Abstract
Background
Previous studies investigated the impact of brain-derived neurotrophic factor (BDNF) val66met (rs6265) on hippocampus volumes and neurocognition in bipolar disorders (BD), but the results were not consistent. This study aimed to investigate the effect of BDNF polymorphism on hippocampus volumes and memory performance in well-characterized adult populations diagnosed with type I BD (BD-I) and major depressive disorder (MDD) compared with healthy controls (HC).
Methods
48 BD-I patients, 33 MDD patients and 60 HC were genotyped for BDNF rs6265 using DNA isolated from white blood cells. Individuals with val/met and met/met genotypes were grouped as met carriers and compared to those with the val/val. Brain segmentations were obtained from structural magnetic resonance imaging (MRI) using the Freesurfer. Memory performance was assessed with the California Verbal Learning Task (CVLT).
Results
We found a significant diagnosis effect and marginal interaction between diagnosis and BDNF genotype group for both hippocampus volumes and memory performance. BDNF met allele carrier BD patients had smaller hippocampus volumes and reduced performance on multiple CVLT scores compared to MDD patients and HC.
Conclusions
We provide strong evidence for the BDNF val66met polymorphism as a putative biological signature for the neuroanatomical and cognitive abnormalities commonly observed in BD patients.
Keywords: Bipolar disorder Type I, BDNF val66met, polymorphism, hippocampus, volume, memory, MRI, bipolar, major depressive disorder
Introduction
Bipolar Disorder (BD) is a significantly disabling chronic psychiatric condition with characteristic depressive and manic phases and lifetime prevalence of 3.9% (Kessler et al., 2005; Yatham et al., 2009). BD has long been sought to be associated with a number of neuroanatomical changes in the fronto-limbic regions, hippocampus, basal ganglia and corpus callosum (Chepenik et al., 2009b; Lavagnino et al., 2015; Radaelli et al., 2015; Selek et al., 2013). Notably, the hippocampus - a brain region widely acknowledged for its role in declarative memory (Eldridge et al., 2000) and emotion processing (Houenou et al., 2011) - has been reported to be either reduced (Bearden et al., 2008; Blumberg et al., 2003a; Chepenik et al., 2009b; Hartberg et al., 2011), enlarged (Beyer et al., 2004; Javadapour et al., 2010; van Erp et al., 2012) or unchanged (Altshuler et al., 2000; Bertolino et al., 2003; Brambilla et al., 2003; Chen et al., 2004; Delaloye et al., 2009) compared to healthy controls (HC). The reported discrepancies could arguably be attributed to methodological factors, such as the variety of techniques used to demarcate brain structures and demographic and clinical characteristics of the sample, such as age and gender related brain characteristics (Blumberg et al., 2003b), BD subtype (Strasser et al., 2005) and medication history (Yucel et al., 2008). Thus, further investigation of the hippocampus volume alterations in BD with careful control of the above factors is warranted.
In addition to potential brain abnormalities, the majority of BD patients have significant cognitive impairments that persist during the euthymic and acute phases of the illness (APA, 2002; Bora et al., 2009; MacQueen et al., 2001; Quraishi and Frangou, 2002). Overall, there is evidence of deficits in visuomotor processing speed, verbal memory and executive functioning (van der Werf-Eldering et al., 2011). Impairments of smaller effect size in working memory, and sustained attention have also been reported (Cohen’s d=0.6 to 0.7) (Albus et al., 1996; Bora et al., 2009; Goldberg et al., 1993; Martínez-Arán et al., 2004; Quraishi and Frangou, 2002). The presence of verbal memory impairment across mood phases may indicate that these deficits are trait markers of the bipolar illness (Gualtieri and Johnson, 2006). In particular, a study comparing manic, hypomanic, depressive and euthymic BD patients showed that manic BD patients had the most robust cognitive deficits of all treatment groups and presented poor immediate and delayed verbal memory performance on the California Verbal Learning Test (CVLT) and executive tasks (Sweeney et al., 2000).
Along with neurocognitive deficits, BD is characterized by high peripheral levels of proinflammatory markers (Barbosa et al., 2011) and decreased brain-derived neurotrophic factor (BDNF) levels (Bourne et al., 2013; Cunha et al., 2006). BDNF is a member of the neurotrophin family of growth factors and regulates major cellular processes such as neurogenesis, development, dendritic growth, survival and maturation (Kuipers and Bramham, 2006) as well as complex neuronal processes such as synaptic plasticity and memory consolidation (Post, 2007). The BDNF val66met polymorphism (rs6265) has been consistently implicated in candidate gene studies examining the pathophysiology of BD (Bourne et al., 2013; Cunha et al., 2006). This single nucleotide polymorphism is located on chromosome 11p13 and results in a valine (G) to methionine (A) substitution at codon 66 (Bath and Lee, 2006; Green et al., 2006; Hwang et al., 2006; Liu et al., 2008; Neves-Pereira et al., 2002; Sklar et al., 2002). Further, in neuronal and neurosecretory cells the BDNF val66met polymorphism can lead to a significant reduction in BDNF trafficking to secretory granules, and as a result, to a reduced BDNF production by the secretory granules (Benjamin et al., 2010; Egan et al., 2003).
A number of studies have linked the BDNF val/met polymorphism to abnormal hippocampus volumes across the mood disorder spectrum in both adults (Benjamin et al., 2010; Chepenik et al., 2008; Gatt et al., 2009; Montag et al., 2009; Pezawas et al., 2004) and pediatric populations with BD (Inal-Emiroglu et al., 2015; Peruzzolo et al.). However, findings in this field remain controversial as a recent systematic and meta-analysis review based on 18 independent clinical cohorts comprising 1695 participants showed that the decreased hippocampus volume observed in patients with mood disorders, psychosis and schizophrenia relative to healthy controls did not depend on the BDNF polymorphism (Harrisberger et al., 2015). Notably, the authors reported that most studies were underpowered and did not provide information about treatment regimen, comorbidities, and progress/severity of the disease. These factors should therefore be taken into account in future investigations. To date it is still unclear whether the relationship between BDNF polymorphisms and hippocampus volumes is specific to BD as a broad diagnostic group, or whether it is specific to a bipolar subtype. For instance BD type I is viewed as being the most severe form of BD as it involves more pronounced anatomical (Maller et al., 2014) and cognitive impairments (Bourne et al., 2015) than BD type II. In addition a study showed that the number of manic episodes –observed in BD-I and not BD-II- is inversely correlated with performance in learning and memory tests (Bourne et al., 2015).
Thus, in the current study we recruited a well-characterized group of individuals with BD type I and major depressive disorder (MDD) compared to healthy controls (HC). Based on previous studies, we hypothesized that the BDNF val66met variant would be associated with abnormalities in hippocampus volumes and memory performance – as measured by the CVLT - in both individuals with BD type I and MDD, as well as healthy controls (HC).
Materials and methods
Participants
The sample included 48 BD-I patients (32 females, age (Mean±S.D.)=41.02±12.65 years), 33 MDD patients (23 females, age (Mean±S.D.)=39.17±12.42 years) and 60 HC (39 females, age (Mean±S.D.)=40.57±12.95 years). Patients were recruited from inpatient and outpatient clinics at the University of Texas Health Science Center at San Antonio campus. HC were recruited through local media advertisements and flyers posted in public areas. All patients met the DSM-IV-R criteria for BD-I or MDD. The diagnosis of BD-I and MDD and the absence of mental disorders among controls were ascertained by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Axis I (SCID I), which was administered to all participants by an independent psychiatrist or trained research assistant. Based on the SCID assessment 16 BD and 18 MDD patients were classified as euthymic and 20 BD and 15 MDD patients as depressed at the time of testing. Fourteen MDD patients had achieved complete remission, while the remnant of the sample experienced episodes of depression of mild to moderate intensity (based on the SCID diagnosis). The interview also included the Young Mania Rating Scale (YMRS)(Young et al., 1978) and Hamilton Rating Scale for Depression (HAM-D) (Hamilton, 1960). All participants had no history of substance abuse in the previous 6-months and no chronic medical issues including cardiovascular and neurological disorders. At the time of enrollment 44 of the 48 participants with BD type I reported taking one or more psychotropics. Primary types of medication included lithium (n=2), antidepressant (n=5), anticonvulsants (n=5), atypical antipsychotics (n=1) and benzodiazepines (n=3). Eighteen participants took two or more medications concurrently. There was no medication history data available for 10 of the 44 medicated BD patients. One of the 33 patients with MDD took lithium. HC with a history of any Axis I disorder in the first-degree relatives and use of psychoactive medication less than 2-weeks prior to the start of the study were excluded. All female participants underwent a urine pregnancy test and urine drug screen to exclude participants with pregnancy and illegal drug use. The study protocol was approved by the local Institutional Review Board and informed consent was obtained from all the participants.
Genotyping
DNA was extracted from white blood cells from blood samples collected from study participants with the Gentra Puregene blood kit (Qiagen, Germantown, MD). val66met (rs6265) genotyping was performed with a 5′-fluorogenic exonuclease assay (C__11592758_10) (TaqMan®, Applied Biosystems, Foster City, CA) or an ABI 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) in duplicates by an individual blind to participants clinical status. Platinum® quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA) on a GeneAmp PCR system 9700 was employed for PCR amplification. Applied Biosystems Prism® 7900 sequence detection system and SDS 2.2 software (Applied Biosystems) were used for analysis of amplification products. In subsequent analyses individuals with val/met or met/met genotypes were combined (met carriers) and compared with individuals with the val/val genotype, because met/met carriers only occupy about 6% of the total sample.
Hippocampus volume
High resolution T1-weighted brain images were acquired on a Philips 1.5 Tesla MR system (Philips Medical System, Andover, MA, USA). Images were collected by means of an axial three-dimensional fast field echo sequence (field of view 256 mm × 256 mm; repetition time 24 ms; echo time 5 ms; flip angle 40 degrees; slice thickness 1 mm).
After all scans were visually inspected to be valid, cortical and subcortical reconstruction and volumetric segmentation were performed with the Freesurfer software version 5.3.0 (http://surfer.nmr.mgh.harvard.edu). The whole procedure included motion correction, intensity normalization, automated topology corrections and automatic segmentations of cortical and subcortical regions, as documented elsewhere (Dale et al., 1999; Dale and Sereno, 1993; Fischl and Dale, 2000; Fischl et al., 2001; Fischl et al., 2002; Fischl et al., 2004; Fischl et al., 1999; Han et al., 2006; Jovicich et al., 2006; Ségonne et al., 2004). The regions labeled as left and right hippocampus were extracted, and the corresponding volumes were calculated (Fischl et al., 2002). Both left and right hippocampus volumes were scaled by the estimated total intracranial volume for each subject to control for differences in head size.
Cognitive performance
All participants were administered the Wechsler Abbreviated Scale of Intelligence (WASI), which is a screener of verbal, non-verbal, and general cognitive ability [41], and the Wechsler Test of Adult Reading (WTAR), which is a measure of premorbid intellectual quotient (IQ) (Wechsler, 2001). 116 out of the total 141 participants were administered a revised version of the CVLT – a standardized test measuring verbal learning and declarative memory via a trial list-learning paradigm (Donders, 2008). This version of the CVLT was part of the South Texas Assessment of Neurocognition (STAN) which includes both standardized and computerized neurocognitive tasks (Glahn et al., 2007). In the CVLT task participants are presented orally with 16 words for 5 times and asked to recall as many words as possible in any order (immediate recall). After being presented with an intrusion list (List B), participants are asked to recall the words included in the List A one more time via cues (short delay cued recall) and without cues CVLT short-delay (free recall). After a 20-minute delay participants are asked to recall words from List A, both with the aid of categorical cues (long delay cued recall) and spontaneously (long delay free recall). Scores of each CVLT variable represented the number of correctly recalled words. Four subjects were excluded from analyses because they did not complete the whole or part of the CVLT.
Statistical analyses
Statistical analyses were performed using IBM SPSS statistics (Version 21.0). Normality of each variable was verified. One subject was excluded as an outlier (outside 3 standard deviations) in hippocampus volume. Demographic, clinical and cognitive differences between groups were assessed with chi-square and a series of univariate analyses of variance (ANOVA). A general linear model (GLM) was used with bilateral hippocampus volumes as the dependent variable, and two between-subject factors: diagnosis (BD-I vs. MDD vs. HC) and genotype (met-allele carrier vs. val/val genotype). Hippocampus laterality was a within-subject variable, and age and gender were entered to the model as covariates. The threshold of statistical significance was set at p < 0.05 and a Bonferroni correction for multiple comparisons was performed across the three diagnosis groups for post hoc tests.
The CVLT data were analyzed using a GLM with two between-subject independent factors: diagnosis (BD-I vs MDD vs. HC) and genotype (0=met-allele carrier vs. 1=val/val genotype). Five CVLT performance measures (CVLT trial-1-to-5, short delay cued recall, short delay free recall, long-delay cued recall and long-delay free recall) served as within subject factors. Age and gender were entered as covariates. Linear regression analyses were performed to examine the link between hippocampus volume, CVLT scores, diagnosis, BDNF genotype, and the interactions between diagnosis and BDNF genotype. Age and gender were entered as predictors and CVLT scores were the outcomes measures.
Results
Demographics and clinical description
Demographics and clinical features for BD, MDD and HC are reported in Table 1. There was no significant difference in age, gender and genotype distribution between the three groups. However, there were different levels of educational attainment across groups (p<.05) with BD-I individuals reporting less years of education than HC. Overall HAM-D scores were significantly higher in BD-I, and to a lesser extent MDD patients, compared to HC. YMRS scores were higher in BD-I patients compared to HC and MDD patients.
Table 1.
Demographic and clinical features of study participants (Mean ± standard deviation (SD)
| Healthy Control (n=60) | BD-I (n=48) | MDD (n=33) | F/X2 | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 40.57±12.95 | 41.02±12.65 | 39.17±12.42 | 0.21 | 0.81 |
|
| |||||
| Gender (n of females) | 39 | 32 | 23 | 0.21 | 0.9 |
|
| |||||
| BDNF met carriers | 19 | 13 | 8 | 0.63 | 0.72 |
|
| |||||
| Ethnicity | |||||
| Caucasian | 25 | 35 | 20 | ||
| Hispanic | 26 | 12 | 13 | ||
| Asian | 6 | 0 | 0 | 18.14 | 0.02 |
| Other | 1 | 0 | 0 | ||
| Multi-racial | 1 | 0 | 0 | ||
| Missing | 1 | 1 | 0 | ||
|
| |||||
| Education (years) # | 16.51±3.34 | 13.9±1.87** | 15.03±2.99* | 9.70 | <0.01 |
|
| |||||
| WTAR## | 38.15±9.33 | 37.5±7.84 | 39.29±8.44 | 0.37 | 0.7 |
|
| |||||
| YMRS | 0.28±.71 | 6.65±7.26** | 1.94±2.08 | 8.44 | <0.01 |
|
| |||||
| HAM-weD | 0.93±1.27 | 12.67±8.11** | 10.39±8.77** | 50.8 | <0.01 |
Note:
p<0.05;
p<0.01
Total n for the variable “Years of education” is 21;
Total n for the variable “WRAT” is 111
Hippocampus volume
We found a main effect of diagnosis on the hippocampus volumes (F(2,133)=9.527, p<0.001), a marginal effect of BDNF genotype [F(1,133)=3.762, p=0.055] and a marginal interaction between diagnosis and BDNF genotype [F(2,133)=3.040, p=0.051] (Figure 1a). Post-hoc tests with the Bonferroni correction showed that within BDNF met carriers, BD-I patients displayed smaller hippocampus volumes compared to HC (p=0.01) and MDD (p=0.003), and MDD patients have similar hippocampus volumes with HC (p=0.725). BDNF val/val genotype BD-I patients had similar hippocampus sizes as HC (p=0.257) and MDD patients (p=0.747), and MDD patients have similar hippocampus volumes with HC (p=1). There was no main effect of laterality on the hippocampus [F(1,133)=0.072, p=0.788]. Further, we did not find an interaction between laterality and diagnosis [F(1,133)=0.149, p=0.861] and BDNF genotype [F(1,133)=0.001, p=0.971] and no triple interaction between laterality, diagnosis and BDNF genotype [F(1,133)=0.003, p=0.796].
Figure 1.
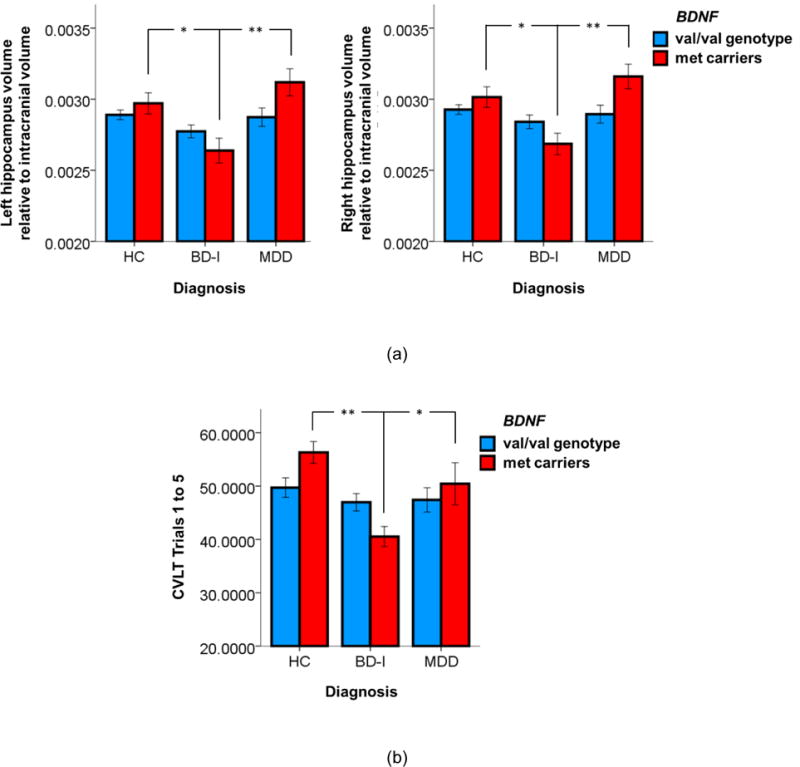
Reduced hippocampus volume and memory performance in bipolar disorder patients carrying the BDNF val66met met allele. (a) Ratios of bilateral hippocampus volumes relative to the intracranial volume in HC, BD-I and MDD patients; (b) Total number of correctly recalled words on the CVLT Trials 1 to 5. *, p<0.05; **, p<0.005.
Cognitive performance
We found a main effect of diagnosis on all CVLT measures [F(2,108)=8.139, p=0.001] and a significant interaction between diagnosis and BDNF genotype [F(2,108)=3.509, p=0.033]. However, the BDNF genotype main effect did not reach significance [F(1,108)=0.702, p=0.404]. Bonferroni-corrected post-hoc tests showed that among BDNF val/val genotype BD-I patients showed similar memory performance compared with HC (p=1) and MDD patients (p=1). However, BDNF met carrier BD-I patients showed worse memory performance compared to HC (p<0.001) and MDD (p=0.019). Figure 1b shows the scores of CVLT trials 1 to 5. The other four CVLT scores had a similar pattern (see supplementary materials Figure S1). Although there was a main effect of both diagnosis and BDNF genotype on the hippocampus volumes and memory performance measured by CVLT tests, a partial correlation analysis adjusted for diagnosis, BDNF genotype, age and gender revealed a trend toward a positive correlation between the right hippocampus volume and the long delay free recall score marginally (r=0.166, p=0.081).
Further regression analysis found that across clinical populations the left and right hippocampus volumes explain an additional variance of less than 0.6% for the immediate learning and short delay scores, and less than 3.5% for long delay scores. By comparison a regression model including the predictors diagnosis, BDNF genotype, age and gender explains 20% to 25% of the total variance in CVLT scores. Across groups the hippocampus volumes did not explain additional variance in CVLT scores (p>0.05). Except the long delay cued recall, the variance in CVLT scores explained by the interaction between diagnosis and BDNF genotype was significant for BD and HC (p<0.05), but not for MDD and HC (p>0.05).
Discussion
The current study examined the effect of the BDNF val66met genotype on hippocampus volumes in patients with BD-I and MDD compared to demographically matched healthy controls. Given the role of the hippocampus in information encoding and memory storage, we also explored the relationship between BDNF genotype on memory performance measured by the CVLT. The most important finding of this study is the association between the BDNF val66met polymorphism and reduced hippocampus volumes in BD-I patients. Indeed, in our sample, BD patients carrying the met allele of the BDNF gene were found to have smaller hippocampus volumes and reduced memory performance compared to HC. This result corroborates previous findings on the link between the val66met polymorphism and BD-I (Lohoff et al., 2005) and reduced hippocampus volumes and BDNF expression in BD (Chepenik et al., 2009a).
We observed a significant reduction in hippocampus volumes in met carrier BD-I patients. Traditionally, studies on hippocampus abnormalities in BD have been inconsistent. Previous in vivo studies showed either no differences in hippocampus volumes between BD patients and HC (Frey et al., 2007), or smaller hippocampus volumes in BD patients (Bearden et al., 2008; Frazier et al., 2005). A post-mortem study demonstrated reduced pyramidal cell size in the hippocampus of BD patients compared with HC (Liu et al., 2007). Given the dearth of genetic studies integrating neuroanatomical data further investigations are needed to determine whether the BDNF polymorphism mediates these anatomical abnormalities. An alternative explanation for the divergent findings may be related to the administration of psychotropic medication such as lithium and valproate that are hypothesized to reduce oxidative stress possibly by boosting the expression of the BDNF protein expression (Frey et al., 2006) in a number of brain regions including the hippocampus (Nibuya et al., 1996). It is also compelling that antidepressants and lithium lead to volumetric changes possibly induced by the increased proliferation and maturation of hippocampus cells (Foland et al., 2008; Yucel et al., 2007). Although in our sample, 44 of the 48 BD-I patients were medicated at the time of testing, the significant reductions in hippocampus volumes were observed only in met carrier BD-I patients, and therefore were unlikely to be the effect of medication.
While the presence of memory impairment in BD is well-established (Bearden et al., 2006), less is known about the association between the BDNF val66met polymorphism and cognition in BD and related mood disorders. Our lab has previously shown that met carriers displayed a poorer (albeit not statistically significant) memory performance compared to BD patients and HC with the val/val genotype with a smaller sample (Matsuo et al., 2009). Further, cognitive differences between met carriers and those with a val/val genotype have been found on the Wisconsin Card Sorting Test – a test of abstraction and inhibition (Rybakowski et al., 2006; Rybakowski et al., 2003). Studies of patients with schizophrenia showed that met carriers have poorer spatial and verbal memory performance (Egan et al., 2003; Ho et al., 2006) along with smaller hippocampus (Egan et al., 2003; Szeszko et al., 2005), parahippocampus and supramarginal gyrus volumes when compared with val/val carriers (Ho et al., 2006). Notably, we found that BD carrying the BDNF met allele displayed worse neurocognitive performance than MDD and HC. By contrast, the memory performance of MDD patients did not differ from that of HC. The latter finding is likely to be due to the fact that the majority of the MDD patients (n=18) included in this study were euthymic, and that approximately half of them (n=14) were in full remission at the time of testing.
The current findings appear to be in line with the neuroprogression model of BD (Berk et al., 2011; Fries et al., 2012). This model proposes to explain the pathological brain rewiring that takes place in the context of severe mental disorders (Berk et al., 2011; Fries et al., 2012; Kapczinski and Streb, 2014), and links recurrent mood episodes with the disruption of the homeostasis between inflammatory mechanisms, oxidative processes, and neuroprotective mechanisms (e.g., BDNF). This disruption has been linked to an increase in the individual’s vulnerability to psychological stress, brain atrophy and ultimately cognitive impairment (Berk et al., 2010; Kapczinski et al., 2008). Notably, in BD neuroprogression has been associated with cognitive decline, refractoriness, brain volume changes and a more severe course of illness. (Bauer et al., 2014; Cao et al., 2016). Further these results highlight the importance of BDNF on the hippocampus function and may provide a rationale for individualized pharmacological interventions targeting inflammation in subjects carrying the met allele. For instance, met carriers may be more at risk for inflammation and resulting consequences in terms of neuroprogression. Thus, interventions aimed to reduce physiological stress may help reverse neuroanatomical abnormalities.
Our findings supports further investigation into the link between memory performance and hippocampus volumes (Otten and Meeter, 2015). Indeed our regression analyses showed that in BD the hippocampus volume did not significantly contribute to the explained variance in memory performance. Thus, other factors may mediate the link between the hippocampus volumes and memory performance, and may differ across the mood disorder spectrum. Future studies with factorized models are therefore necessary to reveal the specific connections between BD, BDNF genotypes, hippocampus volumes and neurocognitive performance.
Our sample has few limitations, such as the MDD sample might not be completely homogeneous (14 out of 33 MDD patients were in remission), and there was a small but significant difference in education levels between groups. Given the high number of missing data (n=20) for the variable education we decided against adding it as a covariate. However, exploratory analyses showed that after adding education as an additional covariate along with age and gender, the main effect of diagnosis on the hippocampus volumes remained significant (F(2,112)=7.225, p=0.001) and the interaction between diagnosis and BDNF genotype approached significance (F(2,112)=3.060, p=0.051). Similarly, for CVLT, both the main effect of diagnosis (F(2,91)=4.426, p=0.015) and the interaction between diagnosis and BDNF genotype were significant (F(2,91)=3.254, p=0.043). Furthermore, the BD-I group was the only one who received medication and psychotropic drugs have well-known effects on BDNF expression (Notaras et al., 2015). For instance antidepressant medication leads to increased brain-derived neurotrophic factor (BDNF) receptor trkB signaling (Chi et al., 2010), and lithium has been shown to alter the magnetic resonance imaging (MRI) signal and lead to potential misinterpretation of gray matter volume changes. However, these medicines usually increase the hippocampus volume, and thus, the decreased hippocampus volume observed in our BD-I patients with the met genotype was in the opposite direction of the volumetric effect of these medicines and was less likely to be caused by them. In contrast to previous studies, we did not find significant reduction of hippocampus volumes in healthy met carriers (Bueller et al., 2006; Egan et al., 2003; Pezawas et al., 2004). A potential explanation for the divergence in findings may be due to the different types of image processing and selection of brain structures and reconstruction methods between our study and those studies e.g. manual tracing (Bueller et al., 2006) vs. automatic reconstruction with Freesurfer in our study, and grey matters selected in the regions of interest (ROI) by voxel-based morphometry (VBM) (Pezawas et al., 2004) vs. whole hippocampal volume segmented by Freesurfer in our study. Manual tracing is a rather time-consuming approach that is suitable for large rather than small brain structures. VBM registers every brain to a template and is therefore sensitive to errors such as misalignment of brain structures and tissues. While VBM uses less a priori information, but an efficient image segmentation algorithm (Zhang et al., 2001), Freesurfer relies on a priori information (Fischl et al., 2002). Notably, a recent study showed that Freesurfer yields more valid results than VBM in terms of hippocampus segmentation (Grimm et al., 2015). Furthermore, in our study, we scaled the hippocampus volumes by the estimated total intracranial volume to control for differences in head size, and corrected our analyses for both age and gender. By comparison, Pezawas et al. (2004) reported grey matter ratio to val/val mean and Bueller et al. (2006) adjusted hippocampal differences only for gender. The results of our study still need to be interpreted with caution due to these limits.
To the best of our knowledge, this is the first study to view anatomical and cognitive features as a function of BDNF polymorphisms in individuals with BD type I, MDD and HC. Our study provides evidence that the val66met polymorphism is associated with reduced memory performance in BD-I patients. The current findings highlight potential differences in the role of BDNF val66met polymorphisms in neurocognition across the mood disorder spectrum (MDD vs. BD). Furthermore, they raise questions on the possible link between neurocognitive impairment and BDNF variants across BD subtypes.
Supplementary Material
Highlights.
The effect of BDNF val66met (rs6265) polymorphism on hippocampus volumes and verbal memory performance in patients with type I BD (BD-I) and major depressive disorder (MDD) is not consistent.
We found that BDNF met allele carrier BD patients had smaller hippocampus volumes and reduced performance on multiple CVLT scores compared to MDD patients and HC.
We provided strong evidence that the BDNF val66met polymorphism was a putative biological signature for the neuroanatomical and cognitive abnormalities in BD patients.
Acknowledgments
Funding sources
The current work was supported by: NIH/NIDA 5 P50 DA018197-05 (DN), through MD Anderson’s Cancer Center Support Grant NIH/NIDA DA026120 (DN), and the Toomim Family Fund (David A. Nielsen), NIMH grant R01 085667, the Dunn Research foundation and the Pat Rutherford, Jr. Endowed Chair in Psychiatry (Jair C. Soares). The genetic findings are the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX (David Nielsen).
Financial Disclosure
Dr. Kapczinski has received grants/research support from AstraZeneca, Eli Lilly, Janssen-Cilag, Servier, CNPq, CAPES, NARSAD, and the Stanley Medical Research Institute; has been a member of speakers’ boards for AstraZeneca, Eli Lilly, Janssen and Servier; and has served as a consultant for Servier. Dr. Soares participated in research funded by Forest, Merck, BMS, GSK and has been a speaker for Pfizer and Abbott.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Study concept and design: Cao, Bauer, Sharma.
Acquisition, analysis, or interpretation of data: Cao, Bauer, Sharma, Frazier, Nielsen, Mwangi, Lavagnino, Soares.
Drafting of the manuscript: Cao, Bauer, Sharma.
Critical revision of the manuscript for important intellectual content: Cao, Bauer, Sharma, Mwangi, Frazier, Lavagnino, Walss-Bass, Glahn, Kapczinski, Nielsen, Soares.
Statistical analysis: Cao, Bauer.
Administrative, technical, and material support: Zunta-Soares.
Study supervision: Kapczinski, Soares.
Contributor Information
Bo Cao, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States.
Isabelle E. Bauer, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States.
Ajaykumar N. Sharma, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States.
Benson Mwangi, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States
Thomas Frazier, The Center for Pediatric Behavioral Health and Center for Autism, Cleveland Clinic, Cleveland, OH, United States
Luca Lavagnino, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States
Giovana B. Zunta-Soares, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States
Consuelo Walss-Bass, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States
David C. Glahn, The Olin Neuropsychiatry Research Center, Institute of Living, and Department of Psychiatry, Yale University School of Medicine, CT, United States
Flavio Kapczinski, Department of Psychiatry, Universidade Federal Rio Grande do Sul, Rua Ramiro Barcelos, 2350, 90035-903, Rio Grande do Sul, Brazil
David A. Nielsen, Department of Psychiatry and Behavioral Sciences, Michael E. DeBakey V.A. Medical Center, Baylor College of Medicine, Houston, TX, United States
Jair C. Soares, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States
References
- Albus M, Hubmann W, Wahlheim C, Sobizack N, Franz U, Mohr F. Contrasts in neuropsychological test profile between patients with first-episode schizophienia and first-episode affective disorders. Acta Psychiatrica Scandinavica. 1996;94:87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- APA. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159 [PubMed] [Google Scholar]
- Barbosa IG, Huguet RB, Sousa LP, Abreu MNS, Rocha NP, Bauer ME, Carvalho LA, Teixeira AL. Circulating levels of GDNF in bipolar disorder. Neuroscience letters. 2011;502:103–106. doi: 10.1016/j.neulet.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, affective & behavioral neuroscience. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Bauer IE, Pascoe MC, Wollenhaupt-Aguiar B, Kapczinski F, Soares JC. Inflammatory mediators of cognitive impairment in bipolar disorder. Journal of psychiatric research. 2014;56:18–27. doi: 10.1016/j.jpsychires.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Kaur S, Sanches M, Villarreal V, Bowden C, Soares JC. Sources of declarative memory impairment in bipolar disorder: mnemonic processes and clinical features. Journal of psychiatric research. 2006;40:47–58. doi: 10.1016/j.jpsychires.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Soares JC. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin S, McQuoid DR, Potter GG, Payne ME, MacFall JR, Steffens DC, Taylor WD. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2010;18:323–331. doi: 10.1097/JGP.0b013e3181cabd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Conus P, Kapczinski F, Andreazza AC, Yücel M, Wood SJ, Pantelis C, Malhi GS, Dodd S, Bechdolf A. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193:S36–40. [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza A, Dean O, Giorlando F, Maes M, Yücel M, Gama C, Dodd S, Dean B. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience & biobehavioral reviews. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Frye M, Callicott JH, Mattay VS, Rakow R, Shelton-Repella J, Post R, Weinberger DR. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biological psychiatry. 2003;53:906–913. doi: 10.1016/s0006-3223(02)01911-x. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Kuchibhatla M, Payne ME, Moo-Young M, Cassidy F, Macfall J, Krishnan KR. Hippocampal volume measurement in older adults with bipolar disorder. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2004;12:613–620. doi: 10.1176/appi.ajgp.12.6.613. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of general psychiatry. 2003a;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of general psychiatry. 2003b;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of affective disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Bourne C, Aydemir Ö, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh J, Clark L, Cubukcuoglu Z, Dias V, Dittmann S. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatrica Scandinavica. 2013;128:149–162. doi: 10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- Bourne C, Bilderbeck A, Drennan R, Atkinson L, Price J, Geddes JR, Goodwin GM. Verbal learning impairment in euthymic bipolar disorder: BDI v BDII. Journal of Affective Disorders. 2015;182:95–100. doi: 10.1016/j.jad.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. Journal of psychiatric research. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Cao B, Passos IC, Mwangi B, Bauer IE, Zunta-Soares GB, Kapczinski F, Soares JC. Hippocampal volume and verbal memory performance in late-stage bipolar disorder. Journal of psychiatric research. 2016;73:102–107. doi: 10.1016/j.jpsychires.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biological psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009a;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS, Gelernter J, Blumberg HP. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009b;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi MH, Chang HH, Lee SY, Lee IH, Gean PW, Yang YK, Lu RB, Chen PS. Brain derived neurotrophic factor gene polymorphism (Val66Met) and short-term antidepressant response in major depressive disorder. Journal of affective disorders. 2010;126:430–435. doi: 10.1016/j.jad.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Cunha A, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, Santin A, Kapczinski F. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neuroscience letters. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. Journal of cognitive neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Delaloye C, de Bilbao F, Moy G, Baudois S, Weber K, Campos L, Canuto A, Giardini U, von Gunten A, Stancu RI, Scheltens P, Lazeyras F, Millet P, Giannakopoulos P, Gold G. Neuroanatomical and neuropsychological features of euthymic patients with bipolar disorder. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2009;17:1012–1021. doi: 10.1097/JGP.0b013e3181b7f0e2. [DOI] [PubMed] [Google Scholar]
- Donders J. Subtypes of learning and memory on the California Verbal Learning Test–Second Edition (CVLT–II) in the standardization sample. Journal of clinical and experimental neuropsychology. 2008;30:741–748. doi: 10.1080/13803390701689595. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. Medical Imaging, IEEE Transactions on. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19:221. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behavioural pharmacology. 2007;18:419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. Journal of Psychiatry and Neuroscience. 2006;31:326. [PMC free article] [PubMed] [Google Scholar]
- Fries GR, Pfaffenseller B, Stertz L, Paz AVC, Dargél AA, Kunz M, Kapczinski F. Staging and neuroprogression in bipolar disorder. Current psychiatry reports. 2012;14:667–675. doi: 10.1007/s11920-012-0319-2. [DOI] [PubMed] [Google Scholar]
- Gatt J, Nemeroff C, Dobson-Stone C, Paul R, Bryant R, Schofield P, Gordon E, Kemp A, Williams L. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The Neurocognitive Signature of Psychotic Bipolar Disorder. Biological psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Gold JM, Greenberg R, Griffin S, Schulz SC, Pickar D, Kleinman JE, Weinberger DR. Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry. 1993;150:1355–1362. doi: 10.1176/ajp.150.9.1355. [DOI] [PubMed] [Google Scholar]
- Green EK, Raybould R, Macgregor S, Hyde S, Young AH, O’Donovan MC, Owen MJ, Kirov G, Jones L, Jones I, Craddock N. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. The British journal of psychiatry: the journal of mental science. 2006;188:21–25. doi: 10.1192/bjp.bp.105.009969. [DOI] [PubMed] [Google Scholar]
- Grimm O, Pohlack S, Cacciaglia R, Winkelmann T, Plichta MM, Demirakca T, Flor H. Amygdalar and hippocampal volume: A comparison between manual segmentation, Freesurfer and VBM. Journal of Neuroscience Methods. 2015;253:254–261. doi: 10.1016/j.jneumeth.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG. Comparative neurocognitive effects of 5 psychotropic anticonvulsants and lithium. Medscape General Medicine. 2006;8:46. [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harrisberger F, Smieskova R, Schmidt A, Lenz C, Walter A, Wittfeld K, Grabe H, Lang U, Fusar-Poli P, Borgwardt S. BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2015;55:107–118. doi: 10.1016/j.neubiorev.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Sundet K, Rimol LM, Haukvik UK, Lange EH, Nesvag R, Melle I, Andreassen OA, Agartz I. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1122–1130. doi: 10.1016/j.pnpbp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Archives of general psychiatry. 2006;63:731–740. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, Wessa M. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. Journal of affective disorders. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiology of aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Inal-Emiroglu FN, Resmi H, Karabay N, Guleryuz H, Baykara B, Cevher N, Akay A. Decreased Right Hippocampal Volumes and Neuroprogression Markers in Adolescents with Bipolar Disorder. Neuropsychobiology. 2015;71:140–148. doi: 10.1159/000375311. [DOI] [PubMed] [Google Scholar]
- Javadapour A, Malhi GS, Ivanovski B, Chen X, Wen W, Sachdev P. Hippocampal volumes in adults with bipolar disorder. The Journal of neuropsychiatry and clinical neurosciences. 2010;22:55–62. doi: 10.1176/jnp.2010.22.1.55. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, MacFall J. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Streb LG. Neuroprogression and staging in psychiatry: historical considerations. Revista Brasileira de Psiquiatria. 2014;36:187–188. doi: 10.1590/1516-4446-2014-3605. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, Kauer-Sant’Anna M, Grassi-Oliveira R, Post RM. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neuroscience & Biobehavioral Reviews. 2008;32:675–692. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Current opinion in drug discovery & development. 2006;9:580–586. [PubMed] [Google Scholar]
- Lavagnino L, Cao B, Mwangi B, Wu MJ, Sanches M, Zunta-Soares G, Kapczinski F, Soares J. Changes in the corpus callosum in women with late-stage bipolar disorder. Acta Psychiatrica Scandinavica. 2015;131:458–464. doi: 10.1111/acps.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Foroud T, Xuei X, Berrettini W, Byerley W, Coryell W, El-Mallakh R, Gershon ES, Kelsoe JR, Lawson WB, MacKinnon DF, McInnis M, McMahon FJ, Murphy DL, Rice J, Scheftner W, Zandi PP, Lohoff FW, Niculescu AB, Meyer ET, Edenberg HJ, Nurnberger JI., Jr Evidence of association between brain-derived neurotrophic factor gene and bipolar disorder. Psychiatric genetics. 2008;18:267–274. doi: 10.1097/YPG.0b013e3283060f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Schulz SC, Lee S, Reutiman TJ, Fatemi SH. Hippocampal CA1 pyramidal cell size is reduced in bipolar disorder. Cellular and molecular neurobiology. 2007;27:351–358. doi: 10.1007/s10571-006-9128-7. [DOI] [PubMed] [Google Scholar]
- Lohoff F, Sander T, Ferraro T, Dahl J, Gallinat J, Berrettini W. Confirmation of association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene and bipolar I disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;139:51–53. doi: 10.1002/ajmg.b.30215. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatrica Scandinavica. 2001;103:163–170. doi: 10.1034/j.1600-0447.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. Journal of Affective Disorders. 2014;169:118–127. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Walss-Bass C, Nery FG, Nicoletti MA, Hatch JP, Frey BN, Monkul ES, Zunta-Soares GB, Bowden CL, Escamilla MA. Neuronal correlates of brain-derived neurotrophic factor Val66Met polymorphism and morphometric abnormalities in bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1904–1913. doi: 10.1038/npp.2009.23. [DOI] [PubMed] [Google Scholar]
- Montag C, Weber B, Fliessbach K, Elger C, Reuter M. The BDNF Val66Met polymorphism impacts parahippocampal and amygdala volume in healthy humans: incremental support for a genetic risk factor for depression. Psychological medicine. 2009;39:1831–1839. doi: 10.1017/S0033291709005509. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. American journal of human genetics. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- Otten M, Meeter M. Hippocampal structure and function in individuals with bipolar disorder: A systematic review. Journal of affective disorders. 2015;174:113–125. doi: 10.1016/j.jad.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Peruzzolo TL, Anes M, de Moura Kohmann A, Souza ACML, Rodrigues RB, Brun JB, Peters R, de Aguiar BW, Kapczinski F, Tramontina S. Correlation between Peripheral Levels of Brain-Derived Neurotrophic Factor and Hippocampal Volume in Children and Adolescents with Bipolar Disorder. Neural Plasticity. 501:324825. doi: 10.1155/2015/324825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. Journal of psychiatric research. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. Journal of affective disorders. 2002;72:209. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Radaelli D, Papa GS, Vai B, Poletti S, Smeraldi E, Colombo C, Benedetti F. Fronto-limbic disconnection in bipolar disorder. European Psychiatry. 2015;30:82–88. doi: 10.1016/j.eurpsy.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Rybakowski J, Borkowska A, Skibinska M, Hauser J. Illness-specific association of val66met BDNF polymorphism with performance on Wisconsin Card Sorting Test in bipolar mood disorder. Molecular psychiatry. 2006;11:122–124. doi: 10.1038/sj.mp.4001765. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Skibińska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar disorders. 2003;5:468–472. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale A, Busa E, Glessner M, Salat D, Hahn H, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Selek S, Nicoletti M, Zunta-Soares GB, Hatch JP, Nery FG, Matsuo K, Sanches M, Soares JC. A longitudinal study of fronto-limbic brain structures in patients with bipolar I disorder during lithium treatment. Journal of affective disorders. 2013;150:629–633. doi: 10.1016/j.jad.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Molecular psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: A pilot study. Biological psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Molecular psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- van der Werf-Eldering MJ, van der Meer L, Burger H, Holthausen EA, Nolen WA, Aleman A. Insight in bipolar disorder: associations with cognitive and emotional processing and illness characteristics. Bipolar disorders. 2011;13:343–354. doi: 10.1111/j.1399-5618.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Thompson PM, Kieseppa T, Bearden CE, Marino AC, Hoftman GD, Haukka J, Partonen T, Huttunen M, Kaprio J, Lonnqvist J, Poutanen VP, Toga AW, Cannon TD. Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar cotwins, and control twins. Human brain mapping. 2012;33:501–510. doi: 10.1002/hbm.21239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- Yatham LN, Kennedy SH, Schaffer A, Parikh SV, Beaulieu S, O’Donovan C, MacQueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Young AH, Alda M, Milev R, Vieta E, Calabrese JR, Berk M, Ha K, Kapczinski F. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2009. Bipolar disorders. 2009;11:225–255. doi: 10.1111/j.1399-5618.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacology. 2007;195:357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]
- Yucel K, Taylor VH, McKinnon MC, MacDonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Medical Imaging, IEEE Transactions on. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


