emulsifier consumption at day 63 (Figure 4E). Such alterations were characterized, in emulsifier-treated animals, by a decrease in the richness of signaling pathways, in accord with the observed decrease in bacterial community richness (Figure S5). Moreover, the analysis of significantly altered metabolic pathways revealed that emulsifier consumption led to an increased proportion of bacterial genes involved in LPS biosynthesis, bacterial motility and secretion systems (Figure 4F–G, S8 and S9), correlating with the observation of an increased pro-inflammatory microbiota under emulsifier consumption. Altogether, these data demonstrate that emulsifier consumption drastically altered the intestinal microbiota composition resulting in a basal low-grade pro-inflammatory environment in the intestine that predisposed to subsequent tumorigenesis.
Figure 4. Profound metagenome alteration following CMC and P80 consumption.
WT mice were exposed to drinking water containing CMC or P80 (1.0%) for 13 weeks. PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) was used to predict the metagenomes, subsequently analyzed by principal coordinates analysis of the beta diversity using binary jaccard method at day 0 (A–B) and day 63 (C–D). (E) Kyoto Encyclopedia of genes and genomes (KEGG) pathways were visualized on a volcano plot. From left to right: water-treated versus CMC-treated at day0; water-treated versus CMC-treated at day63; water-treated versus P80-treated at day0; water-treated versus P80-treated at day63. For each KEGG identifier, the difference in abundance between the two groups is indicated in log2 fold change on x-axis (with positive values corresponding to an increase in emulsifier-treated group compare to water-treated group, and negative values corresponding to a decrease in emulsifier-treated group compare to water-treated group), and significance between the two groups is indicated by log10 p-value on the y-axis. Red dots correspond to KEGG identifiers with a p-value <0.05 between emulsifier-treated and water-treated groups. Orange dots correspond to KEGG identifiers with at least a 2-fold decreased or increased abundance in emulsifier-treated group compare to water-treated group. Green dots correspond to KEGG identifiers with at least a 2-fold decreased or increased abundance in emulsifier-treated group compare to water-treated group and with a p-value <0.05. (F–G) Predicted metagenomes were categorized at level 3 of the KEGG pathways, and pathways involved in lipopolysaccharide biosynthesis, secretion system synthesis and motility were graphed. Data are the means +/− S.E.M. (n=5–8). Significance was determined using t-test (* indicates p<0.05).
Dietary emulsifying agent disturb the proliferation/apoptosis balance of epithelial cells
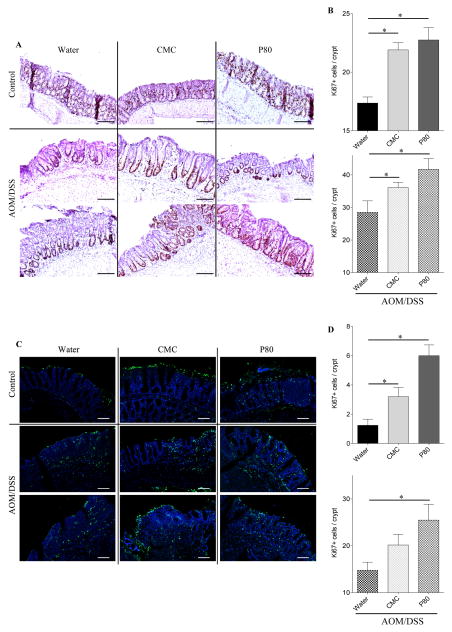
The increased tumor burden observed in emulsifier-treated animals suggested the possibility of increased cell proliferation in those animals. Hence, proliferation of colonic epithelial cells was analyzed by Ki67 staining, which revealed that the consumption of emulsifiers by itself (i.e. no AOM/DSS) resulted in increased cell proliferation relative to the water-treated control group (Figure 5). AOM/DSS treatment increased the number of Ki67 positive cells in all groups of mice, but the proliferation level remained significantly higher in AOM/DSS-treated mice that had consumed emulsifiers, in accord with the notion that emulsifier promotion of tumorigeneis involves increased cell proliferation (Figure 5). To further address the role of cell turnover in this process, we performed TUNEL-based quantification of apoptosis in colonic sections from emulsifier-treated mice. Analogous to our results for cell proliferation, we observed that consumption of emulsifiers, alone, increased the basal level of TUNEL+ cells. Moreover, this difference between the water and emulsifier-consuming groups was further increased in response to AOM/DSS treatment. For both proliferative and apoptotic cells counts, similar results were obtained when subdividing the crypts (top/middle/bottom, data not shown). Together, these results indicate that emulsifier consumption upregulates both apoptosis/proliferation in the intestinal epithelium, resulting in an increased cell turnover, and creating an opportune milieu for the tumorigenesis.
Figure 5. Dietary emulsifiers alter epithelial cell proliferation and apoptosis during colitis-associated cancer development.
(A–B) Epithelial cell proliferation was analyzed by immunohistochemistry using the proliferation marker Ki67 in colonic tissue sections. (A) Representative images of Ki67 staining. Scale bar, 200μm. (B) Ki67+ cells were counted and averaged per crypt. (C–D) Epithelial cell apoptosis was analyzed by terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL). (C) Representative confocal images of TUNEL assay: TUNEL, green; DNA, blue. Scale bar, 25μm. (D) TUNEL+ DAPI+ cells were counted and averaged per crypt. Data are the means +/− S.E.M. (n=5–8). Significance was determined using t-test (* indicates p<0.05).
Dietary emulsifiers alter epithelial cell proliferation and apoptosis in a microbiota-dependent manner
To further investigate how emulsifier consumption impacted proliferation/apoptosis, we next analyzed by q-RT-PCR the expression levels of genes that control proliferation (Cyclin D1, D2, Ki67), apoptosis (BCL2 and BAD), and angiogenesis (VEGFA). As shown in figure 7, we observed that, following dietary emulsifier consumption, the expression of CyclinD1, CyclinD2 and Ki67-encoding genes were significantly deregulated (Figure 7A–C), while the β-catenin pathway were found unaltered (Figure S10). In the AOM/DSS treated groups, the expression levels of these genes were further deregulated, without any difference observed between water- and emulsifier-treated groups (data not shown). The anti- and pro-apoptotic encoding genes Bcl2 and Bad (Figure 7D–E) and the marker of angiogenesis VEGFA (Figure 7F) remained unaltered under emulsifier’s consumption.
We next sought to investigate whether the unbalanced proliferation/apoptosis and the molecular alterations observed in emulsifier treated mice were driven by alterations in gut microbiota. We therefore analyzed the expression of the same genes in germfree animals treated with dietary emulsifiers, and importantly found that none of those genes have an altered expression under germfree conditions (Figure 7A–F), thus indicating that the presence of an altered microbiota is a prerequisite for subsequent perturbations in proliferation and apoptosis processes. Lastly, we investigated whether the altered microbiota driven by emulsifier consumption was sufficient to alter intestinal expression of genes involved in the proliferative/apoptosis balance. As presented in figure 7G–L, we found that transfer of microbiota from emulsifier consuming mice to germ free mice, that were not fed emulsifiers, recapitulated perturbations of Cyclin D1 and D2 expression, thus suggesting that emulsifier-induced alterations of intestinal microbiota composition plays a central and direct role in the promotion of carcinogenesis.
Dietary emulsifiers induce carcinogenesis in AOM-treated animals
We next sought to investigate whether emulsifier consumption may be sufficient to drive colonic carcinogenesis in animals treated with AOM but without exogenously-induced severe intestinal inflammation. For this purpose, WT mice were exposed to drinking water containing CMC or P80 (1.0%) for 12 weeks, and were injected intraperitoneally with AOM (10 mg/kg body weight) weekly, for a total of seven injections. The combination of AOM injections and emulsifier consumption leads to substantial intestinal inflammation, as characterized by increase in colon and spleen weights and by increased pro-inflammatory cytokine expressions and fecal Lcn2 levels (Figure 7A–L). Importantly, CMC-induced intestinal inflammation was sufficient to induce carcinogenesis in a subset of animals, that was associated with alterations of proliferative pathways (Figure 7M–R), suggesting that, at least in presence of some mutagens, emulsifier-induced low grade inflammation and/or alteration of proliferation pathways is sufficient to drive colon carcinogenesis.
Discussion
Mounting evidence implicates alteration of the gut microbiota, i.e. dysbiosis, as an important determinant of colon cancer. A major tenet in this indictment is that the microbial dysbiosis is a major driver of gut inflammation, which, when occurring chronically, is strongly associated with an increased incidence of colon cancer. More recently, it has been shown that the microbiota composition can influence tumor development beyond merely driving inflammation (11,14,33,35). Herein, we add to this body of knowledge in several ways thus providing new insights into factors that may drive microbial dysbiosis in the first place and, moreover, elucidate how an altered microbiota can result in increased tumor development. Specifically, we report that, in mice, consumption of dietary emulsifiers resulted in an altered gut microbiota composition associated with increased levels of flagellin and LPS, hence creating a low-grade pro-inflammatory environment. The latter was associated with altered rates of proliferation/apoptosis that predisposed animals to exacerbated tumor development when subjected to a chemical-induced model of colitis-associated cancer (CAC). In addition, emulsifier consumption was sufficient to drive development of colonic tumors in some animals treated with AOM (without DSS) and was associated with altered proliferation. The level of exposure used herein in mice is intended to approximately model the case of humans who eat large amounts of processed foods, many of which contain CMC, P80, and/or other emulsifiers exceeding 2% of the product by weight (23).
While human studies of microbial dysbiosis, inflammation, and cancer are primarily limited to associations, that germfree mice are protected against colonic carcinogenesis supports the notion that dysbiosis is not purely a consequence of disease but, rather, also plays a role in driving development of colorectal cancer (9,36–38). Moreover, in this study, we demonstrated that microbiota dysbiosis that precedes the initiation of colonic tumorigenesis was sufficient to promote cancer development. Importantly, the induction of colonic tumorigenesis similarly induced intestinal inflammation in emulsifier- and water-treated groups, showing that the observed phenotypes were not only due to an exacerbation of inflammation during DSS, but that the low-grade intestinal inflammation that precedes the initiation of carcinogenesis was playing a central role. While, in mice, emulsifier consumption promotes increased food consumption and adiposity but not obesity and, in humans, obesity itself is associated with colon cancer, we speculate that the link between emulsifiers and cancer is not obesity per se but rather low-grade inflammation. Accordingly, emulsifiers increased food consumption in AOM/DSS-treated mice without a corresponding increase in weight, likely reflecting increased energy demand, suggest that inflammation and associated increases in cell turnover are more germane. However, it is possible that emulsifier-induced alterations in microbiota can cause increased energy intake that might promote carcinogenesis irrespective of adiposity.
One mechanism by which microbiota was previously reported to promote colorectal cancer is via a transient contact between colibactin-producing E. coli and epithelial cells, that subsequently became malignant (11,14,33,35). We thus hypothesized that colibactin-producing E. coli could be involved in the emulsifier-induced exacerbation of tumorigenesis. However, the quantification of Proteobacteria, Enterobacteriaceae, Escherichia coli and ClbB encoding gene negated this possibility. Although emulsifiers did not drive substantial changes in abundance of such pathovars, inflammation, alteration of microbiota and its capacity to generate pro-inflammatory signals were critical for tumor development. Indeed, upon emulsifier’s exposure, the diversity of microbiota was decreased and the abundance of some phyla, class and orders was altered (decrease of Firmicutes, notably Clostridiales class and Lactobacillus member, and increase of Bacteroidetes). Lactobacilli have been associated with protection against colonic carcinogenesis via anti-oxidant, anti-proliferation properties and immunomodulatory and antitumor effects (39–41). We observed a greater abundance of bacteroidetes in both CMC and P80 treated mice, correlating with the observation that intestinal mucosal surface of patients with adenoma displayed increased abundance of Bacteroidetes (42). However, this same study reported higher abundance of Firmicutes in adenoma patients while we observed a decrease of this phylum upon emulsifier’s consumption (42). While little is known about how emulsifiers impact microbiota, the findings of our present study, in conjunction with previous reports, suggest that the consumption of emulsifiers induce a shift of the gut microbial population creating a favorable environment for colonic carcinogenesis (22,23). Gut microbes could indeed act through various pathways including proliferation, immune system or inflammation. We quantified the pro-inflammatory potential of the microbiota associated with the consumption of CMC or P80 and showed increased level of bioactive flagellin and lipopolysaccharide. It was recently reported that, in human, serum anti-flagellin and anti- lipopolysaccharide antibodies concentration positively correlate with colorectal cancer risk (43). Importantly, TLR4-deficient mice, unable to recognize bacterial lipopolysaccharide, are protected from colon carcinogenesis (44), further highlighting the central role played by increased fecal flagellin and/or lipopolysaccharide loads in the carcinogenesis initiation and promotion.
Comparative metagenomes analyses of CMC- or P80-treated mice and water control mice showed that the shift of bacteria was accompanied by alterations of several metabolic pathways, with a decrease in overall metabolic pathway richness and an increased proportion of bacterial genes involved in LPS biosynthesis, as well as bacterial motility and secretion systems. These observations were correlating with the increased pro-inflammatory ability of microbiota measured under emulsifier consumption. Importantly, in addition of promoting and maintaining low-grade intestinal inflammation, we observed in the current study that the consumption of dietary emulsifiers also induced alterations of some major proliferation and apoptosis actors in a microbiota-dependent manner. The observation that such disruptions were efficiently transferred to mice receiving fecal microbiota of emulsifier-treated donors revealed that emulsifier-induced alteration of microbiota composition plays a central role in the promotion of carcinogenesis. The previous observation that genetically engineered animals that develop intestinal inflammation, such as TLR5KO and NLRP3 mice, are not necessarily predisposed to colonic carcinogenesis (unpublished data and (45) respectively), highlights the concept that alterations of proliferation and apoptosis pathways in a microbiota-dependent manner may be the central mechanism that mediates the emulsifier-dependent increase of carcinogenesis.
An important distinction between this study and others examining microbiota, inflammation, and cancer, is that emulsifier consumption does not induce histopathologically evident (i.e. classic) inflammation, but rather induces only low-grade inflammation. Such low-grade inflammation is associated with and may cause obesity and its interrelated metabolic diseases, i.e. metabolic syndrome. Hence, the mechanisms described herein may be relevant not only to the potential promotion of colon cancer by one class of food additive, emulsifiers, but may be a broad mechanism whereby any inducer of low-grade inflammation, including obesity itself, may increase potential for cancer development. While the increased risk of cancer development in obese are modestly less than that of IBD patients (46), given the very high and increasing prevalence of obesity in all developed countries, low-grade inflammation may prove to be a major factor that underlies the increasing incidence of colon cancer. Hence, we propose that numerous factors that induce low-grade inflammation, including consumption of dietary emulsifiers, may promote a hostile environment in the colon by modifying the microbiota composition, leading to low-grade intestinal inflammation and alterations in the colonic proliferation/apoptosis balance, therefore creating a favorable niche for colonic tumorigenesis.
Supplementary Material
Acknowledgments
Financial support: This work was supported by NIH grant DK099071 and DK083890 (A.T. Gewirtz). B. Chassaing is a recipient of the Career Development Award from the Crohn’s and Colitis Foundation of America (CCFA). E. Viennois is a recipient of the Research Fellowship Award from the CCFA. D. Merlin is a recipient of a Research Career Scientist Award from the Department of Veterans Affairs.
Abbreviations
- AOM
azoxymethane
- CAC
colitis-associated cancer
- CMC
carboxymethylcellulose
- CRC
colorectal cancer
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- IL
interleukin
- KEGG
Kyoto Encyclopedia of genes and genomes
- Lcn-2
lipocalin-2
- LEfSe
LDA Effect Size
- LPS
lipopolysaccharide
- MPO
colonic myeloperoxidase
- P80
polysorbate-80
- PICRUSt
Phylogenetic investigation of communities by reconstruction of unobserved states
- TLR4
Toll-like receptor 4
- TLR5
Toll-like receptor 5
- TUNEL
terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling
- UC
ulcerative colitis
Footnotes
Conflict of interest: none.
References
- 1.Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 2.Merlin D, Si-Tahar M, Sitaraman SV, Eastburn K, Williams I, Liu X, et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120(7):1666–79. doi: 10.1053/gast.2001.24845. [DOI] [PubMed] [Google Scholar]
- 3.Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37(9):1871–7. doi: 10.1124/dmd.109.027367. [DOI] [PubMed] [Google Scholar]
- 4.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi A, Polyak S, Draganov PV. Colorectal cancer surveillance in inflammatory bowel disease: the search continues. World J Gastroenterol. 2009;15(1):61–6. doi: 10.3748/wjg.15.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336(8711):357–9. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 7.Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100(12):2724–9. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Thorsteinsdottir S, Gudjonsson T, Nielsen OH, Vainer B, Seidelin JB. Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2011;8(7):395–404. doi: 10.1038/nrgastro.2011.96. [DOI] [PubMed] [Google Scholar]
- 9.Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1720–28. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Arthur JC, Jobin C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes. 2013;4(3):253–8. doi: 10.4161/gmic.24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–3. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132(2):551–61. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Maddocks OD, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS One. 2009;4(5):e5517. doi: 10.1371/journal.pone.0005517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313(5788):848–51. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 15.Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8(2):e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut. 2014;63(5):761–70. doi: 10.1136/gutjnl-2013-304739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimesova K, Kverka M, Zakostelska Z, Hudcovic T, Hrncir T, Stepankova R, et al. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflammatory bowel diseases. 2013;19(6):1266–77. doi: 10.1097/MIB.0b013e318281330a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4(6):e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014;42(1):49–53. doi: 10.1177/0192623313508481. [DOI] [PubMed] [Google Scholar]
- 20.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 22.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–6. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swidsinski A, Ung V, Sydora BC, Loening-Baucke V, Doerffel Y, Verstraelen H, et al. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis. 2009;15(3):359–64. doi: 10.1002/ibd.20763. [DOI] [PubMed] [Google Scholar]
- 24.Roberts CL, Keita AV, Duncan SH, O’Kennedy N, Soderholm JD, Rhodes JM, et al. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59(10):1331–9. doi: 10.1136/gut.2009.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J Crohns Colitis. 2013;7(4):338–41. doi: 10.1016/j.crohns.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Bar A, Van Ommen B, Timonen M. Metabolic disposition in rats of regular and enzymatically depolymerized sodium carboxymethylcellulose. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1995;33(11):901–7. doi: 10.1016/0278-6915(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 27.Organization WH. Safety evaluation of certain food additives. International programme on chemical safety. 1999 [Google Scholar]
- 28.https://www.fsc.go.jp/english/evaluationreports/foodadditive/polysorbate_report.pdf.
- 29.Treon J, Gongwer L, Nelson M, Kirschman J. Chemistry, physics, and application of surface active substances. Gordon and Breach. 1967;III(381):381. [Google Scholar]
- 30.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63(7):1069–80. doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7(9):e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014;63(12):1932–42. doi: 10.1136/gutjnl-2013-305257. [DOI] [PubMed] [Google Scholar]
- 34.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chassaing B, Gewirtz AT. Pathobiont hypnotises enterocytes to promote tumour development. Gut. 2014;63(12):1837–8. doi: 10.1136/gutjnl-2014-306890. [DOI] [PubMed] [Google Scholar]
- 36.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15(3):317–28. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, et al. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62(22):6362–6. [PubMed] [Google Scholar]
- 38.Laqueur GL, Matsumoto H, Yamamoto RS. Comparison of the carcinogenicity of methylazoxymethanol-beta-D-glucosiduronic acid in conventional and germfree Sprague-Dawley rats. J Natl Cancer Inst. 1981;67(5):1053–5. [PubMed] [Google Scholar]
- 39.Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol. 2014;20(24):7878–86. doi: 10.3748/wjg.v20.i24.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaki T, Yokokura T, Mutai M. Antitumor effect of intrapleural administration of Lactobacillus casei in mice. Cancer Immunol Immunother. 1988;26(3):209–14. doi: 10.1007/BF00199931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thirabunyanon M, Hongwittayakorn P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl Biochem Biotechnol. 2013;169(2):511–25. doi: 10.1007/s12010-012-9995-y. [DOI] [PubMed] [Google Scholar]
- 42.Sanapareddy N, Legge RM, Jovov B, McCoy A, Burcal L, Araujo-Perez F, et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6(10):1858–68. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong SY, Tran HQ, Gewirtz AT, McKeown-Eyssen G, Fedirko V, Romieu I, et al. Serum Endotoxins and Flagellin and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Cancer Epidemiol Biomarkers Prev. 2016;25(2):291–301. doi: 10.1158/1055-9965.EPI-15-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133(6):1869–81. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow MT, Sceneay J, Paget C, Wong CS, Duret H, Tschopp J, et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72(22):5721–32. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 46.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.