Abstract
Self-management is becoming increasingly important in COPD health care although it remains difficult to embed self-management into routine clinical care. The implementation of self-management is understood as a complex interaction at the level of patient, health care provider (HCP), and health system. Nonetheless there is still a poor understanding of the barriers and effective facilitators. Comprehension of these determinants can have significant implications in optimizing self-management implementation and give further directions for the development of self-management interventions. Data were collected among COPD patients (N=46) and their HCPs (N=11) in three general practices and their collaborating affiliated hospitals. Mixed methods exploration of the data was conducted and collected by interviews, video-recorded consultations (N=50), and questionnaires on consultation skills. Influencing determinants were monitored by 1) interaction and communication between the patient and HCP, 2) visible and invisible competencies of both the patient and the HCP, and 3) degree of embedding self-management into the health care system. Video observations showed little emphasis on effective behavioral change and follow-up of given lifestyle advice during consultation. A strong presence of COPD assessment and monitoring negatively affects the patient-centered communication. Both patients and HCPs experience difficulties in defining personalized goals. The satisfaction of both patients and HCPs concerning patient centeredness during consultation was measured by the patient feedback questionnaire on consultation skills. The patients scored high (84.3% maximum score) and differed from the HCPs (26.5% maximum score). Although the patient-centered approach accentuating self-management is one of the dominant paradigms in modern medicine, our observations show several influencing determinants causing difficulties in daily practice implementation. This research is a first step unravelling the determinants of self-management leading to a better understanding.
Keywords: self-management, health communication, chronic disease management, chronic obstructive pulmonary disease, mixed methods, barriers and facilitators, primary health care, specialist care
Introduction
According to the World Health Organization, COPD is listed among the top three leading causes of death in 2030.1 COPD is currently ranked sixth in the Dutch mortality ranking list.2,3 Because of the aging population, it is expected that the number of COPD patients will increase by 38% between 2005 and 2025.4 Apart from the expected loss in both years of life and health-related quality of life among many patients, this development influences both the accessibility and affordability of COPD care. Consequently, self-management is becoming increasingly more important,5 referring to the individual’s ability to manage symptoms, treatment, physical and psychosocial consequences, and lifestyle changes inherent in living with a chronic disease.6 Over the last decade, an increase is seen in general practice-based nurse specialists, improving consultation to chronic care patients, transferring knowledge of lifestyle awareness and optimized prevention.7 Furthermore, a variety of (e-health) interventions and tools are available for COPD patients, supporting in handling symptoms, maintenance of physical functioning, and medical management.8 These self-management interventions are understood as all programs and techniques enabling patients to assume responsibility for managing one or more aspects of COPD.9 The majority of those interventions are based on the cognitive behavioral therapy10–15 increasing problem solving and gaining confidence in one’s own ability to perform a particular behavior, called self-efficacy.16
Another used model refers to the willingness and motivation of individuals to change behavior.17,18 Interventions based on this theory focus on exploring the motivation for behavior change and next adjusting the treatment to this.
Although achieving behavior change is the ultimate goal, a most challenging task is influencing it. There are numerous determinants affecting health behavior and lifestyle, which can be explained by a complex interaction at the level of the patient, health care provider (HCP), health care system, and community.19,20 First, it is essential that patients feel supported in the clinical encounter, increase their motivation and self-efficacy preferably in an equal patient–HCP partnership making shared decisions possible.21 The patient’s perspective is essential, and the role of the HCP shifts from a leading to a more coaching role during consultation.22 This commitment to equality in which there occurs a rearrangement of tasks and responsibilities is also called “patient empowerment”.23
Second, both the patients and the HCPs need competencies to optimally interact during this encounter. Spencer and Spencer24 distinguish visible and less-visible underlying competencies indicating ways of behaving or thinking, which generalizes across a wide range of situations and endures for long period of time. Among these visible competencies are the degree of knowledge, general and communication skills of both patients and HCPs. Examples of less visible underlying competencies of both patients and HCPs are: beliefs (attitudes), motivation, values, motives, and personality characteristics. These can influence the consultation encounter to a large extent. The degree of self-efficacy can also be seen as one of these invisible competencies. Within chronic disease management, it is seen as one of the most essential competencies to achieve behavioral change.25,9
A third important mechanism empowering patients is the embedding of self-management in the organizational care process26 as understood according to the chronic care model.27,28 Because of the numerous health care professionals involved in COPD treatment in both primary care and hospital care, good cooperation and coordination between the patient and the various levels of health care are essential. Another less accentuated influencing organizational factor is the duration of the consultation time on self-management.
Despite the many new developments, the wide range of available (e-health) tools and the efforts made by the patients and HCPs, accomplishing changes in lifestyle and optimal self-management, seem not feasible for everyone. This is evidenced by the high level of medication noncompliance,29–33 the lower psychic well-being, and decreased quality of life, reported among people with a wide range of chronic diseases.34–38 Improving the patient’s motivation must always be the first step in developing an intervention promoting self-management.39 Yet, this critical area of concern is often lacking in today’s self-management interventions.40 Moreover, the implementation of (e-health) self-management interventions seems to be still having difficulties embedding into routine health care and normal standard care.41,42 While effectiveness has been demonstrated of using action plans in early detection of exacerbations,43,44 just a minority of 14% of the COPD patients in the Netherlands actually use them. The action plans are still highly focused on medical regimes and to a lesser extent on adapting lifestyle and setting up personal goals.45 Similar modest numbers apply to used motivational interviewing techniques during consultation by general practitioners and community nurses.46 There is still a poor understanding of both the effective facilitators as barriers concerning the implementation of self-management. A better comprehension of these facilitators and barriers can have significant implications for the direction of further development of self-management interventions and the optimization of the implementation of self-management in health care.
In the current study, we adopted a mixed methods approach investigating possible effective facilitators and barriers for the implementation of self-management in both general practices and their affiliated hospitals. The focus of the study lied on the extension of self-management implementation and was affected by
Methods
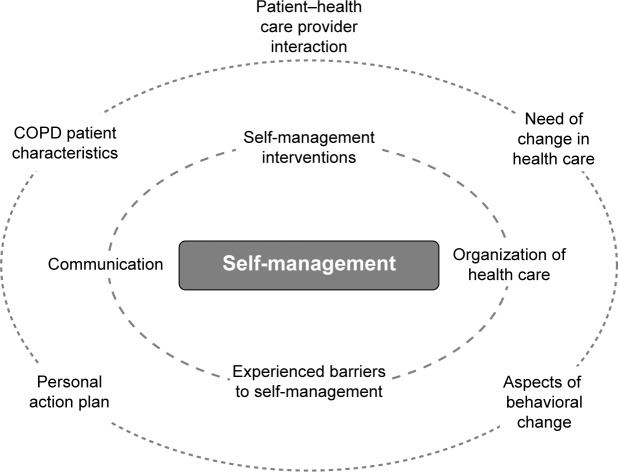
The theoretical model is based on the chronic care model, providing a framework for understanding and addressing chronic illness care, whereas self-management is further explained according to the social cognitive behavioral theory.27,16 A model is used in identifying the numerous determinants affecting health behavior and lifestyle. Influencing factors are subdivided at patient, HCP, health care system, and community level (Figure 1).47 Participants of the research were both patients and their HCPs of Dutch general practices and their affiliated outpatient lung clinics with a follow-up period of a year. Data were used from baseline questionnaires, interviews, and observations of consultations with patients and HCPs; collected sequentially in three different phases (exploratory, inventory, and feedback phase) according to the three-dimensional typology of mixed methods designs (Figure 2).48 The study protocol was approved by the Medical Ethical Board of the University Medical Center Utrecht (UMC Utrecht) prior to commencement.
Figure 1.
Influencing factors of self-management.
Figure 2.
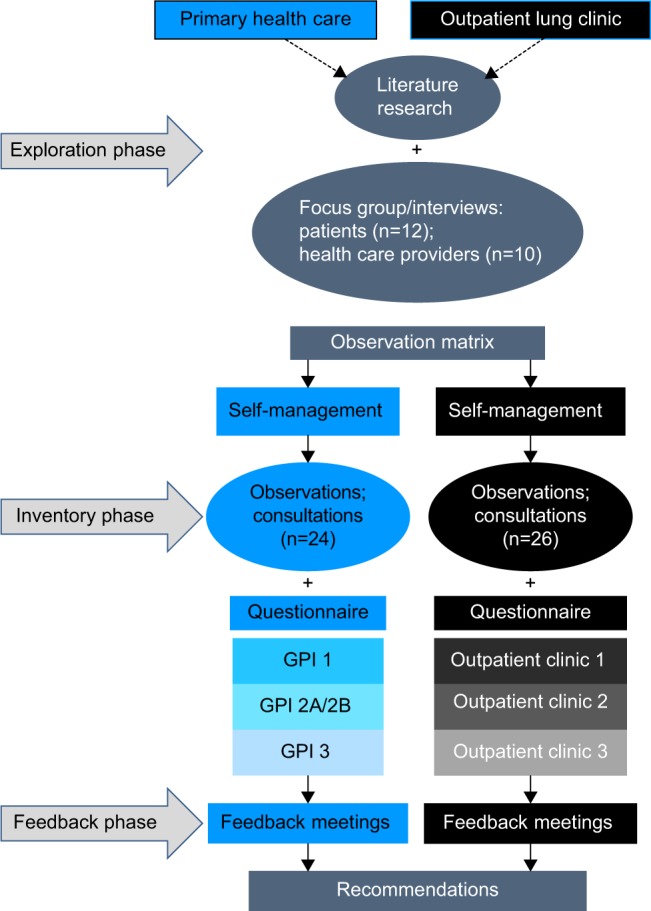
Flowchart of research approach.
Abbreviation: GPI, general practice institute.
Participant selection and setting
General practices and outpatient clinics were recruited by contacting the medical director or highest responsible person asking to participate in the research. Three matching couples were formed based on their regional collaboration, each couple consisting of a general practice with their affiliated hospital. The HCPs of the participating organizations recruited primary care patients and hospital outpatients according to the inclusion criteria doctor-diagnosed COPD, aged 18 years or older and willingness to participate. The HCPs informed the patients by telephone; subsequently detailed information about the study was sent by post. Written informed consent was obtained from every participant, and the research assistant double-checked the compliance with the study criteria.
Data collection and analysis
Exploratory phase
Insight is gained of the perspectives of both patients and caregivers about possible influencing factors to promote and implement self-management in daily practice. The initially planned patient focus groups in each participating primary care and outpatient clinic were rescheduled into individual interviews, after one focus group of five persons was held. In the interviews, it was easier to elaborate on the topics.
Ten caregivers with different professional backgrounds and 12 patients from primary care and outpatient clinics were purposively selected and participated in semistructured interviews. All interviews were conducted by the same researcher using a topic list developed according to the principles of Kvale.49 Recordings were transcribed verbatim by an independent transcription service and subsequently compared with the audio recordings for completeness and accuracy. Both content and thematic analyses were used based on the framework approach.50 Interviews were coded by two separately working researchers with the use of the data analysis software of Dedoose, a web-based program for mixed methods research. The top 9 most-coded key concepts (%) from both the patients and the HCPs were collected and compared.
Inventory phase
Influencing factors of implementing and promoting self-management were observed in real-life settings by videotaping consultations (N=50) obtained by 46 patients and 11 HCPs (4 patients had a second consult) both from the participating general practices and their affiliated outpatient clinics. Perceived patient-centeredness during consultation was measured afterwards by both the patients and the HCPs by the validated 16-item (4-point scale) dyadic patient feedback questionnaire on consultation skills (PFC; Table 1).51 The PFC questionnaire has two parallel versions: a patient feedback version and a HCP self-assessment version.51
Table 1.
Patient feedback questionnaire on consultation skills; 4-point scale (completely, mostly, a little, not at all)
| 1. To what extent was your main problem(s) discussed today? |
| 2. How satisfied were you with the discussion of your problem? |
| To what extent did: |
| 3. The health care provider listen to what you had to say? |
| 4. The health care provider explain this problem to you? |
| 5. You and the health care provider discuss your respective roles? |
| 6. The health care provider explain the treatment? |
| 7. The health care provider explore how manageable this treatmentwould be for you? |
| 8. How well do you think your health care provider understood you today? |
| 9. To what extent did the health care provider discuss personal or family issues that might affect your health? |
| 10. To what extent was there an atmosphere of trust during the consultation? |
| To what extent did: |
| 11. The health care provider show his/her concern? |
| 12. The health care provider invite you to ask all the questions you wanted to ask? |
| 13. The health care provider give you clear information and explanation? |
| 14. The health care provider act in a structured way? |
| 15. The health care provider give you new or better insight into your problem? |
| 16. The health care provider give you clear treatment advice? |
An observation matrix was used analyzing the video content of the consultations. Development of the matrix was based on the most common-coded key concepts extracted from HCPs and patients’ interviews in the exploratory phase, combined with the foremost contemporary literature concerning self-management. Newman’s subdivision of essential components of self-management was used in “giving information”, “behavior change”, “maintaining behavior change”, “enhancing social support”, “managing emotions”, “skills training”, “enhancing communication skills”, and “self-monitoring”.8 Selections of video clips were encoded in accordance with the observation matrix, using the analysis program Dedoose.
Baseline demographic, clinical and contextual measures of the 46 patients and 11 HCPs were obtained by a self-administered questionnaire, including the dyadic PFC.51 Descriptive analyses were used to describe the characteristics of the patients and HCPs, to calculate the mean PFC score per possible confounder, and to provide insight into the percentage of patients and HCPs who were fully satisfied with the consultation. Questions with a score of <90% full satisfaction were used in extra analyses. Bivariate and multivariate logistic regression analyses were used to find any covariates that might explain the variance in PFC scores for the patients and HCPs. Dummies were created for employment status. All covariates were recoded into 2 classes, for example age <70 years and age ≥70 years. The mean score on the PFC questionnaire was only calculated if a patient or HCP had answered 10 or more questions out of the 16. All data were analyzed in SPSS version 19.
Feedback phase
The results of the research were shared with participating patients and HCPs in five feedback meetings (two general practices and three outpatient clinics). The responses and comments were integrated into the discussion of the research.
Results
Patient and HCP characteristics
The characteristics of the studied group of patients and observed HCPs consultations are shown in Tables 2 and 3. A total of 60.9% patients were male, low-educated (50.0%), had an mean age of 70 years, and were mostly of Dutch ethnicity (93.5%). Most of the participating HCPs were women (81.8%) with a mean overall work experience in their profession of 20 years, compared with 9 years specifically in pulmonary medicine.
Table 2.
Patients’ social and demographic characteristics (N=46)
| Patients’ characteristics | Patients |
|---|---|
| Institute, n (%) | |
| General practice 1 | 3 (6.5) |
| General practice 2A | 6 (13.0) |
| General practice 2B | 4 (8.7) |
| General practice 3 | 9 (19.6) |
| Outpatient clinic 1 | 9 (19.6) |
| Outpatient clinic 2 | 8 (17.4) |
| Outpatient clinic 3 | 7 (15.2) |
| Gender, n (%) | |
| Male | 28 (60.9) |
| Female | 18 (39.1) |
| Age in years, mean (range) | 70 (53–87) |
| Duration of disease in years, mean (range) | 6.3 (0.2–20) |
| CCQ score, mean (range) | 1.9 (0.3–4.7) |
| GOLD scale, n (%) | |
| GOLD 1 | 10 (21.7) |
| GOLD 2 | 18 (39.1) |
| GOLD 3 | 12 (26.1) |
| GOLD 4 | 5 (10.9) |
| Missing data | 1 (2.2) |
| Employment status, n (%) | |
| Working | 9 (19.6) |
| Retired | 22 (47.8) |
| Not working, not retired | 13 (28.3) |
| Missing data | 2 (4.3) |
| Marital situation, n (%) | |
| Single | 15 (32.6) |
| Living together | 29 (63.0) |
| Missing data | 2 (4.3) |
| Ethnicity, n (%) | |
| Dutch | 43 (93.5) |
| Missing data | 3 (6.5) |
| Exercise, n (%) | |
| Less than 30 minutes per day | 7 (15.2) |
| 30 minutes per day | 10 (21.7) |
| More than 30 minutes per day | 26 (56.5) |
| Missing data | 3 (6.5) |
| Educational level, n (%) | |
| None | 2 (4.3) |
| Elementary school or low vocational education | 23 (50.0) |
| Secondary school or intermediate vocational education | 9 (19.6) |
| Higher vocational education or university | 10 (21.7) |
| Missing data | 2 (4.3) |
| Support from caregiver, n (%) | |
| Poor support | 3 (6.5) |
| Moderate support | 8 (17.4) |
| Support | 6 (13.0) |
| Good support | 16 (34.8) |
| Missing data | 13 (28.3) |
Abbreviations: CCQ, Clinical COPD Questionnaire; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 3.
Health care providers’ social and demographic characteristics
| Health care providers’ characteristics | Total health care providers (N=11) | Health care providers (%) | Total observations (N=50)* |
Observations (%) |
|---|---|---|---|---|
| Institute | ||||
| General practice 1 | 2 | 18.2 | 5 | 10.0 |
| General practice 2A | 1 | 9.1 | 6 | 12.0 |
| General practice 2B | 1 | 9.1 | 4 | 8.0 |
| General practice 3 | 1 | 9.1 | 9 | 18.0 |
| Outpatient clinic 1 | 2 | 18.2 | 9 | 18.0 |
| Outpatient clinic 2 | 2 | 18.2 | 10 | 20.0 |
| Outpatient clinic 3 | 2 | 18.2 | 7 | 14.0 |
| Occupation | ||||
| Pulmonologist | 3 | 27.3 | 13 | 26.0 |
| Pulmonary nurse specialist | 3 | 27.3 | 13 | 26.0 |
| General practitioner | 1 | 9.1 | 2 | 4.0 |
| Nurse practitioner | 2 | 18.2 | 10 | 20.0 |
| Practice nurse | 2 | 18.2 | 12 | 24.0 |
| Gender | ||||
| Male | 2 | 18.2 | ||
| Female | 9 | 81.8 | ||
| Age in years, mean (range) | 44 (28–56) | |||
| Work experience in years, mean (range) | 20 (7–39) | |||
| Work experience in pulmonary disease in years, mean (range) | 9 (3–20) | |||
| Working hours per week, mean (range) | 33 (20–50) |
Note:
Fifty videotaped observed consultations were obtained from 46 patients and 11 HCPs (4 patients had a second consult).
Qualitative results
Figures 3 and 4 represent the top 9 most coded key concepts (%; inner circle top 4) analyzed from the interviews with the patients and the HCPs. The most coded concepts in common were “organization of care”, “interventions self-management”, “perceived barriers to self-management”, “patient, caregiver interaction”, “communication”, and “need for change in care”. These concepts were used in developing the observational matrix, together with the essential self-management concepts according to Newman et al.8 After analyzing the consultations using the observation matrix, simultaneous triangulation was applied with the outcome of the analyzed interviews,52 resulting in the following seven main themes.
Figure 3.
Key topics of interviewed patients.
Figure 4.
Key topics of interviewed health care providers (HCPs).
Use of self-management tools
Both professionals from the primary care and outpatient clinics stated not often using self-management interventions. This corresponds with the observed consultations, revealing no use is made of an individual care plan, e-health application, or any other intervention, though there is a desire among some organizations to apply e-health applications in the nearby future. Although the patients have heard the possibility of using e-health applications, not many actually use it for several reasons. Some patients say they are too old to learn new things. Some have tried but found the confrontation with their disease unpleasant, having to log into the e-health application daily.
Organization of health care
Participating general practices and outpatient clinics had not yet imbedded self-management at the organizational level supported by an integrated approach, such as a COPD program. However, some transmural agreements were made between organizations concerning the referral of patients or the use of a uniform referral form. The majority of the participating organizations had a specialized COPD consultation service in both primary care and outpatient clinics.
Patients did not elaborate much about the organization of health care, but the patients who did were positive about the collaboration between the general practitioner and the pulmonologist.
Consultation structure
The focus of the consultations accentuated on information and knowledge exchange, where controlling and monitoring health-related outcomes were emphasized. We observed no difference between primary care and outpatient clinics, (pulmonary) physicians, nurse practitioners, or community nurses. Aspects of changing lifestyle were discussed thoroughly by nurse practitioners and community nurses, in a lesser extent observed by physicians. The degree of shared decision making is low; the HCPs had a proactive role during consultations. Some HCPs continue their own consultation structure without considering the type of questions patients may have. Others adapt their structure and topics according to the patients’ input.
Patients stress the importance of the HCP giving personal attention in an atmosphere where there is enough time taken for the person beside the disease. Patients indicate that they appreciate the information and advice given during consultation.
Self-management components
Compared to the various essential components of self-management8 during consultations, much attention is given to the components: “information provision”, “training skills”, and “self-monitoring”. Other components, such as “maintaining behavior change”, “social support”, and “managing emotions” are to a lesser extent raised in the consultation by either the HCP or the patient.
Connecting to patients’ goals and expectations
In 54% of the observed consultations (N=26), HCPs made an inventory of patient-centered goals or asked patients if they had any general questions. The majority of the patients (88.5%) responded not having questions or goals to mention at all. It is one of the reasons HCPs report having difficulties in formulating patient-centered goals with the patients.
Indeed I try with a patient sometimes like […] “Do you have a goal or activity you would like to do, or cannot do anymore for the last five years?” But actually […] it doesn’t work […] because they are quite satisfied with their life now. I think it is pretty difficult! [HCP 0]
Used resources for information transfer
Despite the observed great emphasis on transferring information and knowledge during consultation, little use was made of assistive devices, such as written information, brochures, decision aids, or video materials. The exchange of information between HCPs and patients remains mainly verbal.
Patient-centered communication
Both HCPs and patients express the importance of accessibility and communication on the basis of equality. Connecting with the patients’ social background usually occurred on an intuitive basis. In the interviews, some HCPs expressed experiencing difficulties in estimating what motivated patients to take an active or inactive role in their self-management.
Me searching for the connection with the patient […]is sometimes like a blackbox. [HCP 1]
Where do you find your motivation [as a patient] when you don’t feel related to your disease? [HCP 2]
Also patients explained several difficulties taking the self-management role. After analyzing they were subdivided into three categories:
low level of experienced burden of the disease,
wishing not to be confronted with disease,
dealing with external social problems.
I don’t want to be confronted with my disease constantly. I do not need to know everything about my illness, but prefer the HCP to dose this information when explaining it to me. [Patient 1]
Having a disease […] costs a lot of time. You have to go to the doctor, nurse, dietician, general practitioner […] rehabilitation care […]. At some point you think your whole life is centered around that. [Patient 2]
Quantitative results
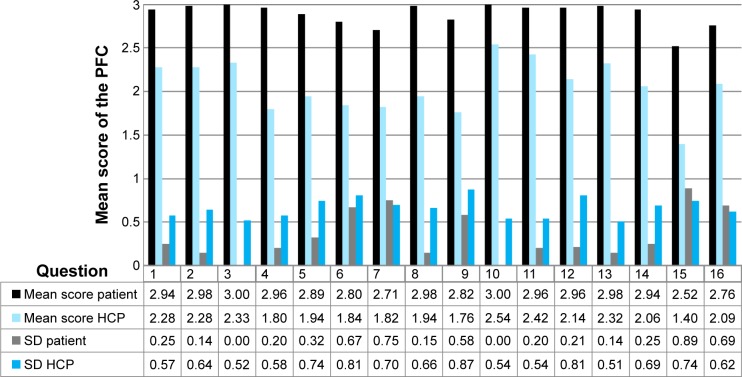
Table 4 provides insight into the response pattern of the patients and HCPs (in %) of the PFC questionnaire, subdivided into the response categories “Completely”, “Mostly”, “A little”, and “Not at all”. It can be seen that the mean score in the highest category “Completely” is much higher among the patients (84.3%) than the mean score of the HCPs (26.5%). Of all patients, 40% (N=20) answered with the maximum score for all questions. Furthermore, 64% of all patients (N=32) had the maximum score and/or did not answer some questions. Further analyses of the patient scores per question item with an mean score of <90% full satisfaction (questions 5, 6, 7, 9, 12, 15, and 16) did not significantly explain variance in communication scores (P=0.05; Figure 5). In a bivariate model the variance (P=0.09, 0.056, and 0.039) could be explained by the variables duration of illness, gender, and HCPs' experience in lung diseases. However multivariate regression analysis did not show any significant results.
Table 4.
Answering pattern and response rate for the patient feedback questionnaire on consultation skills (%)
| Question | Completely
|
Mostly
|
A little
|
Not at all
|
Nonresponse
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | HCP | Pt | HCP | Pt | HCP | Pt | HCP | Pt | HCP | |
| 1 | 90 | 34 | 6 | 60 | 0 | 6 | 0 | 0 | 4 | 0 |
| 2 | 96 | 38 | 2 | 52 | 0 | 10 | 0 | 0 | 2 | 0 |
| 3 | 96 | 34 | 0 | 62 | 0 | 2 | 0 | 0 | 4 | 2 |
| 4 | 92 | 8 | 4 | 62 | 0 | 28 | 0 | 0 | 4 | 2 |
| 5 | 80 | 24 | 10 | 46 | 0 | 30 | 0 | 0 | 10 | 0 |
| 6 | 78 | 16 | 6 | 48 | 0 | 18 | 4 | 6 | 12 | 12 |
| 7 | 58 | 12 | 8 | 60 | 0 | 22 | 4 | 4 | 30 | 2 |
| 8 | 92 | 18 | 2 | 56 | 0 | 24 | 0 | 0 | 6 | 2 |
| 9 | 78 | 18 | 6 | 50 | 2 | 22 | 2 | 10 | 12 | 0 |
| 10 | 96 | 56 | 0 | 42 | 0 | 2 | 0 | 0 | 4 | 0 |
| 11 | 90 | 44 | 4 | 54 | 0 | 2 | 0 | 0 | 6 | 0 |
| 12 | 86 | 36 | 4 | 46 | 0 | 14 | 0 | 4 | 10 | 0 |
| 13 | 94 | 34 | 2 | 64 | 0 | 2 | 0 | 0 | 4 | 0 |
| 14 | 90 | 26 | 6 | 52 | 0 | 20 | 0 | 0 | 4 | 2 |
| 15 | 60 | 4 | 14 | 40 | 4 | 40 | 6 | 10 | 16 | 6 |
| 16 | 72 | 22 | 8 | 58 | 0 | 14 | 4 | 0 | 16 | 6 |
| Mean (%) | 84.3 | 26.5 | 5.1 | 53.3 | 0.4 | 16.0 | 1.3 | 2.1 | 9.0 | 2.1 |
Notes: N=50 consultations. Question details are available in Table 1.
Abbreviations: HCP, health care provider; Pt, patient.
Figure 5.
PFC scores of patient and HCP (mean/SD).
Note: Question details are available in Table 1.
Abbreviations: HCP, health care provider; PFC, patient feedback questionnaire on consultation skills; SD, standard deviation.
Discussion and practice implications
The study identified a number of complex underlying mechanisms and determinants at patient, HCP, and organizational levels mutually interacting with each other as described in Figure 1. These insights yield important conclusions and have significant practice implications which we will discuss.
We can conclude that during consultation a traditional health care approach is still commonly used in which HCPs take the leading role. Despite the great amount of attention paid to lifestyle change and advice during consultation, less emphasis was seen on effective behavioral change and follow-up of the advice given, which is quite understandable given the fact that patients see their HCP once or twice a year. It can be questioned if the low frequency in patient–HCP contact is a good breeding ground for building mutual trust and effectively achieving behavior change.
Earlier research has shown that solely providing information and lifestyle advice are not sufficient enough to actually change lifestyle and behavior.53–55 Emphasis should be placed on connecting to the goals and motivation of the patient using shared decision making.21 From the qualitative data-exploration it can be seen that both patients and HCPs experience difficulties defining personalized goals and expectations. Significant influencing factors are causing this disadvantage. Newman’s self-management component, social support, is little observed8 in the consultations, which relates to the extent social context and participation level in everyday life have been explored. This basic understanding of one’s personal life, daily activity level, motivation, and values is essential to make a personalized plan in improving health, which are in tune with the perceived limitations and possibilities in daily living activities. Verhage et al56 point out the necessity of a detailed patient assessment with the recognition of the diversity of patients in their knowledge and skills, health perception, level of communication, and the motivation to work on their self-management. A customized self-management approach where the patient’s social context, individual background, desires and capabilities are taken into account is lacking.5
Another negatively influencing aspect observed in patient–HCP interaction is the profoundly structured consultation, where explicit attention is given to monitoring and assessment aspects of the COPD. This tendency toward a more rationalized, biomedical medicine, based on protocols and guidelines is a movement which has distinguished itself in the last 15 years.57 The patient–HCP interaction is subsequently changing where the HCP is more proactive, whereas the patient is unwittingly pushed into a more passive role. This is a very contradictory development. Equality between patient and HCP during consultation must be pursued and is most essential in patient-centered communication. Consequently patients feel more uninhibited, which results in them taking a proactive role, sharing their ideas, worries, and questions, and making shared decisions possible.
One success factor seems to be the high satisfaction of the patients with the experienced patient-centered communication during consultation, where a total of 84.3% of the patients (N=46) gave full maximum scores against 26.5% of the HCPs (N=11) answering the PFC. However the high percentage of maximum patient scores made it difficult to measure effects (ceiling effect). While there is no straightforward answer for this observed discrepancy between the perceived patient-centered communication between patient and HCP, one interpretation could be that the HCPs are more critical about themselves concerning their consultation compared with how patients judge this. On one hand, we see satisfied patients with the traditional consultation structure; and on the other hand, we have HCPs indicating having difficulties in formulating patient-orientated goals. We can identify the fact that patients are not yet well accustomed to their new role as a proactive patient, which is also hampered by the overall observed traditional and medically oriented type of consultation structure. Further research is necessary for evaluating the needs of patients regarding self-direction, interaction, and communication with HCPs.
This study has both strengths and limitations. The strength of this study lies in the inclusion of both the patients’ and HCPs’ perspectives gaining insight into the similarities and differences of mutual opinions concerning self-management.
Limitations are the small simple size, which restricted performing subgroup analyses. Patients and HCPs took part in the research on a voluntary basis. It is, therefore, likely that we only reached patients and HCPs who appreciate the importance of supporting self-management. The single recruitment source, via the respiratory community nurse, limited the degree of generalizing the results of the study. No conclusions can be drawn about self-management among various ethnic populations because the majority of the participants had Dutch ethnicity. Finally, we identified high patient scores on the PFC, from which we can conclude patients being very satisfied with the degree of patient-centered communication in the consultation. But giving socially desirable answers to the questions must be considered as another possible explanation.58–60
In summary, although the patient-centered approach being one of the dominant paradigms in modern medicine61–64 our observations accentuate difficulties implementing self-management in daily practice. Both patients and HCPs are still very much framed in a traditional consultation structure according to the biomedical perspective though an observed discrepancy in the satisfaction level of the consultation was seen between patients and their HCPs.
Influencing determinants were identified from the interviews and consultations affecting implementation of self-management at patient, HCP, and organizational level. Seven main themes could be identified: “use of self-management tools”, “organization of care”, “consultation structure”, “self-management components”, “connecting to patients’ goals and expectations”, “used resources for information transfer”, and “patient-centered communication”.
More research is needed of the influencing factors of self-management and for gaining insight into finding the right balance between the implementation of patient-centered medicine in a predominant evidence-based setting. To our knowledge this research was the first study focusing on the interaction between patients and HCPs according to the implementation of self-management, integrating the opinion and perspectives of both, and is a first step in better understanding and unravelling the determinants of self-management.
Acknowledgments
The Patient Empowerment Programme is a cooperative venture between patient organization the Dutch Lung Foundation (Longfonds), the health insurance company Achmea, and AstraZeneca to improve the position of patients with COPD in the care process by means of shared decision making and patient empowerment. We would like to express our warm thanks to the participating patients, the HCPs, and the members of the Patient Empowerment Programme who contributed to the research.
Footnotes
Disclosure
NHC has had temporary consulting roles on the advisory boards of Novartis, Chiesi, Boehringer Ingelheim, and Pfizer. OCPvS has received unrestricted research grants from Pfizer and Boehringer Ingelheim. NHC and OCPvS have had temporary consulting roles on the advisory board of the Patient Empowerment Programme. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organization, editor. World health statistics 2008. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 2.Gommer A, Poos M, editors. Cijfers COPD (prevalentie, incidentie en sterfte) uit de VTV 2010. Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid [Data COPD (prevalence, incidence and mortality) from the VTV 2010. Future Public Health Exploration, National Compass Public Health] Bilthoven, the Netherlands: RIVM; 2010. Dutch. [Google Scholar]
- 3.Gijsen R, Oostrom Sv, Schellevis F, Hoeymans N, editors. Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid [Chronic diseases and multimorbidity summarized, Future Public Health Exploration, National Compass Public Health] Bilthoven, the Netherlands: RIVM; 2013. Chronische ziekten en multimorbiditeit samengevat. Dutch. [Google Scholar]
- 4.Boezen H, Postma D, Smit H, Poos M, editors. Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid. [What is the prevalence and mortality of COPD? Future Public Health Exploration, National Compass Public Health] Bilthoven, the Netherlands: RIVM; 2006. Hoe vaak komt COPD voor en hoeveel mensen sterven eraan? Dutch. [Google Scholar]
- 5.Effing TW, Bourbeau J, Vercoulen J, et al. Self-management programmes for COPD moving forward. Chron Respir Dis. 2012;9(1):27–35. doi: 10.1177/1479972311433574. [DOI] [PubMed] [Google Scholar]
- 6.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 7.Heiligers PJM, Noordman J, Korevaar JC, et al., editors. Kennisvraag: praktijkondersteuners in de huisartspraktijk (POH’s), klaar voor de toekomst? [Knowledge question: Practice-based nurse specialists in the general practice center, ready for the future?] Utrecht, the Netherlands: NIVEL; 2012. p. 124. Dutch. [Google Scholar]
- 8.Newman S, Steed L, Mulligan K, editors. Chronic Physical Illness: Self Management and Behavioural Interventions. Berkshire, UK: McGraw-Hill Education; 2009. [Google Scholar]
- 9.Bourbeau J, Van der Palen J. Promoting effective self-management programmes to improve COPD. Eur Respir J. 2009;33(3):461–463. doi: 10.1183/09031936.00001309. [DOI] [PubMed] [Google Scholar]
- 10.Fekete EM, Antoni MH, Schneiderman N. Psychosocial and behavioral interventions for chronic medical conditions. Curr Opin Psychiatry. 2007;20(2):152–157. doi: 10.1097/YCO.0b013e3280147724. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa-Moseley C, Jean-Pierre P, Roscoe JA, et al. Behavioral interventions in treating anticipatory nausea and vomiting. J Natl Compr Canc Netw. 2007;5(1):44–50. doi: 10.6004/jnccn.2007.0006. [DOI] [PubMed] [Google Scholar]
- 12.O’Hea E, Houseman J, Bedek K, Sposato R. The use of cognitive behavioral therapy in the treatment of depression for individuals with CHF. Heart Fail Rev. 2009;14(1):13–20. doi: 10.1007/s10741-008-9081-2. [DOI] [PubMed] [Google Scholar]
- 13.Tazaki M, Landlaw K. Behavioural mechanisms and cognitive-behavioural interventions of somatoform disorders. Int Rev Psychiatry. 2006;18(1):67–73. doi: 10.1080/09540260500467046. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Thomas S, Hillier C, Galvin K, Baker R. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;1:CD004431. doi: 10.1002/14651858.CD004431.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters SJ, McKee DC, Keefe FJ. Cognitive behavioral approaches to the treatment of pain. Psychopharmacol Bull. 2007;40(4):74–88. [PubMed] [Google Scholar]
- 16.Bandura A, editor. Social Foundations of Thought and Action. Englewood Cliffs, NJ: Prentice-Hall inc; 1986. [Google Scholar]
- 17.Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Criticisms and concerns of the transtheoretical model in light of recent research. Br J Addict. 1992;87(6):825–828. doi: 10.1111/j.1360-0443.1992.tb01973.x. [DOI] [PubMed] [Google Scholar]
- 18.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 20.Ouwens M, Wollersheim H, Hermens R, Hulscher M, Grol R. Integrated care programmes for chronically ill patients: a review of systematic reviews. Int J Qual Health Care. 2005;17(2):141–146. doi: 10.1093/intqhc/mzi016. [DOI] [PubMed] [Google Scholar]
- 21.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brink-Muinen A, Van Dulmen A, editors. Tweede Nationale studie naar ziekten en verrichtingen in de huisartspraktijk: oog voor communicatie: huisarts-patiënt communicatie in Nederland [Second national study of diseases and proceedings in general practices: emphasizing communication: doctor-patient communication in the Netherlands] Utrecht, the Netherlands: NIVEL; 2004. Dutch. [Google Scholar]
- 23.Aujoulat I, d’Hoore W, Deccache A. Patient empowerment in theory and practice: polysemy or cacophony? Patient Educ Couns. 2007;66(1):13–20. doi: 10.1016/j.pec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Spencer L, Spencer P, editors. Competence at work models for superior performance. New Jersey, NJ: John Wiley and Sons; 2008. [Google Scholar]
- 25.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271–277. doi: 10.1016/S0738-3991(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 26.Alpay LL, Henkemans OB, Otten W, Rövekamp TA, Dumay AC. E-health applications and services for patient empowerment: directions for best practices in The Netherlands. Telemed J E Health. 2010;16(7):787–791. doi: 10.1089/tmj.2009.0156. [DOI] [PubMed] [Google Scholar]
- 27.Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv. 2001;27(2):63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- 28.Vrijhoef HJM, editor. Dutch Chronic Care Model-Model voor geïntegreerde, chronische zorg [Dutch Chronic Care Model for integrated chronic care] Seattle: Improving Chronic Illness Care (ICIC); 2008. Dutch. [Google Scholar]
- 29.Cline CMJ, Björck-Linné AK, Israelsson BYA, Willenheimer RB, Erhardt LR. Non-compliance and knowledge of prescribed medication in elderly patients with heart failure. Eur J Heart Fail. 1999;1(2):145–149. doi: 10.1016/s1388-9842(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 30.Cochrane GM, Horne R, Chanez P. Compliance in asthma. Respir Med. 1999;93(11):763–769. doi: 10.1016/s0954-6111(99)90260-3. [DOI] [PubMed] [Google Scholar]
- 31.Glasgow RE. Compliance to diabetes regimens: conceptualization, complexity, and determinants. In: Cramer JA, Spilker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York, NY: Raven Press; 1991. pp. 209–224. [Google Scholar]
- 32.Kravitz RL, Hays RD, Sherbourne CD, et al. Recall of recommendations and adherence to advice among patients with chronic medical conditions. Arch Intern Med. 1993;153(16):1869–1878. [PubMed] [Google Scholar]
- 33.Peterman AH, Cella DF. Adherence Issues Among Cancer Patients. The Handbook of Health Behavior Change. New York, NY: Springer Publishing Company; 1998. pp. 462–482. [Google Scholar]
- 34.Hawley DJ, Wolfe F. Depression is not more common in rheumatoid arthritis: a 10-year longitudinal study of 6,153 patients with rheumatic disease. J Rheumatol. 1993;20(12):2025–2031. [PubMed] [Google Scholar]
- 35.Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–241. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehrer P, Feldman J, Giardino N, Song HS, Schmaling K. Psychological aspects of asthma. J Cons Clin Psychol. 2002;70(3):691. doi: 10.1037//0022-006x.70.3.691. [DOI] [PubMed] [Google Scholar]
- 37.Pincus T, Griffith J, Pearce S, Isenberg D. Prevalence of self-reported depression in patients with rheumatoid arthritis. Rheumatol. 1996;35(9):879–883. doi: 10.1093/rheumatology/35.9.879. [DOI] [PubMed] [Google Scholar]
- 38.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Vercoulen JH. A simple method to enable patient-tailored treatment and to motivate the patient to change behaviour. Chron Respir Dis. 2012;9(4):259–268. doi: 10.1177/1479972312459974. [DOI] [PubMed] [Google Scholar]
- 40.Efraimsson EO, Fossum B, Ehrenberg A, Larsson K, Klang B. Use of motivational interviewing in smoking cessation at nurse-led chronic obstructive pulmonary disease clinics. J Adv Nurs. 2007;68(4):767–782. doi: 10.1111/j.1365-2648.2011.05766.x. [DOI] [PubMed] [Google Scholar]
- 41.Finch T, May C, Mair F, Mort M, Gask L. Integrating service development with evaluation in telehealthcare: an ethnographic study. BMJ. 2003;327(7425):1205–1209. doi: 10.1136/bmj.327.7425.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May C, Harrison R, Finch T, MacFarlane A, Mair F, Wallace P. Understanding the normalization of telemedicine services through qualitative evaluation. J Am Med Inform Assoc. 2003;10(6):596–604. doi: 10.1197/jamia.M1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trappenburg JC, Koevoets L, de Weert-van Oene GH, et al. Action plan to enhance self-management and early detection of exacerbations in COPD patients; a multicenter RCT. BMC Pulm Med. 2009;9(1):52. doi: 10.1186/1471-2466-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnock AC, Walters EH, Walters JA, Wood-Baker R. Action plans for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;4:CD005074. doi: 10.1002/14651858.CD005074.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Baan D, Heijmans M, Spreeuwenberg P, Rijken M. Zelfmanagement vanuit het perspectief van mensen met astma of COPD [Self-management from the perspective of patients with Asthma and COPD] Utrecht, the Netherlands: NIVEL; 2012. Dutch. [Google Scholar]
- 46.Noordman J, Koopmans B, Korevaar JC, van der Weijden T, van Dulmen S. Exploring lifestyle counselling in routine primary care consultations: the professionals’ role. Fam Pract. 2013;30(3):332–340. doi: 10.1093/fampra/cms077. [DOI] [PubMed] [Google Scholar]
- 47.Wallace LM, Turner A, Kosmala-Anderson J, et al. Evidence: Co-Creating Health: Evaluation of First Phase. London, UK: Applied Research Centre in Health and Lifestyle Interventions; 2012. [Google Scholar]
- 48.Leech NL, Onwuegbuzie AJ. A typology of mixed methods research designs. Qual Quant. 2009;43(2):265–275. [Google Scholar]
- 49.Kvale S, editor. Doing Interviews. London, UK: Sage publications; 2008. [Google Scholar]
- 50.Ritchie J, Spencer L, Bryman A, Burgess R, editors. Analysing Qualitative Data. London: Routledge; 1994. [Google Scholar]
- 51.Reinders ME, Blankenstein AH, Knol DL, de Vet HC, van Marwijk HW. Validity aspects of the patient feedback questionnaire on consultation skills (PFC), a promising learning instrument in medical education. Patient Educ Couns. 2009;76(2):202–206. doi: 10.1016/j.pec.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Morse JM. Principles of mixed methods and multimethod research design. In: Tashakkori A, Teddlie C, editors. Handbook of Mixed Methods in Social and Behavioral Research. Thousands Oaks, CA: Sage; 2003. pp. 189–208. [Google Scholar]
- 53.Coates VE, Boore JR. Knowledge and diabetes self-management. Patient Educ Couns. 1996;29(1):99–108. doi: 10.1016/0738-3991(96)00938-x. [DOI] [PubMed] [Google Scholar]
- 54.Gibson P, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;1:CD001117. doi: 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- 55.Taal E, Rasker JJ, Wiegman O. Group education for rheumatoid arthritis patients. Semin Arthritis Rheum. 1997;26(6):805–816. doi: 10.1016/s0049-0172(97)80024-8. [DOI] [PubMed] [Google Scholar]
- 56.Verhage TL, Heijdra YF, Molema J, Daudey L, Dekhuijzen PR, Vercoulen JH. Adequate patient characterization in COPD: reasons to go beyond GOLD classification. Open Respir Med J. 2009;3:1–9. doi: 10.2174/1874306400903010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bensing JM, Tromp F, Van Dulmen S, Van den Brink-Muinen A, Verheul W, Schellevis F. De zakelijke huisarts en de niet-mondige patiënt: veranderingen in communicatie [The businesslike general practitioner and the non-assertive patient: changes in communication] Huisarts en wetenschap. 2008;51(1):6–12. Dutch. [Google Scholar]
- 58.Campbell C, Lockyer J, Laidlaw T, MacLeod H. Assessment of a matched-pair instrument to examine doctor–patient communication skills in practising doctors. Med Educ. 2007;41(2):123–129. doi: 10.1111/j.1365-2929.2006.02657.x. [DOI] [PubMed] [Google Scholar]
- 59.Greco M, Brownlea A, McGovern J. Impact of patient feedback on the interpersonal skills of general practice registrars: results of a longitudinal study. Med Educ. 2001;35(8):748–756. doi: 10.1046/j.1365-2923.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 60.Makoul G, Krupat E, Chang C. Measuring patient views of physician communication skills: development and testing of the communication assessment tool. Patient Educ Couns. 2007;67(3):333–342. doi: 10.1016/j.pec.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Kinnersley P, Stott N, Peters TJ, Harvey I. The patient-centredness of consultations and outcome in primary care. Br J Gen Pract. 1999;49(446):711–716. [PMC free article] [PubMed] [Google Scholar]
- 62.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51(7):1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 63.Stewart M. Towards a global definition of patient centred care. BMJ. 2001;322(7284):444–445. doi: 10.1136/bmj.322.7284.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart M, editor. Patient-Centered Medicine: Transforming the Clinical M. Abingdon, UK: Radcliffe Medical Press; 2003. [Google Scholar]