Abstract
Importance
This study provides the first data showing that rural African Americans' participation, during childhood at age 11, in a preventive intervention designed to enhance supportive parenting ameliorates the association of poverty with hippocampal and amygdalar volumes during adulthood at age 25.
Objective
To determine whether participation in an efficacious prevention program designed to enhance supportive parenting for rural African American children will ameliorate the association between living in poverty and reduced hippocampal and amygdalar volumes that are evident at age 25.
Design, Setting, and Participants
In the rural southeastern United States, African American parents and their 11-year-old children were assigned randomly to the Strong African American Families (SAAF) program or to a control condition. Parents provided data used to calculate income-to-needs ratios when children were 11 to 13 and 16 to 18 years of age. When the children were 25 years of age, hippocampal and amygdalar volumes were measured using magnetic resonance imaging.
Exposure
Household poverty was measured by income-to-needs ratios.
Main Outcomes and Measures
Young adults' whole hippocampal, dentate gyrus and CA3 hippocampal subfields, and amygdalar volumes were assessed using magnetic resonance imaging.
Results
Years lived in poverty across ages 11 to 18 years forecast diminished left dentate gyrus and CA3 hippocampal subfields and left amygdalar volumes among young adults in the control condition but not among those who participated in SAAF.
Conclusions and Relevance
Studies suggest that supportive parenting may offset risks to brain development posed by exposure to poverty during childhood and adolescence. Prior to this study, it was less clear whether these findings reflected a causal process. In this study, we described how participation in a randomized, controlled trial designed to enhance supportive parenting ameliorated the association of years lived in poverty with left dentate gyrus and CA3 hippocampal subfields and left amygdalar volumes. These findings are consistent with a causal role for supportive parenting and suggest a strategy for narrowing social disparities.
More than one in five children in the United States lives in poverty.1 Poverty and other markers of disadvantage are powerful variables that forecast developmental trajectories, including cognitive development,2 psychosocial development,3 and physical health4 throughout life. As interest in the effects of poverty and disadvantage has surged in the pediatric research community, a parallel literature has been developing in which scientists have begun to investigate the possibility that growing up in poverty, where stressors are common and resources are scarce, will have implications for the maturation of the hippocampus and amygdala. These temporal lobe structures contribute to various facets of academic functioning and social development, and they support learning, memory, mood, and stress reactivity.5 Despite the importance of the hippocampus and amygdala to mental and physical health across the lifespan,6 little is actually known about the ways in which childhood exposure to poverty is associated with their development.
Reports from initial investigations show diminished hippocampal volumes among children living in poverty.7–12 These reports mirror findings from animal models, which show hippocampal cell proliferation and neurogenesis to be greater among mice and rats reared in stimulating environments compared with those reared in relative deprivation.13 Results are less clear for the amygdala. Child poverty has been found to be associated with both larger11 and smaller9,10 amygdalar volumes, and findings from animal models typically show associations between exposure to chronic stress and larger amygdalar volumes.14 Taken together, these findings underscore the need for further investigations of the association between children's exposure to poverty across time and the development of the hippocampus and amygdala. The current study was designed to address this issue using a longitudinal, prospective design to determine whether duration of life in poverty across ages 11 to 18 years would be associated with whole and subfield hippocampal volumes and amygdalar volumes at age 25. Translational studies show that the key consequences for the hippocampus of stress exposure are suppression of neurogenesis in the dentate gyrus and dendritic remodeling in the CA3 subfield.15,16 In children, exposure to stress is also associated with smaller volumes in these subfields.17,18 Thus, this study examined the hypothesis that exposure to poverty would be associated with smaller volumes in the whole hippocampus, the dentate gyrus and CA3 hippocampal subfields, and the amygdala.
Not all children and adolescents who grow up in poverty, however, experience adverse consequences. Recent research suggests that a subset of youths who receive supportive parenting develop resilience to the consequences of poverty and low-SES environments. Studies show that parenting that includes high levels of warmth, sensitivity, and emotional support can offset many of the psychosocial disadvantages that beset children in poverty.19,20 Mounting evidence also reveals that supportive parenting can favorably mold stress-response tendencies among vulnerable children.21 In fact, supportive parenting may help to mitigate some of the hormonal, metabolic, and cardiovascular changes that follow childhood adversity. In particular, supportive parenting buffers the effects of poverty on adolescents' allostatic load, a measure of cardiometabolic risk.22 Such parenting also buffers the effects of low childhood SES on proinflammatory signaling profiles23 and metabolic profiles in adulthood.24 Similarly, the benefits of supportive parenting may extend to hippocampal and amygdalar development, as vividly illustrated in a recent series of studies.25–27 Among children reared in poverty, those who received supportive parenting had larger hippocampal volumes than did those who received parenting that was not as supportive.
These are important findings. To the extent that they reflect a causal process in which supportive parenting offsets some of the risks to brain maturation associated with poverty, they have implications for numerous pediatric research domains, including those focused on social disparities and resilience to adversity.28 Causal inferences, however, cannot easily be made on the basis of existing studies because their observational designs are prone to residual confounding and reverse causal influences. Here, we avoid those problems by conducting secondary analyses of data from the Strong African American Families (SAAF) randomized, controlled trial.19 SAAF was designed to mitigate the negative impact of life stress on rural African American youths by increasing supportive parenting processes.29 SAAF has demonstrated stress-buffering capacities for a range of psychosocial outcomes such as self-control, drug use, and conduct problems.30 It also has favorable effects on several health-relevant biological processes—including inflammation, catecholamine levels, telomere lengths, and epigenetic aging31—all of which could, in turn, influence patterns of brain development.5 Accordingly, in this study we tested the hypothesis that the cumulative number of years during which African American youths lived in poverty across preadolescence and adolescence would be associated with diminished volumes in the whole hippocampus, the dentate gyrus and CA3 hippocampal subfields, and the amygdala among young adults who had been randomly assigned to the control condition, but not young adults who had been assigned to the SAAF condition.
Summary of this Study
In summary, in this study analyses were performed on data gathered from rural African American youths and their primary caregivers, who had taken part in the SAAF randomized prevention trial when the youths were 11 years of age. When youths were 11 to 13 and 16 to 18 years of age, caregivers provided data that were used to calculate income-to-needs ratios. T-1 weighted MRI data were obtained when the participants were 25 years of age to determine the volumes of their whole hippocampi, dentate gyrus and CA3 hippocampal subfields, and amygdalae.
Methods
Participants
A total of 119 right-handed rural African Americans, age 25 years, were recruited from the 667 participants in the SAAF randomized prevention trial. The SAAF sample was recruited randomly from rural Georgia communities when the participants were 11 years of age (mean age at pretest = 11.2 years, SD = 0.34; see Brody et al.29 for details). At pretest, the SAAF sample could be characterized as working poor; primary caregivers worked an average of 39.4 hours per week, yet 46.3% of the sample lived below federal poverty standards. The age 25 data collection included 408 participants from the original SAAF sample, a retention rate of 60% across 14 years. Random selection of the subsample of 119 participants to take part in a neuroimaging session was made necessary by financial constraints associated with imaging. The subsample was selected randomly from a list of the 408 participants in the age 25 assessment until the targeted sample size was reached. All participants included in the subsample were screened for standard imaging contraindications and right-handedness prior to enrollment. Three participants were excluded due to excess motion (see the Image Analyses section). The remaining 116 participants were included in the analyses. At age 11, 59 of these participants had been assigned randomly to the SAAF condition and 57 had been assigned randomly to the control condition. A two-factor multivariate analysis of variance was executed to evaluate the equivalence of the demographic and study variables for participants who did or did not take part in the imaging study at age 25 by prevention group assignment at age 11. No significant main or interaction effects emerged (see Table 1). The University of Georgia's Institutional Review Board reviewed and approved all study procedures, and all participants provided written informed consent.
Table 1.
Characteristics of Subjects with and without Brain Imaging Data
| With Brain Imaging Data |
Without Brain Imaging Data |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAAF | Control | SAAF | Control | |||||||
|
|
|
|
|
|||||||
| Age 11 | M | SD | M | SD | M | SD | M | SD | F | p |
|
|
|
|
|
|
||||||
| (n = 59) | (n = 57) | (n = 310) | (n = 241) | F (1,663) | ||||||
| Gender, male | 0.46 | 0.50 | 0.51 | 0.50 | 0.46 | 0.50 | 0.48 | 0.50 | 0.092 | .761 |
| Parent age | 37.15 | 8.70 | 37.25 | 5.91 | 37.81 | 7.59 | 37.89 | 7.78 | 0.000 | .993 |
| Family poverty | 0.47 | 0.50 | 0.37 | 0.49 | 0.39 | 0.49 | 0.34 | 0.48 | 0.329 | .566 |
| (n = 59) | (n = 57) | (n = 307) | (n = 241) | F (1,660) | ||||||
| Parent education | 4.53 | 1.17 | 4.61 | 1.13 | 4.57 | 1.33 | 4.73 | 1.50 | 0.080 | .777 |
| (n = 59) | (n = 57) | (n = 308) | (n = 241) | F (1,661) | ||||||
| Parent unemployment | 0.24 | 0.43 | 0.11 | 0.31 | 0.24 | 0.43 | 0.22 | 0.41 | 1.614 | .204 |
| (n = 58) | (n = 57) | (n = 305) | (n = 241) | F (1,657) | ||||||
| Single-parent family | 0.71 | 0.46 | 0.58 | 0.50 | 0.55 | 0.50 | 0.54 | 0.50 | 1.150 | .284 |
| (n = 59) | (n = 310) | t(367) | ||||||||
| Intervention sessions | 5.02 | 2.47 | - | - | 4.64 | 2.63 | - | - | –1.022 | .307 |
| (n = 59) | (n = 57) | (n = 310) | (n = 239) | F (1,661) | ||||||
| Family poverty (ages 11–18) | 2.61 | 1.77 | 2.04 | 1.88 | 2.20 | 1.85 | 2.03 | 1.87 | 1.177 | .278 |
SAAF Intervention Implementation
The SAAF prevention program consisted of seven consecutive, 2-hour weekly meetings held at community facilities, with separate skill-building curricula for youths and for their primary caregivers, and a family curriculum. Caregivers in the prevention condition were taught the consistent provision of instrumental and emotional support, high levels of monitoring and control, adaptive racial socialization strategies, and methods for communicating about sex and alcohol use. Youths learned the importance of forming goals for the future and making plans to attain them, resistance efficacy skills, and adaptive behaviors to use when encountering racism.
Measures
Family Poverty
When participants were 11 to 13 and 16 to 18 years of age, caregivers provided data on their families' income-to-needs ratios, based on family size, that were used to compute household poverty. Poverty statuses at six assessment waves were summed to determine the number of years living below federal poverty guidelines (M = 2.30, SD = 1.83).
Intervention Status and Gender
Intervention status and gender were coded as follows: SAAF participants were coded 1 and control participants were coded 0; male participants were coded 1 and female participants were coded 0.
Psychosocial Variables
At age 25, participants reported their past-month frequencies of cigarette smoking and alcohol use. The response sets ranged from 0 (not at all) to 6 (about two packs a day) for cigarette smoking, and from 0 (none) to 5 (20 or more days) for alcohol use. Because the distributions for smoking and alcohol use were skewed, a log transformation was applied to normalize the ratings. Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies Depression scale.32 Consistent with psychometric studies of the CES–D, 16 was used as the cutoff score to identify clinically significant depression.
MRI Acquisition
Imaging data were collected using a GE Signa HDx 3-Tesla scanner at the University of Georgia's Bio-Imaging Research Center. Structural imaging consisted of a high-resolution T1-weighted, fast spoiled gradient echo scan (repetition time [TR] = 7.8 ms, echo time [TE] = 3.1 ms, flip angle = 20°; field of view [FOV] = 25.6 cm, matrix = 256 × 256, 160 contiguous 1 mm axial slices, voxel size = 1 mm3).
Image Analysis
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer 5.3 image analysis suite, which is documented and freely available online for download (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer morphometric procedures have demonstrated good test-retest reliability across scanner manufacturers and field strengths.33,34 The standard FreeSurfer pipeline (discussed in detail in prior publications, e.g., Reuter et al.34) was utilized to process the MRI data and, specifically, to derive intracranial volume (ICV) and amygdalar volumes for use in this study. Hippocampal subfield segmentation was derived using the new automated algorithm available in FreeSurfer 6.0.35 This method utilizes a refined probabilistic atlas built upon a combination of manual annotations of the hippocampal subregions from 15 ultra-high resolution, ex vivo images and of the neighboring subcortical structures from an independent data set of 39 in-vivo, T1-weighted, 1mm resolution MRI scans. Using Bayesian inference, the constructed atlas is used to segment automatically the hippocampal subregions. Recently published research36 found the new segmentation procedure to have a high degree of test-retest and transplatform reliability across scanning modalities (1.5T vs. 3T scanners). Although this software enables isolation of CA4 and the granule cell layer of the dentate gyrus (GCL), these two subdivisions were combined in this study because they are both components of the dentate gyrus and the ability to distinguish the molecular layer in T1-weighted images is limited.35 The other regions quantified in this study, CA2/CA3, were combined because of a lack of distinguishing T1 weighted MRI contrast. Results were blindly reviewed for surface quality, a process with well-established reliability.37
Results
Family Poverty, the Hippocampi, and the Amygdalae
Our initial analysis was designed to determine whether family poverty was associated with hippocampal and amygdalar volumes among young adults in the control condition. Presumably, young adults in the control condition display normative associations between family poverty and the volumes of the hippocampi and amygdalae. Adjusting for gender and ICV, Table 2 shows the results of partial correlations between family poverty across time and the brain volumes of interest. Consistent with the first study hypothesis, control participants who spent more time in poverty evinced smaller left amygdalae, left CA2/CA3, and left CA4/GCL than did young adults who spent less time in poverty.
Table 2.
Descriptive Statistics and Correlations among Study Variables for Control andSAAF Groups
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| M | 2.61 | 1250.02 | 1318.27 | 3413.38 | 3391.39 | 215.12 | 224.46 | 555.88 | 560.86 |
| SD | 1.77 | 234.33 | 189.87 | 417.00 | 373.68 | 35.83 | 30.94 | 78.12 | 68.40 |
|
| |||||||||
| 1. Family poverty (ages 11–18) | -- | .038 | −.103 | .040 | −.015 | −.042 | −.133 | −.031 | −.073 |
| 2. Left amygdalar volume (age 25) | −.296* | -- | .399** | .530*** | .288* | .426** | .346* | .548*** | .357** |
| 3. Right amygdalar volume (age 25) | −.009 | .335* | -- | .317* | .649*** | .144 | .639*** | .154 | .694*** |
| 4. Left hippocampal volume (age 25) | −.233 | .393** | .308* | -- | .519*** | .777*** | .454*** | .891*** | .502*** |
| 5. Right hippocampal volume (age 25) | −.145 | .119 | .467*** | .573*** | -- | .275* | .804*** | .296* | .925*** |
| 6. Left CA2/CA3 volume (age 25) | −.302* | .440** | .223 | .884*** | .379* | -- | .416** | .920*** | .369** |
| 7. Right CA2/CA3 volume (age 25) | −.170 | .116 | .429** | .499*** | .793*** | .533*** | -- | .340* | .916*** |
| 8. Left CA4/GCL volume (age 25) | −.300* | .489*** | .189 | .936*** | .365** | .960*** | .433** | -- | .366** |
| 9. Right CA4/GCL volume (age 25) | −.119 | .089 | .471*** | .568*** | .909*** | .483*** | .928*** | .431*** | -- |
|
| |||||||||
| M | 2.04 | 1233.68 | 1367.06 | 3460.78 | 3460.69 | 215.49 | 229.84 | 552.05 | 571.33 |
| SD | 1.88 | 215.02 | 198.35 | 383.92 | 316.98 | 38.86 | 31.76 | 85.99 | 63.31 |
Correlations are partial correlations controlling for gender and intracranial volume. Upper diagonal: descriptive statistics and correlations for SAAF group (n = 58 for left and right amygdala and right hippocampal, CA2/CA3, CA4/GCL; n = 59 for left hippocampal, CA2/CA3, CA4/GCL); lower diagonal: descriptive statistics and correlations for control group (n = 57).
p < .05.
p < .01.
p < .001.
Participation in SAAF, Family Poverty, and Volumes of the Hippocampi and Amygdalae
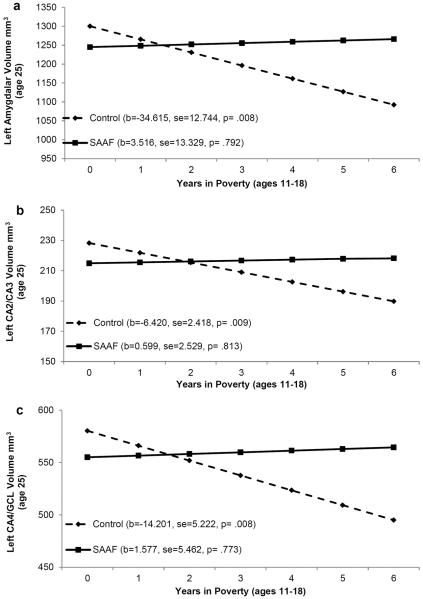
Next, we tested the hypothesis that participation in SAAF would ameliorate the association of family poverty with the left amygdalar, left CA2/CA3, and left CA4/GCL volumes. To do this, we executed hierarchical multiple regression analyses that included main effects for family poverty, prevention status (SAAF = 1, control = 0), and the interaction of family poverty with prevention status. In all models, gender and ICV were controlled. Interactions were interpreted through the plotting of estimated levels of hippocampal and amygdalar volumes by years in poverty and prevention status. The results of these analyses are presented in Table 3. A main effect for family poverty and an interaction effect between family poverty and SAAF participation were found. These interactions are depicted in Figure 1, panels a, b, and c. More time spent living in family poverty from ages 11 to 18 years was associated with a smaller left amygdala volume (simple-slope = −34.615, SE = 12.744, p = .008), a smaller left CA2/CA3 volume (simple-slope = −6.420, SE = 2.418, p = .009), and a smaller left CA4/GCL volume (simple-slope = −14.201, SE = 5.222, p = .008) among participants from the control condition. Family poverty was not associated with the volume of these regions among participants from the SAAF condition (simple-slope = 3.516, SE = 13.329, p = .79 for the left amygdala; simple-slope = 0.599, SE = 2.529, p = .81 for the left CA2/CA3; and simple-slope = 1.577, SE = 5.462, p = .77 for the left CA4/GCL).
Table 3.
Family Poverty and Intervention as Predictors of Left Amygdalar, CA2/CA3, and CA4/GCL Volume at Age 25
| Left Amygdalar Volume | Left CA2/CA3 Volume | Left CA4/GCL Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Predictors | B | SE | β | B | SE | β | B | SE | β |
| 1. Gender (male) | 114.129 | 47.553 | .256* | 2.524 | 9.021 | .034 | 6.711 | 19.485 | .042 |
| 2. Intracranial volume (age 25) | 90.672 | 23.256 | .406*** | 13.190 | 4.412 | .359** | 30.976 | 9.529 | .384** |
| 3. Intervention, SAAF (age 11) | 32.764 | 33.041 | .073 | 2.802 | 6.268 | .038 | 11.091 | 13.539 | .069 |
| 4. Family poverty (ages 11–18) | −34.615 | 12.743 | −.283** | −6.420 | 2.418 | −.319** | −14.201 | 5.222 | −.321** |
| 7. SAAF × poverty | 38.131 | 18.704 | .213* | 7.019 | 3.548 | .238* | 15.778 | 7.664 | .244* |
p < .05.
p < .01.
p < .001.
Figure 1.
The effect of family poverty on youths' left amygdalar volume (a), left CA2/CA3 volume (b), and left CA4/GCL volume (c) by intervention status. Numbers in parentheses refer to simple slopes for the control group and the intervention group.
To describe further the years in poverty × SAAF interaction discussed previously, we conducted planned group comparisons to test the hypothesis that adolescents who spent more years living in poverty and were assigned to the control condition would have smaller mean left amygdala volumes and left CA2/CA3 and CA4/GCL volumes than would (a) similar youths assigned to the SAAF condition and (b) youths who spent less time in poverty who were assigned to either the SAAF or the control condition. Table 4 presents the results of these analyses. The patterning of the means for each analysis conformed to the study hypothesis. Youths assigned to the control condition who spent more years during adolescence living in poverty had smaller left amygdalar and left hippocampal subfield volumes than did youths in the other three groups, who did not differ from one another.
Table 4.
Means of Left Amygdalar, CA2/CA3, and CA4/GCL Volume for Family Poverty by Intervention Condition Groups
| Family Poverty × Intervention Condition Groups |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Poverty | Low Poverty | High Poverty | High Poverty | |||||||
| Variables | Control | SAAF | Control | SAAF | ||||||
|
|
|
|
|
|||||||
| M | SE | M | SE | M | SE | M | SE | F | p | |
|
|
|
|
|
|||||||
| (n = 35) | (n = 29) | (n = 22) | (n = 29) | F (1, 109) | p | |||||
| Left Amygdalar Volume | 1273.62 | 30.76 | 1260.92 | 33.70 | 1160.11 | 39.20 | 1246.73 | 33.82 | 5.256 | .024 |
| (n = 35) | (n = 29) | (n = 22) | (n = 30) | F (1, 110) | p | |||||
| Left CA2/CA3 Volume | 223.86 | 5.85 | 217.13 | 6.42 | 201.31 | 7.47 | 213.80 | 6.34 | 4.147 | .044 |
| Left CA4/GCL Volume | 570.22 | 12.69 | 561.10 | 13.91 | 521.07 | 16.18 | 552.35 | 13.74 | 4.952 | .028 |
Note. Low poverty: ≤ 2 years living in poverty; high poverty: ≥ 3 years living in poverty. F tests refer to planned group comparisons of the high-poverty control group vs. the other three groups. All tests controlled for gender and intracranial volume. SE = standard error.
Hippocampal Subfield Volumes, Amygdalar Volumes, and Psychosocial Functioning
To explore the significance of the study findings, we examined contemporaneous associations of the left hippocampal subfield volumes and left amygdalar volume with depression status, smoking, and alcohol use. To do this, we executed partial correlations and adjusted them for gender, ICV, and prevention condition (SAAF or control). A score of 16 or greater on the CES-D was associated with diminished left CA2/CA3 (r = −.245, p = .009) and left CA4/GCL (r = −.215, p = .022) volumes. Smoking was associated with diminished amygdalar volumes (r = −.225, p = .017). No associations with alcohol use were found.
Discussion
Two important findings emerged from this study. First, we confirmed the association between childhood poverty and diminished volume of limbic regions in adulthood. These diminished volumes were significant to important outcomes, as the associations between the hippocampal subfield volumes and depression indicated. Second, we found evidence suggesting that a parenting-focused intervention during early adolescence attenuated associations between poverty and brain development. These results, made possible by the embedding of MRI assessments of hippocampal and amygdalar volumes in a randomized, parenting-focused prevention trial, increases confidence in the causal nature of the linkages between supportive parenting and brain maturation. Observational research25–27 indicated that associations between living in poverty and reductions in hippocampal volume could be offset by supportive parenting processes. This study confirmed and extended those findings by demonstrating for the first time that exposure to prevention programming at age 11 could have lasting protective effects on brain development into adulthood. Of relevance to pediatric clinical practice, efficacious family-centered programs designed to enhance supportive parenting are available for rural African American preadolescents,29 adolescents,30 and young adults.38 Participation in these programs has demonstrated stress-buffering effects on adolescent catecholamine levels, cytokine levels, telomere lengths, epigenetic aging,31 and, as this report demonstrates, on hippocampal subfield and amygdalar volumes.
The sample in this study was underpowered for detecting precise parenting practices that could be responsible for the buffering effects; additional research with larger samples is needed to identify specific mediators. Candidate parenting processes might include developmentally supportive emotional and instrumental behaviors and household routines, along with avoidance of harsh and coercive parenting processes. Future research should also examine the hypothesis that SAAF helped to ameliorate the impact of stressors common to families coping with economic hardship, such as parental depression and family conflict, both of which have implications for brain maturation.5 In past research, SAAF has been shown to decrease both of these risk factors.30,31
Several limitations of this study must be noted. First, the SAAF trial was not designed to examine change in hippocampal and amygdalar volumes. We did not assess pretrial hippocampal or amygdalar volumes; therefore, we could not determine whether the volumes in these regions changed differentially over time for members of the intervention and control groups. At study entry, the SAAF and control groups were similar in terms of family poverty, parental education, family structure, parental age, and youth gender, suggesting that randomization worked to minimize pretrial group differences. These findings are consistent with the assumption that the groups began the trial with similar hippocampal and amygdalar volumes. Nevertheless, until pretest data become available, conclusions about SAAF's capacity to influence changes in hippocampal and amygdalar volumes must be viewed as tentative. Second, only diminished left-side hippocampal subfield and amygdalar volumes were associated with poverty for control participants. The reasons for this lateralized finding currently are not clear, but these results are consistent with evidence of diminished hippocampal volumes on the left side, but not the right side, in adults with histories of childhood adversity.39–41 Future research should be designed to determine whether links between exposure to poverty and lateralized hippocampal and amygdalar volumes have delayed effects that do not emerge until adulthood.42
Conclusions
This study provides further evidence of the effects of poverty on brain development and initial evidence that a family-oriented intervention can reduce those effects. To the extent that they are substantiated in future research, these strategies may provide a means of narrowing social disparities.
Acknowledgement
This research was supported by Award Number P30 DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr. Brody had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Brody, Yu, Gray.
Acquisition, analysis, or interpretation of data: Brody, Yu, Gray.
Drafting of the manuscript: Brody.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Yu.
Obtained funding: Brody.
Administrative, technical, or material support: Brody, Gray, Sweet.
Study supervision: Brody.
Conflict of Interest Disclosures: None reported.
References
- 1.Macartney S. Child poverty in the United States 2009 and 2010: Selected race groups and Hispanic origin (ACSBR/10-05) U.S. Census Bureau; Washington, DC: Nov, 2011. [Google Scholar]
- 2.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- 3.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 4.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS, Morrison JH. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luby JL, Belden A, Botteron KN, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- 13.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 14.Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neuosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 16.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 17.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109(9):563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagliaccio D, Luby JL, Bogdan R, et al. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39(5):1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brody GH, Kogan SM, Grange CM. Translating longitudinal, developmental research with rural African American families into prevention programs for rural African American youth. In: King RB, Maholmes V, editors. The Oxford handbook of poverty and child development. Oxford University Press-USA; New York, NY: 2012. pp. 553–570. [Google Scholar]
- 20.Rutter ML. Environmentally mediated risks for psychopathology: Research strategies and findings. J Am Acad Child Adolesc Psychiatry. 2005;44(1):3–18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]
- 21.Gunnar MR, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 22.Brody GH, Lei M-K, Chen E, Miller GE. Neighborhood poverty and allostatic load in African American youth. Pediatrics. 2014;134(5):e1362–e1368. doi: 10.1542/peds.2014-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22(12):1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luby JL, Barch DM, Belden A, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A. 2012;109(8):2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luby JL, Belden A, Harms MP, Tillman R, Barch DM. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci U S A. 2016;113(20):5742–5747. doi: 10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao H, Betancourt L, Giannetta JM, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 29.Brody GH, Murry VM, Gerrard M, et al. The Strong African American Families program: Translating research into prevention programming. Child Dev. 2004;75(3):900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 30.Brody GH, Chen Y-f, Kogan SM, et al. Family-centered program to prevent substance use, conduct problems, and depressive symptoms in Black adolescents. Pediatrics. 2012;129(1):108–115. doi: 10.1542/peds.2011-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brody GH, Yu T, Beach SRH. Resilience to adversity and the early origins of disease. Dev Psychopathol. doi: 10.1017/S0954579416000894. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES–D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 33.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 34.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelan CD, Hibar DP, van Velzen LS, et al. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage. 2016;128:125–137. doi: 10.1016/j.neuroimage.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202(2):504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 38.Brody GH, Yu T, Chen Y-f, Kogan SM, Smith K. The Adults in the Making program: Long-term protective stabilizing effects on alcohol use and substance use problems for rural African American emerging adults. J Consult Clin Psychol. 2012;80(1):17–28. doi: 10.1037/a0026592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: A preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44(13):799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 2006;163(4):630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]