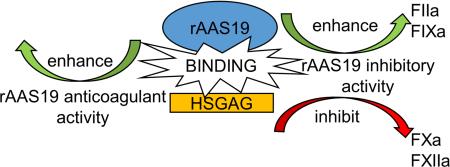
Abstract
Some serine protease inhibitor (serpin) regulators of essential life pathways bind glycosaminoglycans (GAGs) to enhance inhibitory functions and achieve physiologically relevant rates. This study demonstrates that highly conserved Amblyomma americanum tick saliva serpin 19 (AAS19), a broad-spectrum inhibitor of hemostasis and inflammation system proteases and anticoagulant, can bind heparan sulfate/heparin (HS)GAGs and that this interaction alters its function. Substrate hydrolysis and unpaired t-test analyses revealed that HSGAG binding caused rAAS19 inhibitory activity to: (i) significantly increase against blood clotting factors (f) IIa (thrombin) and fIXa, (ii) significantly reduce against fXa and fXIIa and (iii) moderate to no effect against trypsin, kallikrein, papain, and plasmin. Stoichiometry of inhibition (SI) analyses show that HSGAG binding improved the rAAS19 inhibitory efficiency against thrombin 2.7-4.3 folds as revealed by SI of 13.19 in absence of HSGAGs to 4.83-3.04 in their presence. Our data show that HSGAG binding dramatically enhanced rAAS19 anticoagulant function. In the recalcification time assay, rAAS19 pre-incubated with HSGAGs prior to the assay, delayed plasma clotting by an additional 176-457 s above HSGAGs or rAAS19 alone. Our data suggest that formation of the HSGAGs and rAAS19 complex is important for the observed enhanced anticoagulant effect. Delay of plasma clotting was higher when HSGAGs and rAAS19 were co-incubated to allow complex formation prior to blood clotting assay as opposed to no co-incubation. We have discussed our finding with reference to tick feeding physiology and utility of the rAAS19 in blood clotting disorder therapy.
Keywords: Heparin, heparan sulfate, A. americanum serpin 19, tick blood feeding
Graphical abstract
1. Introduction
Hard ticks such as Amblyomma americanum are successful ectoparasites because of their immense capacity to evade the host's defense response to tick feeding. The tick feeding style of lacerating host tissue and sucking up host blood should provoke host defenses that are aimed at stopping further blood loss. To successfully feed and transmit disease agents, ticks inject numerous pharmacologically active compounds into the feeding site to evade host defenses (Ribeiro and Francischetti, 2003). The host's defense responses to tick feeding include hemostasis, complement activation, and inflammation, all of which are primarily serine protease mediated under tight control of serine protease inhibitors (serpins). Numerous human diseases caused by serpin malfunction further validate the importance of this family of proteins (Brouwer et al., 2009; Cui et al., 2014; Davis et al., 1999; Egeberg, 1965; Eriksson, 1964; Kubo et al., 2013). On this basis, it was hypothesized that ticks could inject serpins into the feeding site to thwart host defense (Mulenga et al., 2001b). Multiple serpin encoding cDNAs have been identified in several tick species, including A. americanum (Mulenga et al., 2001a, 2003, 2007, 2009; Porter et al., 2015; Sugino et al., 2003; Tirloni et al., 2014).
In proposing that ticks utilize serpins to evade host defenses, the assumption is that ticks inject functional inhibitory serpins into the feeding site. Tick saliva proteomes (Kim et al., 2016b; Tirloni et al., 2015), immunoproteomes (Radulović et al., 2014), and western blotting analysis approaches (Chalaire et al., 2011; Ibelli et al., 2014; Kim et al., 2015) have confirmed that ticks do inject serpins into animals during feeding. Several studies have established that some tick saliva serpins have inhibitory functions against host defense system proteases (Chmelar et al., 2011; Ibelli et al., 2014; Kim et al., 2015; Mulenga et al., 2013; Prevot et al., 2006, 2009; Xu et al., 2016). Other studies have shown that some of tick saliva serpins can delay blood clotting (Ibelli et al., 2014; Kim et al., 2015; Mulenga et al., 2013), prevent platelet aggregation (Chmelar et al., 2011; Ibelli et al., 2014; Kim et al., 2015; Mulenga et al., 2013), and block inflammation (Prevot et al., 2009) and host immune response (Palenikova et al., 2015).
Important serpin regulators of the mammalian blood clotting system, antithrombin III , protein C inhibitor, and heparin cofactor II need to bind glycosaminoglycans (GAGs) to achieve physiologically relevant rates of activity (Berliner, 2012; Pratt and Church, 1992; Rein et al., 2011; Tollefsen, 1997). In other serpins such as the α1-antitrypsin and plasminogen activator inhibitor 1, GAG binding inhibits their inhibitory functions (Carrell, 2016; Ehrlich et al., 1991). We recently described highly conserved A. americanum serine protease inhibitor 19 (AAS 19) that is characterized by its functional domain being 100% conserved across different hard tick species (Porter et al., 2015). We showed that this protein was injected into the host during tick feeding, and that Pichia pastoris expressed recombinant (r)AAS19 was a broad-spectrum inhibitor of hemostasis and inflammation system proteases with anti-hemostatic functions (Kim et al., 2015). In another study target validation by RNAi silencing and immunization affected tick fitness, prevented successful tick feeding, and female ability to lay eggs (Kim et al., 2016a). Comparative modeling predicted four basic patches on AAS19 tertiary structure (Kim et al., 2015) that were similar to functionally validated GAG binding sites in mammalian serpins (Huntington, 2013; Koide, 1993; Pratt and Church, 1993). The goal of this study was to determine if putative AAS19 GAG binding sites were functional, and if so, to determine the effect of this interaction on rAAS19 function. We report that rAAS19 binds heparan sulfate/heparin (HS)GAGs and that this interaction altered the inhibitory profile and enhanced the anticoagulant function of rAAS19.
2. Materials and Methods
2.1. Validation of AAS19 GAG binding sites
Expression and affinity purification of rAAS19 was previously described (Kim et al., 2015). Comparative modeling predicted four GAG binding sites on AAS19 (Kim et al., 2015). To determine if putative AAS19 GAG binding sites were functional, ~100 μg of affinity purified rAAS19 was bound and eluted on HiTrap Heparin HP Column according to instructions by the manufacturer (GE Healthcare Bio-Sciences, Uppsala, Sweden) using 10 mM sodium phosphate, pH 7 as binding and 10 mM sodium phosphate, 2 M sodium chloride, pH 7 as eluting buffer. All column fractions were concentrated using the Microsep Advance Centrifugal Devices with 10K MWCO filters (Pall Life Sciences, Ann Arbor, MI, USA). On the same column, eluting fractions were desalted and buffer exchanged to binding buffer. Fractions as well as pre-column rAAS19 were subjected to western blotting analysis using the antibody to the C-terminus histidine tag (Life Technologies, Carlsbad, CA, USA). Positive signal was detected using a BioFX chemiluminescent reagent (SurModics, Eden Prairie, MN, USA).
2.2. Effect of HSGAG binding on protease inhibitor activity of AAS19
Proteases, peptide substrates and the methods described elsewhere (Kim et al., 2015) were used with slight modification: substrate hydrolysis was optimized at 37°C as opposed to 33°C. We used four commercially available HSGAGs: heparan sulfate (HS) (Galen Laboratory Supplies, Middletown, CT, USA), low molecular weight heparin (LMWH, the main fraction 3-5 KDa, Fisher Scientific), medium molecular weight heparin (MMWH, ~10KDa, J.T.Baker, Center Valley, PA, USA), and high molecular weight heparin (HMWH, 17-19 KDa, Sigma-Aldrich). The assay was done in four steps. In the first step we determined the lowest effective protease concentration (Table 1). In the second step, we screened inhibitory activity of 1 μM rAAS19 in presence of HSGAGs to identify proteases that were sensitive (Table 1). In the third step, we determined the molar ratio of rAAS19 (I) to target protease (E) for which in absence of HSGAGs less than 80% inhibition of target enzyme activity was observed (Table 2). In the fourth step we conducted substrate hydrolysis in presence of HSGAGs. For each protease (Table 2) rAAS19 was pre-incubated with HSGAG (final concentrations: 5 ng/μL for HS, 10 ng/μL for LMWH and MMWH, and 20 ng/μL for HMWH) for 5 min at 37°C in the reaction buffer (20 mM Tris-HCl, 150 mMNaCl, 0.1% BSA, pH 7.4). Subsequently the protease was added to the reaction, followed by incubation for 15 min at 37°C. Following incubation, appropriate substrates (Table 1) were added and substrate hydrolysis was monitored at A405nm every 30 seconds for 30 min at 37°C using the BioTek Synergy H1 plate reader (BioTek, Winooski, VT, USA). Data were fit onto the Michaelis-Menten kinetics equation to plot progress curves and determine residual enzyme activity or maximum velocity (Vmax) in Prism 6 software (GraphPad Software, La Jolla, CA, USA). With background removed, the % enzyme activity inhibition of rAAS19 with or without HSGAGs was calculated using this formula: 100-[Vmax(TPB or TPE)/Vmax(PC)]X100, where TPB, TPE, and PC represent residual enzyme activity of protease + rAAS19 + substrate without or with HSGAGs and HSGAG + protease + substrate without rAAS19, respectively. Mean (M) ± SEM % inhibition levels of three separate runs are reported. Mean (M) ± SEM % TPB was calculated from a combined twelve runs. Differences between TPB and TPE mean % enzyme activity inhibition levels determined the effect of HSGAG binding on rAAS19 inhibitory function. Positive or negative values denoted enhancement or decrease of rAAS19 inhibitory activity respectively in presence of HSGAGs. The statistical significance of HSGAG binding on rAAS19 inhibitory function was validated using unpaired t-test with Welch's correction in Prism 6 software (GraphPad Software).
Table 1.
List of proteases and their substrates used in substrate hydrolysis assays. Lowest effective protease concentrations (LEPC) and rAAS19 concentrations that inhibit less than 80% of protease activity in specified concentration (IC <80%) are specified.
| Protease (Company) | LEPC | IC <80% | Substrate (Company) |
|---|---|---|---|
| Tryptase from Human Lung (Sigma-Aldrich) | 4.94 nM | NIa | N-α-Benzoyl-DL-Arg-pNA (Sigma-Aldrich) |
| Kallikrein from Porcine Pancreas (Sigma-Aldrich) | 19.05 nM | 1 μM | H-D-Pro-Phe-Arg-pNA×2HCl (Chromogenix) |
| Proteinase 3, Human Neutrophil (Athens Research & Technology) | 779.31 nM | NIa | N-Metoxysuccinyl-Ala-Ala-Pro-Val-pNA (Santa Cruz Biotechnology) |
| Elastase from Porcine Pancreas (Sigma-Aldrich) | 6.18 nM | NIa | N-Succinyl-Ala-Ala-Ala-pNA (Sigma-Aldrich) |
| Recombinant Human HMW Urokinase (Molecular Innovations) | 9.26 nM | NIa | CH3SO2-D-CHG-Gly-Arg-pNA×AcOH (Pentapharm) |
| Human tPA, >85% Single Chain (Molecular Innovations) | 4.73 nM | NIa | CH3SO2-D-CHG-Gly-Arg-pNA×AcOH (Pentapharm) |
| Papain (Sigma-Aldrich) | 2.14 μM | 1 μM | N-Benzoyl-Phe-Val-Arg-pNA×HCl (Sigma-Aldrich) |
| Bovine Factor IXa beta (Enzyme Research Laboratories) | 31.44 nM | 1 μM | CH3SO2-D-CHG-Gly-Arg-pNA×AcOH (Pentapharm) |
| Factor Xa Protease (New England Biolabs) | 4.65 nM | 40 nM | Benzoyl-Ile-Glu(γ-OR)-Gly-Arg-pNA×HCl (Chromogenix) |
| Human Factor XIa (Enzyme Research Laboratories) | 3.69 nM | 200 nM | H-D-Pro-Phe-Arg-pNA×2HCl (Chromogenix) |
| Human Factor alpha-XIIa (Enzyme Research Laboratories) | 2.03 nM | 1 μM | H-D-Pro-Phe-Arg-pNA×2HCl (Chromogenix) |
| Plasmin from Human Plasma (Sigma-Aldrich) | 11.63 nM | 20 nM | H-D-Val-Leu-Lys-pNA×2HCl (Chromogenix) |
| Thrombin from Bovine Plasma (Sigma-Aldrich) | 27.1 nM | 100 nM | H-D-Phe-Pip-Arg-pNA×2HCl (Chromogenix) |
| Trypsin from Bovine Pancreas (Sigma-Aldrich) | 21.01 nM | 4 nM | Benzoyl-Ile-Glu(γ-OR)-Gly-Arg-pNA×HCl (Chromogenix) |
No inhibition
Table 2.
Effect of heparan sulfate/heparin glycosaminoglycans (HSGAGs) binding on rAAS19 inhibitory activity. Minus sign (−) = rAAS19 without HSGAGs, plus sign (+) = rAAS19 + HS (heparan sulfate), LMWH (low molecular weight heparin), MMWH (medium molecular weight heparin), HMWH (high molecular weight heparin), upward (↑) and downward (↓) arrowheads = rAAS19 inhibitory activity is enhanced or inhibited when compared to control −GAG, asterisks sign (*) = statistically significant and NS = not statistically significant differences in inhibitory activity from control reaction (unpaired t-test with Welch's correction).
| Optimized enzyme to rAAS19 molar ratio | Enzyme activity inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Enzyme | Enzyme (E, nM) | rAAS19 (I, μM) | I:E ratio | −GAG | +HS | +LMWH | +MMWH | +HMWH |
| Porcine Pancreas Kallikrein | 19.05 | 1 | 51.3:1 | 24.9 ± 2.1 | 25.3 ± 0.2 (NS) ↑ | 38.7 ± 5.8 (NS)↑ | 26.9 ± 3.6 (NS)↑ | *41.7 ± 1.8 (p=0.0003)↑ |
| Papain | 2.14 μM | 1 | 0.5:1 | 52.9 ± 2.0 | 59.0 ± 5.1 (NS)↑ | 47.7 ± 1.3 (NS) ↓ | 50.4 ± 1.4 (NS) ↓ | *72.2 ± 2.7 (p = 0.0030)↑ |
| Bovine blood clotting factor IXa | 31.44 | 1 | 31.8:1 | 43.1 ± 1.9 | 51.8 ± 3.3 (NS)↑ | *55.7 ± 2.1 (p =0.0047)↑ | *55.9 ± 2.6 (p = 0.0129)↑ | 45.5 ± 1.3 (NS)↑ |
| Bovine blood clotting factor Xa | 4.65 | 0.04 | 8.6:1 | 75.3 ± 4.3 | *17.4 ± 2.6 (p < 0.0001)↓ | *17.4 ± 2.2 (p < 0.0001)↓ | *17.8 ± 1.5 (p < 0.0001)↓ | *19.4 ± 2.0 (p < 0.0001)↓ |
| Human blood clotting factor XIa | 3.69 | 0.2 | 54.2:1 | 36.3 ± 1.8 | 31.6 ± 2.4 (NS) ↓ | NDa | NDa | NDa |
| Human blood clotting factor XIIa | 2.03 | 1 | 492.6:1 | 76.6 ± 1.3 | *50.6 ± 1.8 (p = 0.0001)↓ | *35.9 ± 0.7 (p < 0.0001)↓ | *49.8 ± 4.6 (p = 0.0216)↓ | *39.2 ± 0.2 (p < 0.0001)↓ |
| Human plasmin | 11.63 | 0.02 | 1.72:1 | 45.5 ± 2.5 | 55.4 ± 3.6 (NS)↑ | 45.7 ± 3.8 (NS) | *28.4 ± 4.2 (p =0.0308)↓ | 27.8 ± 6.4 (NS) ↓ |
| Bovine thrombin | 27.1 | 0.1 | 3.7:1 | 49.2 ± 3.5 | *88.2 ± 3.9 (p = 0.0004)↑ | *82.8 ± 5.1 (p = 0.0053)↑ | *90.1 ± 1.2 (p < 0.0001)↑ | *74.0 ± 4.5 (p = 0.0083)↑ |
| Bovine pancreatic trypsin | 21.01 | 0.004 | 0.19:1 | 57.5 ± 2.8 | 62.9 ± 11.1 (NS)↑ | NDa | NDa | 72.7 ± 5.4 (NS)↑ |
Not determined
2.3. Stoichiometry of inhibition (SI)
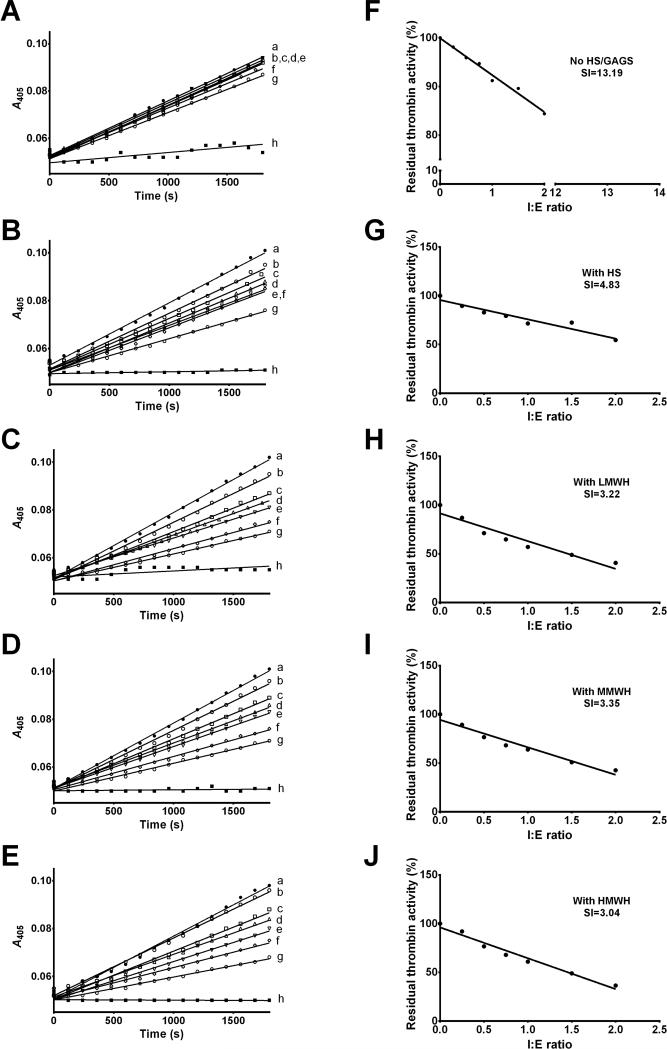
In preliminary analysis, we observed that the effect of HSGAG binding significantly enhanced the rAAS19 inhibitory activity against factor (f) IIa (thrombin). To gauge insight on the effect of HSGAG binding on rAAS19 inhibitory activity efficiency, we determined SI indices for thrombin with and without HSGAGs, as described (Kim et al., 2015). Various concentrations of rAAS19 (0, 6.81, 13.62, 20.44, 27.25, 40.87, and 54.5 nM) pre-incubated with HSGAGs (final concentrations indicated above) for 15 min at 37°C in the reaction buffer, followed by addition of thrombin in the final concentration of 27.25 nM. Reactions were further incubated at 37°C for 3 h. Colorimetric substrate (200 M, Table 1) was then added to a final reaction volume of 100 μL. Residual protease activity was determined by measuring A405nm every 30 seconds for 30 min at 37°C. Data were analyzed using the Michaelis-Menten kinetics equation in Prism 6 software to determine Vmax. Vmax in presence of various rAAS19 concentrations were expressed as percentage of PC. To estimate SI (x = SI when y = 0), a linear regression line was fit to Vmax (y-axis) versus I:E molar ratio [x-axis, rAAS19(I):enzyme(E)]. All I:E molar ratio with Vmax higher than 10% were included in the analysis.
2.4. Effect of HSGAG binding on rAAS19 anti-coagulation function
The effect of HSGAG binding on rAAS19 anti-coagulation function was assessed using recalcification time, activated partial thromboplastin time, and prothrombin time assays in two steps. In the first step, we determined the lowest effective anti-coagulant dosage of rAAS19 and HSGAGs. To determine the lowest effective anti-coagulant dosages, various amounts of rAAS19 (final concentrations 0.25, 0.5, 1, 2, and 4 M) and HSGAGs (0.25, 0.5, 1, 2, 4 and 8 ng/μL) were used in recalcification time, activated partial thromboplastin time, and prothrombin time assays as previously described (Kim et al., 2015). In the second step, we used the lowest effective anti-coagulant dosages to determine the effect of HSGAG binding on rAAS19 anticoagulation function. In the recalcification time assay, we mixed the the lowest effective anti-coagulant dosage of rAAS19 (2 μM) and 0.5 ng/μL for each of the HSGAGs in 40 μL of 20 mM Tris-HCl, 150 mMNaCl, pH 7.4 buffer and incubated for 5 min at 37°C, prior to adding 50 μL of pre-warmed universal coagulation reference plasma. Reactions were further incubated at 37°C for 15 min. Control reactions included rAAS19, or HSGAG the lowest effective anticoagulant dosages, or buffer only, that were pre-incubated at for 5 min at 37°C prior to the addition of universal coagulation reference plasma for each assay. Adding CaCl2 triggered plasma clotting and was monitored at A650nm every 20 s for 20 min using the BioTek Synergy H1 plate reader (BioTek). In preliminary analysis, we observed that pre-incubation of rAAS19 with HSGAGs substantially delayed plasma clotting above rAAS19 or HSGAGs alone. We were curious to investigate whether or not the pre-incubation step was necessary. To clarify, an additional control reaction was performed, with rAAS19 and HSGAGs pre-incubated separately with universal coagulation reference plasma and combined just before addition of CaCl2 to trigger clotting.
In activated partial thromboplastin time and prothrombin time assays, the lowest effective anti-coagulant dosages of rAAS19 and HSGAGs were mixed in 50 μL of 20 mM Tris-HCl, 150 mMNaCl, pH 7.4 buffer and pre-incubated 5 min at 37°C prior to addition of 50 μL of universal coagulation reference plasma or 100 μL of prothrombin time reagent for activated partial thromboplastin time and prothrombin time, respectively as previously described (Kim et al., 2015). Controls containing rAAS19, HSGAGs, or buffer only were included. All assays were performed in triplicate.
For all assays, data analysis was fit onto sigmoid line in Prism 6. Initiation of plasma clotting was interpolated from the sigmoid line when A650nm increased by 10%, with 95% confidence interval. Differences in plasma clotting time between treatment (rAAS19 and HSGAG pre-incubated together) and controls, quantified in seconds the effect of HSGAG binding on rAAS19 anticoagulant function.
3. Results
3.1. Recombinant AAS19 binds heparin
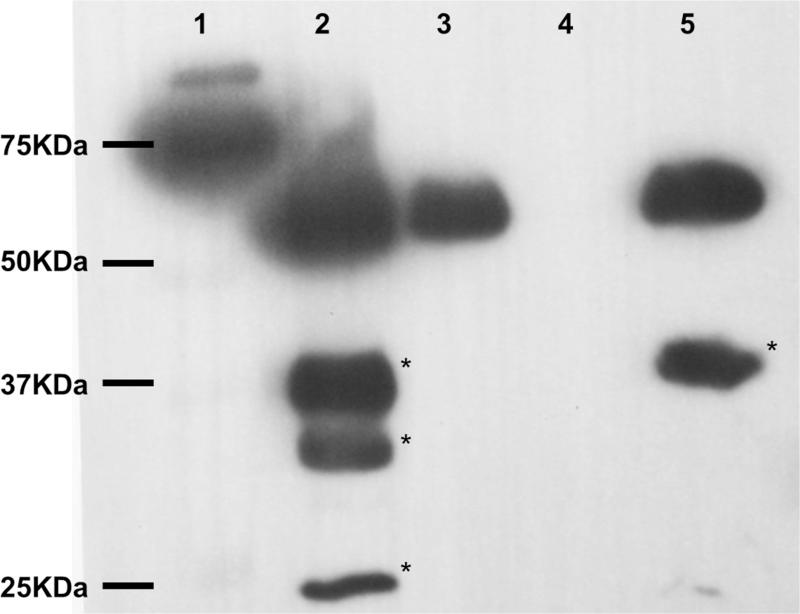
Fig. 1 validates that putative GAG binding sites predicted on AAS19 comparative tertiary structure (Kim et al., 2015) are functional. Fig. 1 shows that rAAS19 bound onto heparin in that rAAS19 was detected in run-through (lane 3) and elution (lane 5) fractions, but not in wash fraction (lane 4).
Fig. 1. Validation of rAAS19 binding to heparin.
Approximately 100 μg of affinity-purified rAAS19 was bound and eluted from a heparin charged column as described in materials and methods. Fractions were subjected to western blotting analysis using the antibody to the C-terminus histidine tag. Lane 1 = ladder, lane 2 = recombinant protein sample before application to the heparin column, lanes 3-5 = unbound run through, wash, and elution fractions, respectively. * - bands corresponding to degradation products of rAAS19.
3.2. HSGAG binding alters rAAS19 inhibitory profile
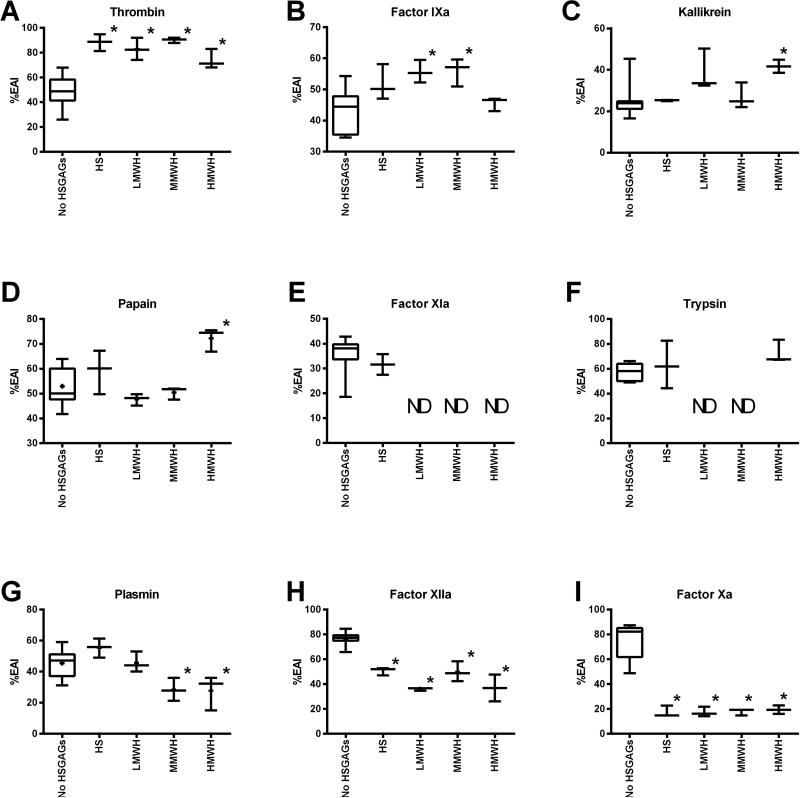
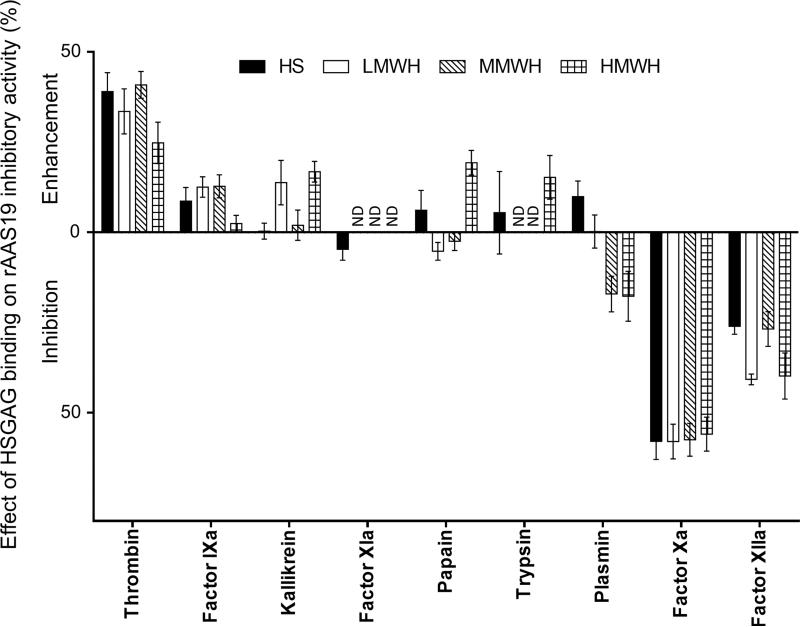
Data summarized in Table 2, Fig. 2 and 3 show that HSGAG binding had three effects on rAAS19 inhibitory function: (i) enhancement (blood clotting factors thrombin and fIXa, (ii) inhibition (blood clotting factors fXa and fXIIa), and (iii) moderate to no effect (kallikrein, papain, trypsin, and plasmin), while effects on fXIa were inconclusive. Unpaired t-test analysis demonstrated that rAAS19 (100 nM) inhibitory activity against thrombin (27.1 nM) was significantly enhanced from 49.2 ± 3.5% in absence of HSGAGS to 88.2 ± 4.0 (p = 0.0004), 82.8 ± 5.1 (p = 0.0303), 90.1 ± 1.2 (p = 0.0053), and 74.0 ± 4.5% (p = 0.0083) when bound with HS, LMWH, MMWH, and HMWH respectively (Fig. 2A, Table 2). For fIXa (31.44nM), binding LMWH and MMWH significantly enhanced rAAS19 (1 μM) inhibitory activity from 43.1 ± 1.9% in absence of HSGAGs to respectively 55.7 ± 2.8 (p = 0.0047) and 55.9 ± 2.6% (p = 0.0129), while binding HMWH and HS also showed enhancement but not statistically significant (Fig. 2B). On the other hand, binding of HSGAGs significantly suppressed rAAS19 (40 nM) inhibitor functions against fXa (4.65 nM), which dropped from 75.3 ± 4.3% in absence of HSGAGs to 17.4 ± 2.6 (p < 0.0001), 17.4 ± 2.2 (p < 0.0001), 17.8 ± 1.5 (p < 0.0001), and 19.4 ± 2.0% (p < 0.0001) in presence of HS, LMWH, MMWH, and HMWH, respectively (Fig. 2I). Similarly, HSGAG binding suppressed rAAS19 (1μM) inhibitory activity against fXIIa (2.03 nM) from 76.7 ± 1.3% in absence of HSGAGs to 50.6 ± 1.8 (p< 0.0001), 35.9 ± 0.7 (p = 0.0001), 49.9 ± 4.6 (P = 0.0216), and 36.8 ± 6.2% (p = 0.0199) in presence of HS, LMWH, MMWH, and HMWH, respectively (Fig. 2H). The effects of HSGAG binding had mixed, or minimal to no effect against kallikrein, trypsin, papain, and plasmin, or results were inconclusive as in the case of fXIa (Fig. 2D-G). Fig. 3 summarizes the effect of HSGAG binding on inhibitory activity of rAAS19 as revealed by differences in mean % enzyme activity inhibition between reactions with and without HSGAGs. Supplemental file S-1 summarizes progress curves of data summarized Table 2, Fig. 2 and 3.
Fig. 2. Effect of HSGAG binding on rAAS19 inhibitor function profile.
We pre-incubated rAAS19 with or without HSGAGS at 37°C for 5 min prior to addition of appropriate proteases and further 15 min incubation at the same temperature. Subsequently, specific colorimetric substrates at optimized concentrations were added and hydrolysis was monitored as described. Control reactions containing protease + rAAS19 + substrate without HSGAGs (TPB), protease + HSGAG + substrate without rAAS19 (PC), and HSGAG + rAAS19 + substrate (background) were included. Data were fit to Michaellis-Menten kinetics equation in PRISM 6 to determine Vmax or residual enzyme activity. With background removed, % enzyme activity inhibition of rAAS19 in presence (TPE) or absence of HSGAGs was calculated using this formula: 100-[Vmax(TPB or TPE)/Vmax(PC)]×100. Mean (M) ± SEM % enzyme activity inhibition of three separate runs are reported, except for TPB, which was calculated from a combined twelve runs. Unpaired t-test in Prism 6 was used to determine statistical significance between TPB and TPE treatments. ND - not defined. * - statistically significant difference between TPB and TPE.
Fig. 3. Summary of effect of HSGAG binding on rAAS19 inhibitor function profile.
Differences between mean % enzyme activity inhibition of rAAS19 with HSGAGS reactions and without HSGAGs calculated in Prism 6. Differences between means (M ± SEM) of three separate runs are presented. ND - not defined.
3.3. HSGAG binding improves rAAS19 inhibitory efficiency against thrombin as revealed by SI
In Fig. 2 and 3 we observed that HSGAG binding dramatically amplified rAAS19 inhibitory functions against thrombin. To gauge insight into whether this is translated to the increased rAAS19 inhibitory efficiency we determined SI indices against thrombin (Fig. 4A-J) in presence of different HSGAGs. As shown in Fig. 4, the inhibitory efficiency of rAAS19 against thrombin improved ~2.7 fold from a SI index of 13.19 in absence of HSGAGs (Fig. 4A and F) to 4.83 when bound to HS (Fig. 4B and G) and ~4.1, 3.9, and 4.3 fold when bound to LMWH (Fig. 4C and H), MMWH (Fig. 4D and I), and HMWH (Fig. 4E and J), respectively.
Fig. 4. Stoichiometry of inhibition analysis of rAAS19 against thrombin in presence of HSGAGs.
Various amounts of rAAS19 was pre-incubated 15 min at 37°C without HSGAGs (A, F), with HS (B, G), LMWH (C, H), MMWH (D, I), and HMWH (E, J) prior to addition of thrombin (27.1 nM) and further incubating at 37°C for an additional 3 h. Specific colorimetric substrate was added and substrate hydrolysis monitored and Vmax or residual enzyme activity were determined as described. The SI index (x coordinate when y = 0) was determined by fitting the linear regression line Vmax (y-axis) versus I:E molar ratio (x-axis) as shown. In progress curves A-E, line graphs “a to g” represent various rAAS19 concentrations used: 0, 6.81, 13.62, 20.44, 27.25, 40.87, and 54.5 nM. Negative controls without thrombin were included (h).
3.4. HSGAG binding enhances rAAS19 anticoagulant function
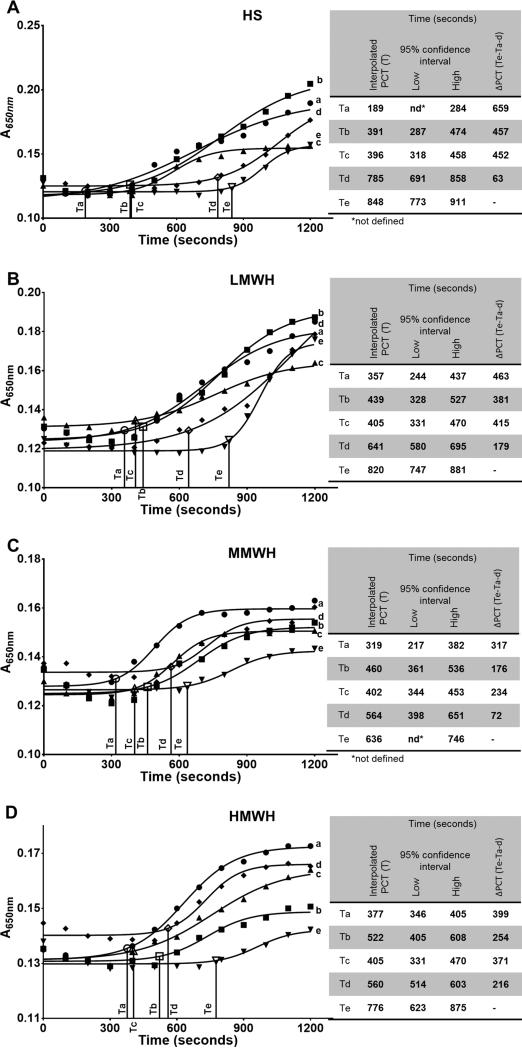
The recalcification time assay summarized in Fig. 5, demonstrate that the anticoagulant effects of rAAS19 in complex with any of the four HSGAGs (Te in Fig. 5) was superior to rAAS19 (Tb in Fig. 5) or any of the four HSGAGs (Tc in Fig. 5) alone. It is interesting to note that when we pre-bound rAAS19 with HS, LMWH, MMWH, and HMWH, plasma clotting was delayed for an additional 176-457 s above HSGAGs or rAAS19 alone (Fig. 5A-D and accompanying table inserts). Another interesting observation from our data is that pre-incubating rAAS19 with HSGAGs together presumably to allow for the complex to form prior to the recalcification time assay was important for the observed enhanced delay of plasma clotting. Plasma clotted faster in reactions where we pre-incubated rAAS19 and HSGAGs separately with plasma (Td in Fig. 5) and mixed just before triggering clotting as opposed to reactions in which rAAS19 and HGAGs were pre-incubated together prior to triggering clotting (Te in Fig. 5). As shown in the table inserts Te reactions delayed plasma clotting for an additional 63-216 s above Td reactions. In the recalcification time assay, firmness of the clot corresponded to higher optical density (A). It is interesting to note that the final A650nm read outs were apparently lower when rAAS19 was mixed with HSGAGs (e in Fig. 5) in comparison with control reactions (a-d in Fig. 5). Under conditions of the assay in this study, pre-binding rAAS19 with HSGAGs did not have any effects on plasma clotting time in the prothrombin time and activated partial thromboplastin time blood clotting assays.
Fig. 5. Effect of HSGAG binding on rAAS19 anticoagulant function in the recalcification time assay.
The lowest effective anti-coagulant dosage of rAAS19 was mixed and pre-incubated with the lowest effective anti-coagulant dosage of HS (A), LMWH (B), MMWH (C), and HMWH (D) in 40 μL reactions for 5 min at 37°C (e). Control reactions containing rAAS19 only (c), HSGAG only (b), and buffer only (a), were also pre-incubated prior to addition to 50 μL of universal coagulation reference plasma and further 15 min incubation at the same temperature. Plasma clotting was triggered by addition 10 μL of pre-warmed CaCl2 (15 mM) and monitored at A650nm every 20 sec over 20 min period at 37°C. An additional control reaction of the lowest effective anti-coagulant dosages of rAAS19 and HSGAGs pre-incubated separately and combined just before CaCl2 addition (d) was included. Data points represent mean of triplicate readings. Data were fitted to a sigmoid line in Prism 6 software. Plasma clotting time was interpolated on the sigmoid line at points when A650nm increased 10%. Drop lines Ta, Tb, Tc and Td = plasma clotting times of control reactions a, b, c, and d. Te = plasma clotting time of reactions containing rAAS19 pre-bound with HSGAGs. PCT = Differences between plasma clotting times of reactions containing rAAS19 pre-bound with HSGAGs (e) and control reactions.
4. Discussion
This study was prompted by functional analysis data that showed that rAAS19 was a functional inhibitory serpin and anticoagulant that was predicted to have putative GAG binding sites (Kim et al., 2015). Key regulatory serpins in the mammalian blood clotting system such as antithrombin III, heparin cofactor II, and protein C inhibitor bind GAGs to achieve physiological inhibitory rates against target proteases (Berliner, 2012; Pratt and Church, 1993; Tollefsen, 1997). We were curious to investigate if putative GAG biting sites on AAS19 tertiary structure (Kim et al., 2015) were functional, and if so, the effect of this interaction on rAAS19 inhibitory and anticoagulant functions. Our data demonstrate that rAAS19 binds HSGAGs and this interaction alters the inhibitory profile and enhances the anticoagulant function of rAAS19. Preventing host blood from clotting is the major tick accomplishment for the simple reason that if blood clots, the tick will starve. The blood-clotting cascade is classified in three pathways: the initiation or tissue factor (classically known as extrinsic), the consolidation or amplification (classically called intrinsic), and the common pathway when the fibrin clot forms to seal off the blood vessel damage (Versteeg et al., 2013; Walsh and Ahmad, 2002). Blood clotting factors have been grouped into those that regulate the initiation (fVa, fVIIa, fIXa, and fXa), the consolidation (thrombin, fVIIIa, fXIa, and fXIIa), and the common pathways (thrombin, fVa, and fXa) (Versteeg et al., 2013). The observation that two blood clotting factors, thrombin and fIXa against which HSGAG binding significantly enhanced rAAS19 inhibitory activity play roles in each of three parts of the blood clotting pathway, suggest that native AAS19 interferes with the blood clotting system at all levels. This may explain the significant delay in plasma clotting time that was observed in the recalcification time assay.
Unlike mammalian serpins for which GAG binding either enhances or blocks inhibitory function of a candidate serpin (Berliner, 2012; Carrell, 2016; Ehrlich et al., 1991), HSGAG binding had dichotomous effect on rAAS19 inhibitory profile. In absence of HSGAGs, rAAS19 inhibited enzyme activity of 11 mammalian serine proteases to variable degrees (Kim et al., 2015) and papain in this study. However in presence of HSGAGs rAAS19 inhibitory activity was enhanced against thrombin and fIXa, inhibited against fXIIa and fXa, and had minimal or no effect against trypsin, plasmin, kallikrein, and papain enzyme activities. Given that native AAS19 protein is injected into the host during tick feeding (Kim et al., 2015), it is likely that this protein will bind mammalian HSGAGs at the feeding site. On this basis, we speculate that native AAS19 is a likely inhibitor of thrombin, fIXa, fXIa, kallikrein, trypsin-, and papain-like proteases. HSGAGs are present on the surface of endothelial cells, which coat blood vessels and other cavities of cardiovascular system (Rosenberg et al., 1997). It is likely that native AAS19 bind HSGAGs on cell and host tissue as way of localizing its effects to the tick-feeding site.
It is important to note here that there are some discrepancies in rAAS19 inhibitory activity between this study and our previous study (Kim et al., 2015). Previously we reported lower rAAS19 % enzyme activity inhibition level against thrombin than in this study. Reasons for this discrepancy could be explained by the fact that in this study we used significantly less amount of thrombin in comparison to previous study (Kim et al., 2015). We believe that this translated to high rAAS19 excesses and thus high inhibitory levels reported here. Additionally substrate hydrolysis assays in this study were optimized at 37°C as opposed to 33°C in previous paper (Kim et al., 2015). We would like to note here that the observed rAAS19 inhibitory activity against thrombin in this study is close to that of its homolog in Rhipicephalus microplus, RmS-15, which was reported to inhibit thrombin with SI of 1.5 (Xu et al., 2016). The observation in this study that HSGAG binding dramatically enhanced rAAS19 against thrombin is not unique as similar observations were reported for a viral serpin (Li et al., 2008).
The rAAS19 inhibitor function profile in this study is partly similar to the inhibitory profile of antithrombin III, which inhibits thrombin, fXa, fIX, fXI, fXII, plasmin, kallikrein, and trypsin (Bock, 2006; Danielsson and Bjork, 1982; Kumar et al., 2013). Heparin binding accelerates antithrombin III inhibitory activity against thrombin, fIXa, and fXa (≥1000-fold) (Bedsted et al., 2003; Jordan et al., 1980; Olson et al., 1992), and to a less extent kallikrein, fXI, and fXII (Holmer et al., 1981; Scott et al., 1982). It is interesting to note that heparin binding enhances rAAS19 inhibitory activity against kallikrein, fIX, and especially against thrombin for which the SI index decreased 2.7-4.3-fold. However, in contrast to antithrombin III, inhibitory activity of rAAS19 against fXa, fXIIa, and plasmin was inhibited after heparin binding. Could native AAS19 represent the tick version of antithrombin III at the feeding site?
Our data suggest the potential of medicinal applications for rAAS19 in treatment of blood clotting disorders. The observation that the significant delay in plasma clotting above those observed with rAAS19 or HSGAGs alone required HSGAGs and rAAS19 to be in complex other than the two molecules simply being in a reaction together, has significant implications toward efforts to improve heparin based treatment of blood clotting disorders. Heparin is used to treat and/or prevent blood-clotting disorders in a wide range of conditions such as preventing thrombi and formation of blood clots in many surgical cases (Gallus et al., 1976; Rasmussen et al., 2009). In addition to potential toxicity due accidental overdosing (Monagle et al., 2012; Monte et al., 2010), patients are at risk of developing life threatening heparin-induced thrombocytopenia a serious immune system-mediated complication that results in depleted platelets (Warkentin, 1998) and was reported to occur in up to 58% of critically ill patients (Gupta et al., 2015). Heparin-induced thrombocytopenia occurs when heparin, a negatively charged molecule, binds to platelet factor 4 (PF4), a positively charged protein found in platelet α-granules, on platelet and some cell surfaces (Warkentin, 1998). Heparin binding exposes antigenic neo-epitopes that act as immunogens leading to production of antibodies to heparin-PF4, which lead to immune mediated depletion of platelets and secretion of factors enhancing thrombin generation which leads to thrombosis (Amiral et al., 1992; Kelton et al., 1994). Our data suggest that the possibility for heparin-induced thrombocytopenia will be eliminated in that heparin will be delivered in complex with rAAS19 and thus may not be biologically available to bind to PF4 and thus no life threatening antibodies will form. Additionally, our data showing that we could achieve significant plasma clotting delay using less heparin suggest that toxicity due to overdose will be prevented.
Heparin based thromboprophylaxis primarily exploits anti-thrombin and fXa effects of this molecule. The risk is that patients face the potential to develop bleeding (Weitz, 2011). Preferred alternative targets for treatment includes fXIa, which impairs thrombus formation with minimal impact on hemostasis (Bane and Gailani, 2014; Muller et al., 2011), and fIXa, which is preferred in treatment of venous thromboembolism (Eikelboom et al., 2010). Data here and elsewhere (Kim et al., 2015) show that rAAS19 is an inhibitor of fXIa and fIXa, suggesting the potential for this molecule to be used in anticoagulant therapy. In addition, the observation that LMWH and MMWH significantly potentiated rAAS19 inhibitory activity against fIXa is interesting. The potential of serpin-heparin covalent complexes to be used in thrombus therapy was successfully demonstrated (Chan et al., 1997; Stević et al., 2013). It will be interesting to investigate if functionally active rAAS19 can form covalent complex with different heparins.
In conclusion, data here suggest that there is likelihood for native AAS19 to undergo post-secretion modification through binding onto GAGs or proteoglycans at the tick-feeding site. On the basis of data in study, we conclude that native AAS19 is a likely inhibitor of thrombin and other trypsin-related proteases. Kim et al., (2016a) showed that RNAi silencing of AAS19 caused deformities in ticks suggesting that this serpin regulated an important protease in the tick. There is evidence that invertebrates do produce heparin-like GAGs (Chavante et al., 2014; Pavão, 2014). It will be interesting to investigate if tick derived GAGs affected inhibitory functions of native AAS19 in the tick.
Supplementary Material
Highlights.
HSGAG binding enhances AAS19 inhibition of thrombin and factor IXa, but inhibits its activity against factors Xa and XIIa.
HSGAG binding improved rAAS19 inhibitory activity 2.7-4.3 folds as determined by stoichiometry of inhibition analysis.
rAAS19 in complex with HSGAGs dramatically delays plasma clotting time much more than rAAS19 or HSGAGs alone.
Acknowledgements
Funding: This research was supported by National Institute of Health grants (AI081093, AI093858, AI074789, and AI074789-01A1S1) to AM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amiral J, Bridey F, Dreyfus M, Vissoc AM, Fressinaud E, Wolf M, Meyer D. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb. Haemost. 1992;68:95–96. [PubMed] [Google Scholar]
- 2.Bane CE, Gailani D. Factor XI as a target for antithrombotic therapy. Drug Discov. Today. 2014;19:1454–1458. doi: 10.1016/j.drudis.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedsted T, Swanson R, Chuang Y, Bock PE, Björk I, Olson ST. Heparin and calcium ions dramatically enhance antithrombin reactivity with factor IXa by generating new interaction exosites. Biochemistry (N. Y.) 2003;42:8143–8152. doi: 10.1021/bi034363y. [DOI] [PubMed] [Google Scholar]
- 4.Berliner L. Thrombin: structure and function. Springer Science & Business Media; New York: 2012. [Google Scholar]
- 5.Bock SC. Antithrombin III and heparin cofactor II. In: Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 235–248. [Google Scholar]
- 6.Brouwer JP, Lijfering WM, ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb. Haemost. 2009;101:93–99. [PubMed] [Google Scholar]
- 7.Carrell R. Alpha-1-Antitrypsin and the Serpins. In: Wanner A, Sandhaus RA, editors. Alpha-1 Antitrypsin. Springer International Publishing; Switzerland: 2016. pp. 1–15. [Google Scholar]
- 8.Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding. J. Exp. Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan A, Berry L, O'Brodovich H, Klement P, Mitchell L, Baranowski B, Monagle P, Andrew M. Covalent antithrombin-heparin complexes with high anticoagulant activity: Intravenous, subcutaneous, and intratracheal administration. J. Biol. Chem. 1997;272:22111–22117. doi: 10.1074/jbc.272.35.22111. [DOI] [PubMed] [Google Scholar]
- 10.Chavante SF, Brito AS, Lima M, Yates E, Nader H, Guerrini M, Torri G, Bisio A. A heparin-like glycosaminoglycan from shrimp containing high levels of 3-O-sulfated D-glucosamine groups in an unusual trisaccharide sequence. Carbohydr. Res. 2014;390:59–66. doi: 10.1016/j.carres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Chmelar J, Oliveira CJ, Rezacova P, Francischetti M, Kovarova Z, Pejler G, Kopacek P, Ribeiro JM, Mares M, Kopecky J, Kotsyfakis M. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood. 2011;117:736–744. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui X, Liu Y, Wan C, Lu C, Cai J, He S, Ni T, Zhu J, Wei L, Zhang Y, Qian H. Decreased expression of SERPINB1 correlates with tumor invasion and poor prognosis in hepatocellular carcinoma. J. Mol. Histol. 2014;45:59–68. doi: 10.1007/s10735-013-9529-0. [DOI] [PubMed] [Google Scholar]
- 13.Danielsson A, Bjork I. Mechanism of inactivation of trypsin by antithrombin. Biochem. J. 1982;207:21–28. doi: 10.1042/bj2070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RL, Shrimpton AE, Holohan PD, Bradshaw C, Feiglin D, Collins GH, Sonderegger P, Kinter J, Becker LM, Lacbawan F, Krasnewich D, Muenke M, Lawrence DA, Yerby MS, Shaw CM, Gooptu B, Elliott PR, Finch JT, Carrell RW, Lomas DA. Familial dementia caused by polymerization of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43894. [DOI] [PubMed] [Google Scholar]
- 15.Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb. Diath. Haemorrh. 1965;13:516–530. [PubMed] [Google Scholar]
- 16.Ehrlich HJ, Keijer J, Preissner KT, Gebbink RK, Pannekoek H. Functional interaction of plasminogen activator inhibitor type 1 (PAI-1) and heparin. Biochemistry (N. Y.) 1991;30:1021–1028. doi: 10.1021/bi00218a020. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Zelenkofske SL, Rusconi CP. Coagulation factor IXa as a target for treatment and prophylaxis of venous thromboembolism. Arterioscl. Throm. Vas. 2010;30:382–387. doi: 10.1161/ATVBAHA.110.203117. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson S. Pulmonary emphysema and alpha1-antitrypsin deficiency. Acta. Med. Scand. 1964;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 19.Gallus AS, Hirsh J, O'Brien SE, McBride JA, Tuttle RJ, Gent M. Prevention of venous thrombosis with small, subcutaneous doses of heparin. JAMA. 1976;235:1980–1982. [PubMed] [Google Scholar]
- 20.Gupta S, Tiruvoipati R, Green C, Botha J, Tran H. Heparin induced thrombocytopenia in critically ill: Diagnostic dilemmas and management conundrums. World J. Critical Care Med. 2015;4:202. doi: 10.5492/wjccm.v4.i3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmer E, Kurachi K, Soderstrom G. The molecular-weight dependence of the rate-enhancing effect of heparin on the inhibition of thrombin, factor Xa, factor IXa, factor XIa, factor XIIa and kallikrein by antithrombin. Biochem. J. 1981;193:395–400. doi: 10.1042/bj1930395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huntington J. Thrombin inhibition by the serpins. J. Thromb. Haemost. 2013;11(s1):254–264. doi: 10.1111/jth.12252. [DOI] [PubMed] [Google Scholar]
- 23.Ibelli AM, Kim TK, Hill CC, Lewis LA, Bakshi M, Miller S, Porter L, Mulenga A. A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting. Int. J. Parasitol. 2014;44:369–379. doi: 10.1016/j.ijpara.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan RE, Oosta GM, Gardner WT, Rosenberg RD. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J. Biol. Chem. 1980;255:10081–10090. [PubMed] [Google Scholar]
- 25.Kelton JG, Smith JW, Warkentin TE, Hayward CP, Denomme GA, Horsewood P. Immunoglobulin G from patients with heparin-induced thrombocytopenia binds to a complex of heparin and platelet factor 4. Blood. 1994;83:3232–3239. [PubMed] [Google Scholar]
- 26.Kim TK, Radulović Ž, Mulenga A. Target validation of highly conserved Amblyomma americanum tick saliva serine protease inhibitor 19. Ticks Tick-Borne Dis. 2016a;7:405–414. doi: 10.1016/j.ttbdis.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TK, Tirloni L, Pinto AF, Moresco J, Yates JR, 3rd, da Silva Vaz I, Jr, Mulenga A. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl. Trop. Dis. 2016b;10:e0004323. doi: 10.1371/journal.pntd.0004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TK, Tirloni L, Radulović Ž, Lewis L, Bakshi M, Hill C, da Silva Vaz I, Jr, Logullo C, Termignoni C, Mulenga A. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 2015;45:613–627. doi: 10.1016/j.ijpara.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koide T. Heparin-binding site(s) of antithrombin III, histidine-rich glycoprotein and activated protein C. In: Shen MC, Teng CM, Takada A, editors. Current Aspects of Blood Coagulation, Fibrinolysis, and Platelets. Springer; Japan: 1993. pp. 23–28. [Google Scholar]
- 30.Kubo A, Shiohama A, Sasaki T, Nakabayashi K, Kawasaki H, Atsugi T, Sato S, Shimizu A, Mikami S, Tanizaki H, Uchiyama M, Maeda T, Ito T, Sakabe J, Heike T, Okuyama T, Kosaki R, Kosaki K, Kudoh J, Hata K, Umezawa A, Tokura Y, Ishiko A, Nizeki H, Kabashima K, Mitsuhashi Y, Amagai M. Mutations in SERPINB7, encoding a member of the serine protease inhibitor superfamily, cause Nagashima-type palmoplantar keratosis. Am. J. Human. Genet. 2013;93:945–956. doi: 10.1016/j.ajhg.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Bhandari A, Sarde SJ, Goswami C. Sequence, phylogenetic and variant analyses of antithrombin III. Biochem. Biophys. Res. Commun. 2013;440:714–724. doi: 10.1016/j.bbrc.2013.09.134. [DOI] [PubMed] [Google Scholar]
- 32.Lewis LA, Radulović ŽM, Kim TK, Porter LM, Mulenga A. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks Tick-Borne Dis. 2015;6:424–434. doi: 10.1016/j.ttbdis.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Schneider H, Peters A, Macaulay C, King E, Sun Y, Liu L, Dai E, Davids JA, McFadden G, Lucas A. Heparin alters viral serpin, serp-1, anti-thrombolytic activity to anti-thrombotic activity. Open Biochem. J. 2008;2:6–15. doi: 10.2174/1874091X00802010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monagle P, Studdert DM, Newall F. Infant deaths due to heparin overdose: time for a concerted action on prevention. J. Paediatr. Child Health. 2012;48:380–381. doi: 10.1111/j.1440-1754.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 35.Monte AA, Bodmer M, Schaeffer TH. Low-molecular-weight heparin overdose: management by observation. Ann. Pharmacother. 2010;44:1836–1839. doi: 10.1345/aph.1P318. [DOI] [PubMed] [Google Scholar]
- 36.Mulenga A, Khumthong R, Blandon MA. Molecular and expression analysis of a family of the Amblyomma americanum tick Lospins. J. Exp. Biol. 2007;210:3188–3198. doi: 10.1242/jeb.006494. [DOI] [PubMed] [Google Scholar]
- 37.Mulenga A, Khumthong R, Chalaire KC. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics. 2009;10:217. doi: 10.1186/1471-2164-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulenga A, Kim T, Ibelli A. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 2013;22:306–319. doi: 10.1111/imb.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulenga A, Sugimoto C, Ingram G, Ohashi K, Misao O. Characterization of two cDNAs encoding serine proteinases from the hard tick Haemaphysalis longicornis. Insect Biochem. Mol. Biol. 2001a;31:817–825. doi: 10.1016/s0965-1748(00)00187-9. [DOI] [PubMed] [Google Scholar]
- 40.Mulenga A, Sugino M, Nakajima M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J. Vet. Med. Sci. 2001b;63:1063–1069. doi: 10.1292/jvms.63.1063. [DOI] [PubMed] [Google Scholar]
- 41.Mulenga A, Tsuda A, Onuma M, Sugimoto C. Four serine proteinase inhibitors (serpin) from the brown ear tick, Rhiphicephalus appendiculatus; cDNA cloning and preliminary characterization. Insect Biochem. Mol. Biol. 2003;33:267–276. doi: 10.1016/s0965-1748(02)00240-0. [DOI] [PubMed] [Google Scholar]
- 42.Muller F, Gailani D, Renne T. Factor XI and XII as antithrombotic targets. Curr. Opin. Hematol. 2011;18:349–355. doi: 10.1097/MOH.0b013e3283497e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson ST, Björk I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J. Biol. Chem. 1992;267:12528–12538. [PubMed] [Google Scholar]
- 44.Palenikova J, Lieskovska J, Langhansova H, Kotsyfakis M, Chmelar J, Kopecky J. Ixodes ricinus salivary serpin IRS-2 affects Th17 differentiation via inhibition of the interleukin-6/STAT-3 signaling pathway. Infect. Immun. 2015;83:1949–1956. doi: 10.1128/IAI.03065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavão MS. Glycosaminoglycans analogs from marine invertebrates: structure, biological effects, and potential as new therapeutics. Front. Cell. Infect. Microbiol. 2014;4:123. doi: 10.3389/fcimb.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porter L, Radulović Ž, Kim T, Braz GR, da Silva Vaz I, Mulenga A. Bioinformatic analyses of male and female Amblyomma americanum tick expressed serine protease inhibitors (serpins). Ticks Tick-Borne Dis. 2015;6:16–30. doi: 10.1016/j.ttbdis.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratt CW, Church FC. Heparin binding to protein C inhibitor. J. Biol. Chem. 1992;267:8789–8794. [PubMed] [Google Scholar]
- 48.Pratt C, Church FC. General features of the heparin-binding serpins antithrombin, heparin cofactor II and protein C inhibitor. Blood Coagulation Fibrinol. 1993;4:479–490. doi: 10.1097/00001721-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J. Biol. Chem. 2006;281:26361–26369. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- 50.Prevot P, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, Adam B, Brossard M, Brasseur R, Zouaoui Boudjeltia K, Vanhamme L, Godfroid E. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J. 2009;276:3235–3246. doi: 10.1111/j.1742-4658.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- 51.Radulović ŽM, Kim TK, Porter LM, Sze S, Lewis L, Mulenga A. A 24-48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics. 2014;15:518. doi: 10.1186/1471-2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmussen MS, Jørgensen LN, Wille-Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst. Rev. 2009 doi: 10.1002/14651858.CD004318.pub2. 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Rein CM, Desai UR, Church FC. Serpin-glycosaminoglycan interactions. Methods Enzymol. 2011;501:105–137. doi: 10.1016/B978-0-12-385950-1.00007-9. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J. Clin. Invest. 1997;99:2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott CF, Schapira M, Colman RW. Effect of heparin on the inactivation rate of human factor XIa by antithrombin-III. Blood. 1982;60:940–947. [PubMed] [Google Scholar]
- 57.Stević I, Chan HH, Berry LR, Chander A, Chan AK. Inhibition of the prothrombinase complex on red blood cells by heparin and covalent antithrombin-heparin complex. J. Biochem. 2013;153:103–110. doi: 10.1093/jb/mvs129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugino M, Imamura S, Mulenga A, Nakajima M, Tsuda A, Ohashi K, Onuma M. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine. 2003;21:2844–2851. doi: 10.1016/s0264-410x(03)00167-1. [DOI] [PubMed] [Google Scholar]
- 59.Tirloni L, Islam MS, Kim TK, Diedrich JK, Yates JR, 3rd, Pinto AF, Mulenga A, You MJ, da Silva Vaz I., Jr Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasite. Vector. 2015;8:338. doi: 10.1186/s13071-015-0918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tirloni L, Seixas A, Mulenga A, da Silva Vaz I, Termignoni C. A family of serine protease inhibitors (serpins) in the cattle tick Rhipicephalus (Boophilus) microplus. Exp. Parasitol. 2014;137:25–34. doi: 10.1016/j.exppara.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Tollefsen DM. Church FC, Cunningham DD, Ginsburg D, Hoffman M, Stone SR, Tollefsen DM, editors. Heparin cofactor II. Chemistry and Biology of Serpins. Adv. Exp. Med. Biol. 1997;425:35–44. doi: 10.1007/978-1-4615-5391-5_4. [DOI] [PubMed] [Google Scholar]
- 62.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol. Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 63.Walsh PN, Ahmad SS. Proteases in blood clotting. Essays Biochem. 2002;38:95–111. doi: 10.1042/bse0380095. [DOI] [PubMed] [Google Scholar]
- 64.Warkentin TE. Heparin-induced thrombocytopenia. In: Ginsberg J, Kearon C, Hirsh J, editors. Critical Decisions in Thrombosis and Haemostasis. BC Decker Inc; Hamilton: 1998. pp. 100–108. [Google Scholar]
- 65.Weitz JI. Factor Xa and thrombin as targets for new oral anticoagulants. Thromb. Res. 2011;127:S5–S12. doi: 10.1016/S0049-3848(10)70147-X. [DOI] [PubMed] [Google Scholar]
- 66.Xu T, Lew-Tabor A, Rodriguez-Valle M. Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris. Ticks Tick-Borne Dis. 2016;7:180–187. doi: 10.1016/j.ttbdis.2015.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.