Abstract
Background
Posterior reversible encephalopathy syndrome (PRES) leads to small- and large-vessel circulatory dysfunction. While aggressive lowering of elevated blood pressure is the usual treatment for PRES, excessive blood pressure reduction may lead to ischemia or infarction, particularly when PRES is accompanied by reversible cerebral vasoconstriction syndrome (RCVS). Regional cerebral oximetry using near-infrared spectroscopy is a noninvasive modality that is commonly used intraoperatively and in intensive care settings to monitor regional cerebral oxygenation (rSO2) and may be useful in guiding treatment in select cases of PRES and RCVS.
Results
We report a case of a patient with PRES complicated by infarction and RCVS where the optimal blood pressure management was unclear. A decision was made to decrease blood pressure which resulted in an improved neurological examination and increase in rSO2 from 40% to 55% in at-risk brain. Infarcted brain as determined by diffusion-weighted magnetic resonance imaging and computed tomography perfusion imaging showed no change in rSO2 during the same time period. Furthermore, there was a qualitative change in the rSO2–mean arterial pressure (MAP) relationship, suggesting an alteration in cerebrovascular autoregulation as a result of lowering blood pressure.
Conclusions
Regional cerebral oximetry can provide valuable diagnostic feedback in complicated cases of PRES and RCVS.
Keywords: brain injury, brain ischemia, hemodynamic monitoring, neurocritical care
Introduction
The posterior reversible encephalopathy syndrome (PRES) is a radiographic and clinical syndrome variably comprised of headache, visual changes, altered mental status, and seizures with characteristic white matter fluid-attenuated inversion recovery (FLAIR) changes on magnetic resonance imaging (MRI) or hypodensity on computed tomography (CT).1 Reversible vasoconstriction has also been described as a component of the syndrome, but there is ongoing debate about whether it represents (1) a component of PRES2; (2) a separate entity from PRES representing the reversible cerebral vasoconstriction syndrome (RCVS), characterized by severe headache and reversible vasospasm3; or (3) a manifestation of a continuum between the diagnoses of PRES and RCVS.4 The semantics are difficult to parse with our current level of knowledge; hence, it is important to be cognizant that the phenomena approximated by the syndromes likely exist on a spectrum of disease and that it is frequently difficult to fully eliminate one or the other diagnosis from the differential. Both PRES and RCVS have been associated with several conditions, notably hypertension, renal failure, immunosuppression, and eclampsia/preeclampsia, and have been rarely associated with blood transfusions.5–7 Although PRES and RCVS usually carry a favorable prognosis, in a minority of cases, they can lead to cerebral infarction and irreversible symptoms. Importantly, treatment of PRES is focused on gradual lowering of blood pressure in cases where hypertension is a prominent factor, whereas the treatment of RCVS typically aims to enhance or maintain high blood pressure. Therefore, the management strategy in cases where there is simultaneous PRES and RCVS presents a serious dilemma. In such instances, real-time measurement of cerebral oxygenation could be helpful in guiding management.
Real-time regional cerebral oximetry using noninvasive near-infrared spectroscopy (NIRS) has been approved by the Food and Drug Administration (FDA) since the 1990s and applied mostly in cardiac and carotid endarterectomy procedures.8 More recently, the technology has been used in the intensive care unit (ICU) setting to follow conditions such as subarachnoid hemorrhage, traumatic brain injury, and postresuscitation cardiac arrest.9,10 The following report describes a novel use of cerebral oximetry to follow the course of a patient with concomitant PRES and RCVS.
Case Description
A 58-year-old woman presented to an outside hospital after being found unresponsive at home. Five days prior to admission, she received 4 units of packed red blood cells for a long-standing history of eosinophilic cystitis with chronic iron deficiency anemia. She developed a headache the day prior to admission. There was no history of hypertension. On initial presentation, she had a normal blood pressure and expressive aphasia. The MRI revealed bilateral occipitoparietal and left temporal FLAIR hyperintensities without restricted diffusion. She developed seizures as manifested by alteration in mental status and was treated with valproic acid and levetiracetam. A repeat MRI on hospital day (HD) #7 revealed worsening FLAIR hyperintensities, and she was transferred to the Columbia neurological ICU (NICU) for further management.
On arrival to the NICU, the patient was afebrile; the blood pressure was 150/80; the heart rate was 96; and she was oriented to person, place, and time. She was unable to count fingers but had intact light perception. There was a left pronator drift, but she otherwise had full strength of all of her extremities. Her hemoglobin concentration was 10.1 g/dL and the rest of her routine laboratory test results and CSF were unremarkable.
Overnight, she had occipital lobe seizures captured on electroencephalogram (EEG), and the levetiracetam dose was increased. Her vision improved to finger counting, but the examination was otherwise unchanged. On HD #9, she was empirically started on intravenous steroids due to a concern for an autoimmune or vasculitic process. On HD #10, a brain biopsy from the left occipital lobe was performed, which revealed a nonspecific chronic small-vessel vasculopathy with multifocal perivascular acute hypoxic–ischemic injury of the cortex and white matter. There was no evidence of vasculitic, infectious, demyelinating, or neoplastic processes.
The patient’s systolic blood pressure (SBP) was maintained at 140–160s. She was more awake on examination and was transferred to a floor bed on HD #12. A follow-up MRI was largely unchanged. On HD #17, the patient decompensated by becoming lethargic and quadriparetic. Her SBP was 170–190 during this time. A repeat MRI was performed which showed interval development of restricted diffusion in the occipital lobes (Figure 1). Magnetic resonance angiography (MRA) showed bilateral anterior and middle cerebral artery and left vertebral artery stenosis. Later in the day, the patient progressed to stupor, stopped following commands, and was readmitted to the NICU for closer monitoring. An arterial line was placed revealing an SBP of 200. A repeat lumbar puncture (LP) was unremarkable. On HD #18, an angiogram was performed which showed vasospasm in all intracranial distributions (Figure 2). Transcranial Doppler measurements demonstrated mild-to-moderate increased velocities in anterior distributions. A repeat EEG was negative for seizures.
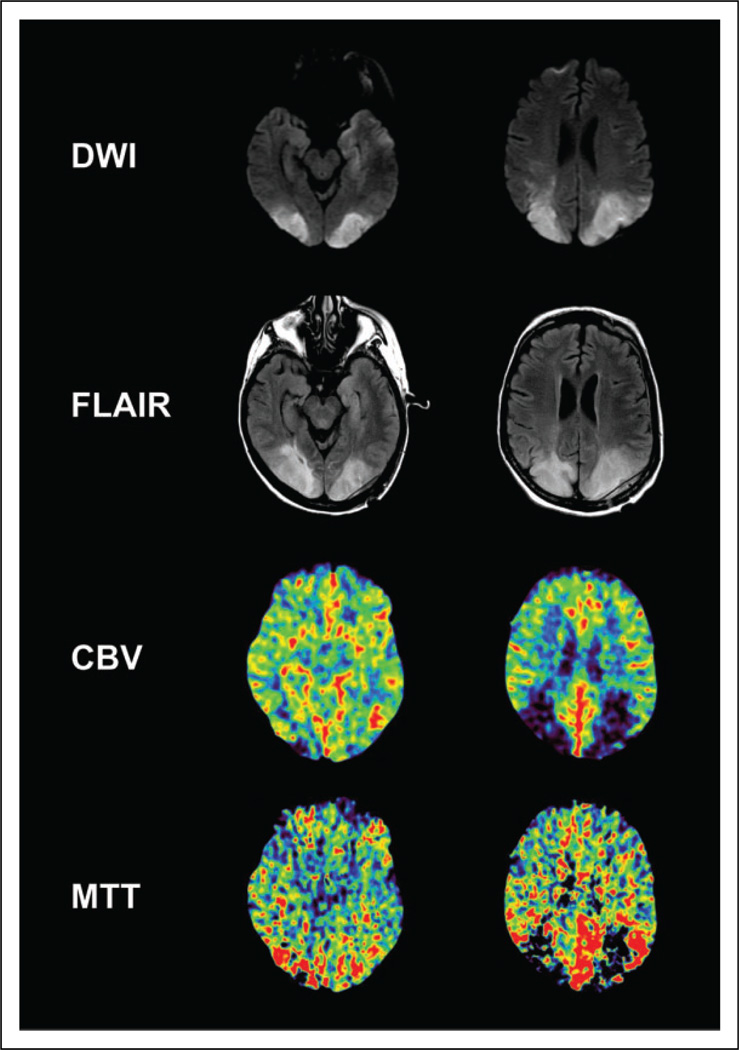
Figure 1.
Magnetic resonance imaging (MRI) and computed tomography (CT) perfusion images of patient after her clinical decompensation on hospital day 17 and 22, respectively. There is an apparent diffusion coefficient (ADC) correlate to the diffusion-weighted imaging (DWI) signal (ADC map not shown). Low axial images on the left. High axial images on the right.
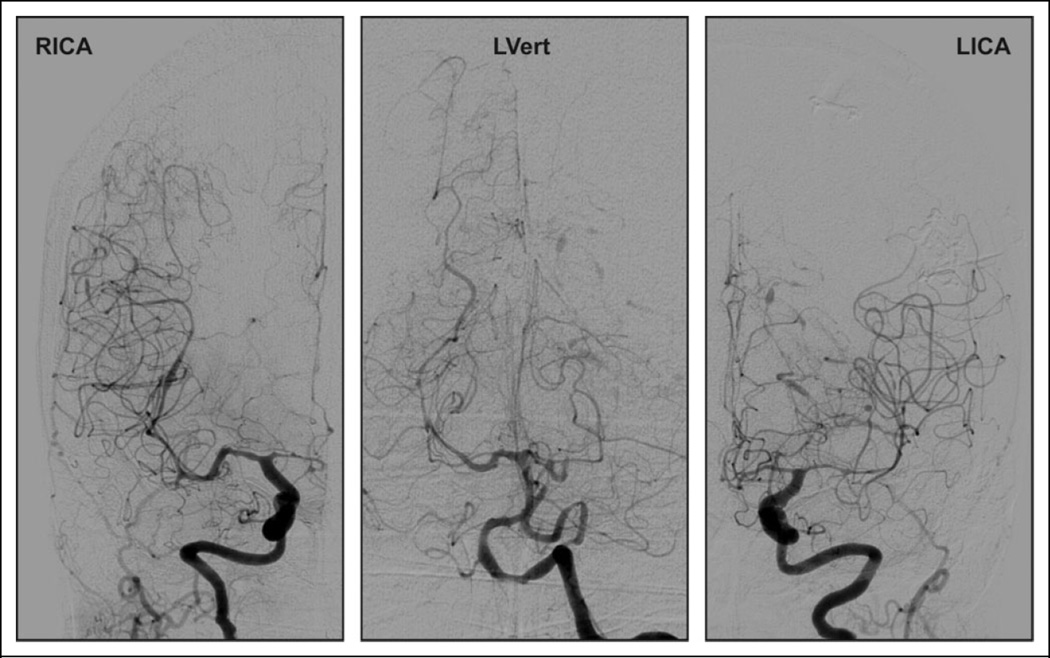
Figure 2.
Conventional cerebral angiography on hospital day 18. A follow-up angiogram on hospital day 24 was largely unchanged.
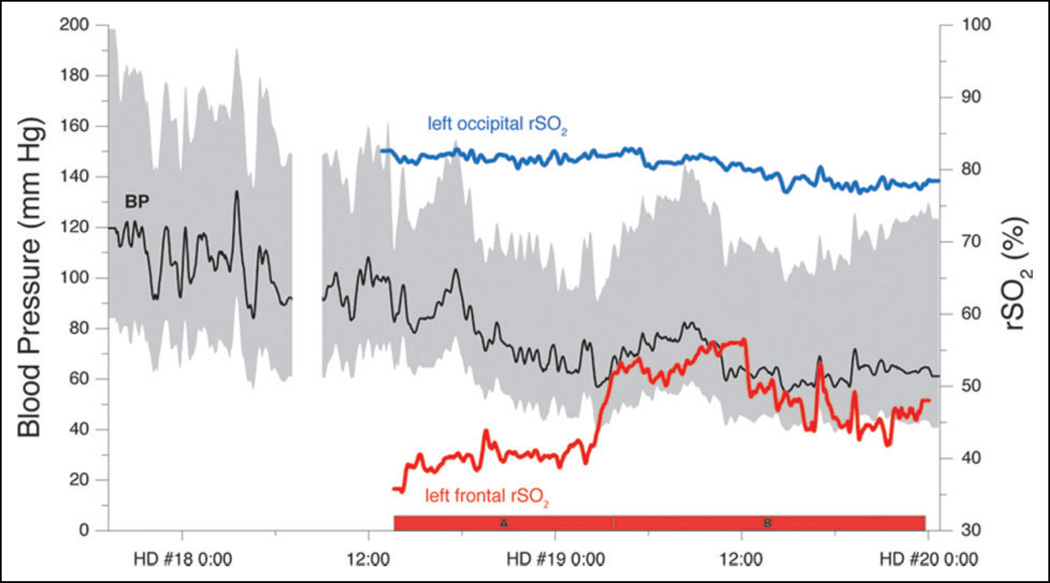
The patient was diagnosed with PRES. Given the presence of vasospasm and simultaneous RCVS, it was unclear whether to raise, lower, or maintain the blood pressure. Therefore, regional cerebral oximeters (Nonin EQUANOX) were placed over the left frontal and left occipital lobes, while the patient’s blood pressure was gradually lowered. Of note, the patient was maintained on room air throughout. The lowering of blood pressure was associated with an increase in regional cerebral oxygenation (rSO2) over the frontal oximeter from 40% to 55% (Figure 3) as well as an improvement in examination. The patient started speaking and moving her upper extremities weakly. In contrast, the occipital rSO2 was ~80% and did not vary with blood pressure for the duration of the measurements, consistent with her known cerebral infarction. Over the remainder of the patient’s hospital course, her neurological course stabilized. She continued to make a steady recovery and was discharged to a rehabilitation facility with a mental status near baseline and cortical blindness.
Figure 3.
Time course of arterial blood pressure and regional cerebral oxygenation (rSO2). The gap in blood pressure data represents the time at the first cerebral angiogram. The red bars, A and B, indicate the time range from which the data for Figure 4A and B were obtained. The upper and lower bounds of gray area represent systolic and diastolic blood pressure. The black line represents MAP.
Methods
Magnetic resonance images were obtained with a GE Signa HDxt 3.0 T scanner (GE Healthcare, USA). The CT perfusion images were obtained with a Toshiba Aquilion ONE volume CT scanner (Toshiba America Medical Systems, Inc., CA, USA) and processed using Vitrea fX 6.1 software. All blood pressure measurements were obtained from a radial arterial line. The patient was spontaneously breathing room air. Regional cerebral oximetry measurements (rSO2) were taken using the Nonin EQUANOX 7600, sensor model 8004ca (Nonin Medical, Inc., USA).11 Leads were applied after cleaning the skin and in the case of the occipital sensor shaving the area prior to application. Data collection was obtained using a centralized server and compiled with ICUpilot v2.0 (CMA/Microdialysis, Sweden). Data analysis including smoothing and chart generation was performed using Origin 7 (OriginLab, Northampton, Massachusetts).
Discussion
We describe a patient who developed a severe case of PRES that led to development of RCVS and infarction of her occipital lobes. Her presentation presents a clinical conundrum: Do we increase, maintain, or lower the blood pressure? The usual treatment for PRES in the setting of hypertension is to gradually lower the blood pressure; however, in the setting of RCVS, an argument could be made to maintain or elevate blood pressure. In addition to the clinical examination, we posited that rSO2 could help inform whether we made the appropriate choice and guide subsequent decision making.
Near-infrared spectroscopy for brain oxygen saturation monitoring initially found use in the operating room with cardiac bypass and carotid endarterectomy cases.9 Subsequent randomized controlled trials have shown an association between active use of NIRS and fewer episodes of cerebral hypoxic events in abdominal and cardiac surgery.12,13 As such, NIRS is often part of routine anesthesia monitoring during cardiac cases at many institutions. More recently, cerebral oximetry has been applied in the ICU to patients who were pericardiac arrest and resuscitated,10 to patients with traumatic brain injury,14–16 and for the detection of vasospasm after aneurysmal subarachnoid hemorrhage17,18; however, NIRS has not found widespread use in the ICU due to limited supportive outcomes data.9
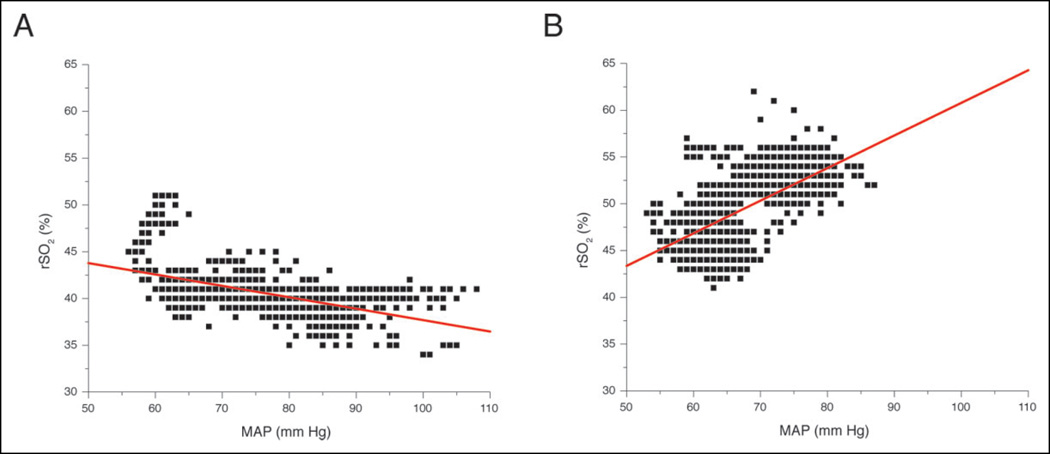
To our knowledge, there has been no prior report of the use of NIRS in the setting of PRES or RCVS. We found that decreasing blood pressure was associated with an improvement in rSO2 and clinical examination. A prior study of NIRS in a population of patients with traumatic brain injury determined that a cutoff rSO2 ≤60% was moderately accurate for detecting severe intracerebral hypoxemia using the invasive LICOX brain tissue monitor (Integra LifeSciences, USA) as a standard.15 In our patient, the rSO2 prior to lowering blood pressure was 40% in viable, at-risk brain tissue (i.e., left frontal lobe). After the blood pressure was lowered, the rSO2 improved to 55%, and the patient’s examination improved, suggesting that intracerebral hypoxemia was symptomatic, reversible, and detectable with noninvasive methods. Furthermore, the rSO2–MAP relationship was reversed indicating a qualitative change in cerebral autoregulation (Figure 4). Notably, in infarcted brain tissue (ie, left occipital lobe), we found a high, fixed rSO2 that did not vary with blood pressure changes—consistent with prior reports in the literature concerning high, invariable rSO2 in dead brain.19 Our case provides proof of principle that noninvasive regional cerebral oximetry can provide clinically relevant information, given the proper context and clinical question.
Figure 4.
Left frontal regional cerebral oxygenation (rSO2) versus MAP (A) before and (B) after clinical improvement. rSO2 in integer values. Red lines are linear regressions.
As for why the patient developed her symptoms, transfusion-associated PRES and RCVS have been described previously in the literature and can be seen in normotensive patients.6 The patient initially stabilized before decompensating and re-presenting to our NICU. It is not entirely clear why she had a second episode. One possibility is that her blood pressure was not adequately controlled on the floor, leading to hypertension-associated PRES.
There are multiple limitations to our report. First, it involves a single patient and suffers from selection bias inherent to all case reports. Second, only one area of at-risk brain was monitored. It would have been ideal to have readings from an additional area of viable brain to validate the assumption that a global ischemic process was taking place, but we were limited to 2 sensors in this particular instance. Third, there is a lack of other objective information such as serial magnetic resonance or CT perfusion, positron emission tomography, microdialysis, invasive parenchymal oxygen monitoring, or jugular venous bulb oximetry to validate our results. Fourth, we observed a qualitative change in autoregulation which was also not validated using established methods such as pressure reactivity index or CO2 reactivity analysis. Many of these limitations could be addressed in future studies. We hope that after reporting this index case, other groups will be encouraged to validate this promising approach.
In conclusion, NIRS has the potential to be used in cases of PRES and RCVS when optimal blood pressure management is unclear. This case presented the dilemma of concomitant PRES, RCVS, hypertension, and infarction. It was not clear whether to increase, maintain, or lower the patient’s blood pressure. In our patient, the improvement in cerebral oxygenation was associated with improvement in clinical examination. In subsequent cases, this modality could be applied in comatose or sedated patients in which following a clinical examination is less informative and where more invasive monitoring is not feasible or available. Further use of NIRS in the setting of PRES and RCVS is extremely promising, as it supplements interval measures such as MRI, CT, or transcranial Doppler by providing valuable real-time, continuous feedback on neurovascular physiology.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 4.Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005–1012. doi: 10.1001/archneurol.2011.68. [DOI] [PubMed] [Google Scholar]
- 5.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito Y, Niwa H, Iida T, et al. Post-transfusion reversible posterior leukoencephalopathy syndrome with cerebral vasoconstriction. Neurology. 1997;49(4):1174–1175. doi: 10.1212/wnl.49.4.1174. [DOI] [PubMed] [Google Scholar]
- 7.Mehdi A, Hajj-Ali RA. Reversible cerebral vasoconstriction syndrome: a comprehensive update. Curr Pain Headache Rep. 2014;18(9):443. doi: 10.1007/s11916-014-0443-2. [DOI] [PubMed] [Google Scholar]
- 8.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(suppl 1):i3–i13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh A, Elwell C, Smith M. Review article: cerebral near-infrared spectroscopy in adults: a work in progress. Anesth Analg. 2012;115(6):1373–1383. doi: 10.1213/ANE.0b013e31826dd6a6. [DOI] [PubMed] [Google Scholar]
- 10.Teschendorf P. Non-invasive monitoring and cardiac arrest: a cautious attempt to view beyond the curtain (skin) Resuscitation. 2012;83(8):926–927. doi: 10.1016/j.resuscitation.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 11.MacLeod DB, Ikeda K, Vacchiano C, Lobbestael A, Wahr JA, Shaw AD. Development and validation of a cerebral oximeter capable of absolute accuracy. J Cardiothorac Vasc Anesth. 2012;26(6):1007–1014. doi: 10.1053/j.jvca.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Casati A, Fanelli G, Pietropaoli P, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101(3):740–747. doi: 10.1213/01.ane.0000166974.96219.cd. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104(1):51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 14.Dunham CM, Ransom KJ, Flowers LL, Siegal JD, Kohli CM. Cerebral hypoxia in severely brain-injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. J Trauma. 2004;56(3):482–489. doi: 10.1097/01.ta.0000114537.52540.95. discussion 9-91. [DOI] [PubMed] [Google Scholar]
- 15.Leal-Noval SR, Cayuela A, Arellano-Orden V, et al. Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med. 2010;36(8):1309–1317. doi: 10.1007/s00134-010-1920-7. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal G, Furmanov A, Itshayek E, Shoshan Y, Singh V. Assessment of a noninvasive cerebral oxygenation monitor in patients with severe traumatic brain injury. J Neurosurg. 2014;120(4):901–907. doi: 10.3171/2013.12.JNS131089. [DOI] [PubMed] [Google Scholar]
- 17.Yokose N, Sakatani K, Murata Y, et al. Bedside monitoring of cerebral blood oxygenation and hemodynamics after aneurysmal subarachnoid hemorrhage by quantitative time-resolved near-infrared spectroscopy. World Neurosurg. 2010;73(5):508–513. doi: 10.1016/j.wneu.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 18.Zweifel C, Castellani G, Czosnyka M, et al. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41(9):1963–1968. doi: 10.1161/STROKEAHA.109.577320. [DOI] [PubMed] [Google Scholar]
- 19.Nemoto EM, Yonas H, Kassam A. Clinical experience with cerebral oximetry in stroke and cardiac arrest. Crit Care Med. 2000;28(4):1052–1054. doi: 10.1097/00003246-200004000-00023. [DOI] [PubMed] [Google Scholar]