Abstract
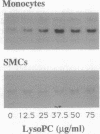
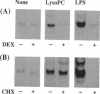
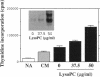
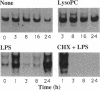
Lysophosphatidylcholine is increased in the plasma of hypercholesterolemic patients, is a component of oxidatively modified low-density lipoprotein, and, as such, may play an important role in atherosclerosis. Here we demonstrate that in human monocytes, lysophosphatidylcholine increases the level of mRNA encoding the heparin-binding epidermal growth factor-like growth factor (HB-EGF), a potent smooth muscle mitogen. Lysophosphatidylcholine treatment also enhances the release of heparin-binding mitogenic activity by these cells in culture. The anti-inflammatory glucocorticoid dexamethasone inhibits the upregulation of HB-EGF mRNA induced by either lysophosphatidylcholine or bacterial lipopolysaccharide in cultured monocytes. However, the responses induced by lysophosphatidylcholine and by lipopolysaccharide differ in their kinetics. In addition, the response to lysophosphatidylcholine is resistant to the action of cycloheximide, whereas the response to lipopolysaccharide is not, suggesting that the activation mechanisms induced by these two stimuli are different. Since a nuclear run-on assay showed no effect of lysophosphatidylcholine on the transcription of the HB-EGF gene, we speculate that lysophosphatidylcholine may increase the level of HB-EGF mRNA by altering the processing or degradation of primary or mature transcripts. Lysophosphatidylcholine enhancement of monocyte production of HB-EGF may represent an important result of the interactions among oxidized low-density lipoprotein and monocyte-derived macrophages and may play a role in initiation of smooth muscle proliferation in atherogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besner G., Higashiyama S., Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990 Oct;1(11):811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Brown G., Albers J. J., Fisher L. D., Schaefer S. M., Lin J. T., Kaplan C., Zhao X. Q., Bisson B. D., Fitzpatrick V. F., Dodge H. T. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990 Nov 8;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corjay M. H., Blank R. S., Owens G. K. Platelet-derived growth factor-induced destabilization of smooth muscle alpha-actin mRNA. J Cell Physiol. 1990 Dec;145(3):391–397. doi: 10.1002/jcp.1041450302. [DOI] [PubMed] [Google Scholar]
- Dluz S. M., Higashiyama S., Damm D., Abraham J. A., Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor expression in cultured fetal human vascular smooth muscle cells. Induction of mRNA levels and secretion of active mitogen. J Biol Chem. 1993 Aug 25;268(24):18330–18334. [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- GLOMSET J. A. The mechanism of the plasma cholesterol esterification reaction: plasma fatty acid transferase. Biochim Biophys Acta. 1962 Nov 19;65:128–135. doi: 10.1016/0006-3002(62)90156-7. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J Cell Biol. 1993 Aug;122(4):933–940. doi: 10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Inkeles S., Eisenberg D. Hyperlipidemia and coronary atherosclerosis: a review. Medicine (Baltimore) 1981 Mar;60(2):110–123. doi: 10.1097/00005792-198103000-00004. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Baird A. A dual receptor system is required for basic fibroblast growth factor activity. Cell. 1991 Oct 18;67(2):229–231. doi: 10.1016/0092-8674(91)90173-v. [DOI] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Malden L. T., Chait A., Raines E. W., Ross R. The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. J Biol Chem. 1991 Jul 25;266(21):13901–13907. [PubMed] [Google Scholar]
- Nakano T., Raines E. W., Abraham J. A., Wenzel F. G., 4th, Higashiyama S., Klagsbrun M., Ross R. Glucocorticoid inhibits thrombin-induced expression of platelet-derived growth factor A-chain and heparin-binding epidermal growth factor-like growth factor in human aortic smooth muscle cells. J Biol Chem. 1993 Oct 25;268(30):22941–22947. [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Steinbrecher U. P., Barnett J., Witztum J. L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 May;82(9):3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Kondratenko N., Parthasarathy S. Analysis of the monocyte chemotactic response to lysophosphatidylcholine: role of lysophospholipase C. Biochim Biophys Acta. 1991 Apr 3;1082(3):293–302. doi: 10.1016/0005-2760(91)90205-v. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Meinkoth J. L., Kimmel A. R. Northern and Southern blots. Methods Enzymol. 1987;152:572–581. doi: 10.1016/0076-6879(87)52064-x. [DOI] [PubMed] [Google Scholar]
- Wells I. C., Peitzmeier G., Vincent J. K. Lecithin: cholesterol acyltransferase and lysolecithin in coronary atherosclerosis. Exp Mol Pathol. 1986 Dec;45(3):303–310. doi: 10.1016/0014-4800(86)90019-5. [DOI] [PubMed] [Google Scholar]