Abstract
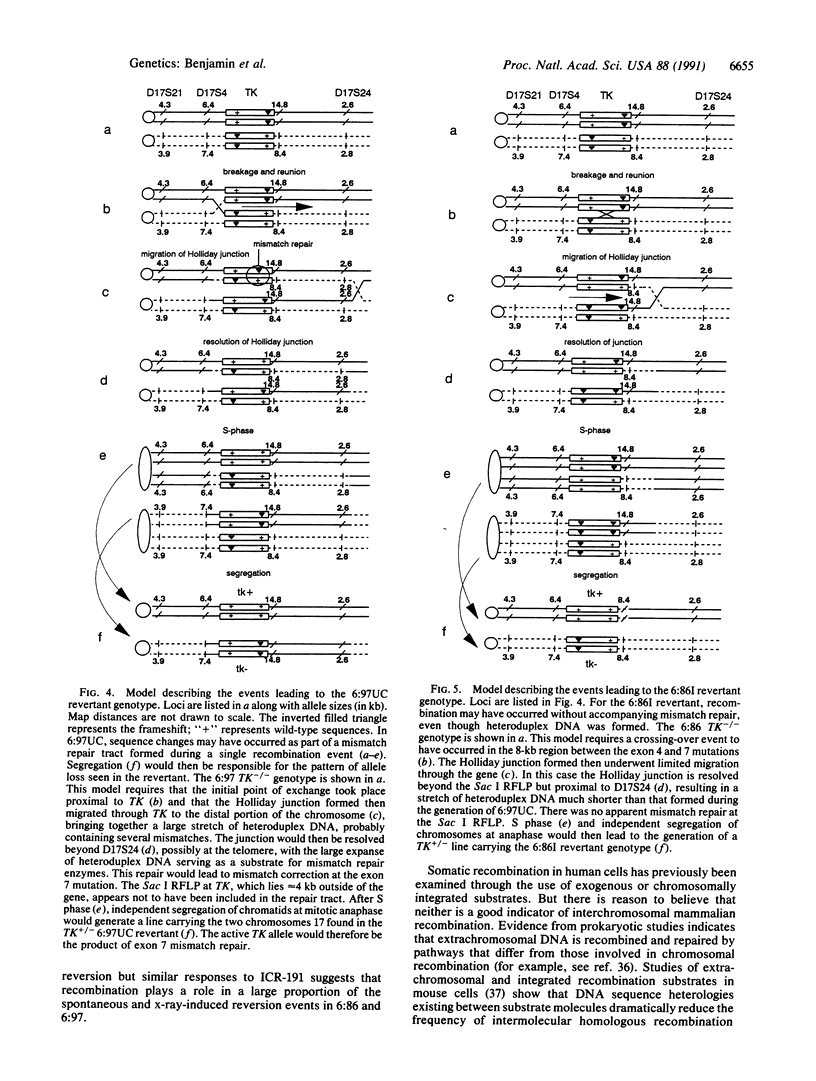
A system for assaying human interchromosomal recombination in vitro was developed, using a cell line containing two different mutant thymidine kinase genes (TK) on chromosomes 17. Heteroalleles were generated in the TK+/+ parent B-lymphoblast cell line WIL-2 by repeated exposure to the alkylating nitrogen mustard ICR-191, which preferentially causes +1 or -1 frameshifts. Resulting TK-/- mutants were selected in medium containing the toxic thymidine analog trifluorothymidine. Mutations were characterized by exon-specific polymerase chain reaction amplification and direct sequencing. In two lines, heterozygous frameshifts were located in exons 4 and 7 of the TK gene separated by approximately 8 kilobases. These lines undergo spontaneous reversion to TK+ at a frequency of less than 10(-7), and revertants can be selected in cytidine/hypoxanthine/aminopterin/thymidine medium. The nature and location of these heteroallelic mutations make large deletions, rearrangements, nondisjunction, and reduplication unlikely mechanisms for reversion to TK+. The mode of reversion to TK+ was specifically assessed by DNA sequencing, use of single-strand conformation polymorphisms, and analysis of various restriction fragment length polymorphisms (RFLPs) linked to the TK gene on chromosome 17. Our data suggest that a proportion of revertants has undergone recombination and gene conversion at the TK locus, with concomitant loss of frameshifts and allele loss at linked RFLPs. Models are presented for the origin of two recombinants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Krakauer T., Camerini-Otero R. D. DNA-mediated gene transfer: recombination between cotransferred DNA sequences and recovery of recombinants in a plasmid. Proc Natl Acad Sci U S A. 1982 May;79(9):2748–2752. doi: 10.1073/pnas.79.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N. P., Maher V. M., McCormick J. J. Intrachromosomal homologous recombination in human cells which differ in nucleotide excision-repair capacity. Mutat Res. 1990 Feb;234(1):31–41. doi: 10.1016/0165-1161(90)90028-m. [DOI] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Deininger P. L. Human thymidine kinase gene: molecular cloning and nucleotide sequence of a cDNA expressible in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2316–2320. doi: 10.1128/mcb.4.11.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P., Miller J., Botchan M. Effects of poly[d(pGpT).d(pApC)] and poly[d(pCpG).d(pCpG)] repeats on homologous recombination in somatic cells. Mol Cell Biol. 1986 Nov;6(11):3948–3953. doi: 10.1128/mcb.6.11.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Dao D. D., Schroeder W. T., Chao L. Y., Kikuchi H., Strong L. C., Riccardi V. M., Pathak S., Nichols W. W., Lewis W. H., Saunders G. F. Genetic mechanisms of tumor-specific loss of 11p DNA sequences in Wilms tumor. Am J Hum Genet. 1987 Aug;41(2):202–217. [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Flemington E., Bradshaw H. D., Jr, Traina-Dorge V., Slagel V., Deininger P. L. Sequence, structure and promoter characterization of the human thymidine kinase gene. Gene. 1987;52(2-3):267–277. doi: 10.1016/0378-1119(87)90053-9. [DOI] [PubMed] [Google Scholar]
- Groden J., Nakamura Y., German J. Molecular evidence that homologous recombination occurs in proliferating human somatic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4315–4319. doi: 10.1073/pnas.87.11.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser H. M., Henderson D. J., Chen C. W., Hopwood D. A. A mutation of Streptomyces lividans which prevents intraplasmid recombination has no effect on chromosomal recombination. Mol Gen Genet. 1989 Dec;220(1):60–64. doi: 10.1007/BF00260856. [DOI] [PubMed] [Google Scholar]
- Kondoleon S., van Tuinen P., Ledbetter D. H., Vissing H., Litt M. An anonymous single-copy clone, pC63, from chromosome 17q23-qter identifies a frequent RFLP [HGM9 No. D17S21]. Nucleic Acids Res. 1987 Nov 11;15(21):9096–9096. doi: 10.1093/nar/15.21.9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Intermolecular recombination between DNAs introduced into mouse L cells is mediated by a nonconservative pathway that leads to crossover products. Mol Cell Biol. 1990 Jan;10(1):103–112. doi: 10.1128/mcb.10.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Repair of double-stranded DNA breaks by homologous DNA fragments during transfer of DNA into mouse L cells. Mol Cell Biol. 1990 Jan;10(1):113–119. doi: 10.1128/mcb.10.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez B., Coppey J. Promotion of double-strand break repair by human nuclear extracts preferentially involves recombination with intact homologous DNA. Nucleic Acids Res. 1987 Sep 11;15(17):6813–6826. doi: 10.1093/nar/15.17.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos G. L., Cotsell J. N. Chromosome structure at interfaces between major chromatin types: alpha- and beta-heterochromatin. Bioessays. 1990 Jan;12(1):1–6. doi: 10.1002/bies.950120102. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Calos M. P., Combépine C., Hofer M. Further correlations of the lacI genetic map with the DNA sequence. J Mol Biol. 1981 Nov 25;153(1):65–66. doi: 10.1016/0022-2836(81)90526-x. [DOI] [PubMed] [Google Scholar]
- Murnane J. P., Young B. R. Nucleotide sequence analysis of novel junctions near an unstable integrated plasmid in human cells. Gene. 1989 Dec 7;84(1):201–205. doi: 10.1016/0378-1119(89)90157-1. [DOI] [PubMed] [Google Scholar]
- Myers R., Nakamura Y., Ballard L., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pRMU3 on chromosome 17q [D17S24]. Nucleic Acids Res. 1988 Jan 25;16(2):784–784. doi: 10.1093/nar/16.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Lathrop M., O'Connell P., Leppert M., Barker D., Wright E., Skolnick M., Kondoleon S., Litt M., Lalouel J. M. A mapped set of DNA markers for human chromosome 17. Genomics. 1988 May;2(4):302–309. doi: 10.1016/0888-7543(88)90018-3. [DOI] [PubMed] [Google Scholar]
- Oeschger N. S., Hartman P. E. ICR-induced frameshift mutations in the histidine operon of Salmonella. J Bacteriol. 1970 Feb;101(2):490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki M., Nishisho I., Tateishi H., Motomura K., Yamamoto M., Miki T., Hayakawa T., Takai S., Honjo T., Mori T. Loss of genes on the long arm of chromosome 22 in human meningiomas. Mol Biol Med. 1988 Feb;5(1):15–22. [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Zeff R. A., Frankel W., Rajan T. V. Mitotic recombination between homologous chromosomes generates H-2 somatic cell variants in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1634–1637. doi: 10.1073/pnas.84.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. Mismatch repair and the fidelity of genetic recombination. Genome. 1989;31(1):68–73. doi: 10.1139/g89-014. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Relative rates of homologous and nonhomologous recombination in transfected DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3355–3359. doi: 10.1073/pnas.82.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Stankowski L. F., Jr, Tindall K. R., Hsie A. W. Quantitative and molecular analyses of ethyl methanesulfonate- and ICR 191-induced mutation in AS52 cells. Mutat Res. 1986 Apr;160(2):133–147. doi: 10.1016/0027-5107(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Wahls W. P., Wallace L. J., Moore P. D. Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell. 1990 Jan 12;60(1):95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Differential effects of base-pair mismatch on intrachromosomal versus extrachromosomal recombination in mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5340–5344. doi: 10.1073/pnas.84.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Maher V. M., Liskay R. M., McCormick J. J. Carcinogens can induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mol Cell Biol. 1988 Jan;8(1):196–202. doi: 10.1128/mcb.8.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Vock Hall L. Genetic demonstration of mitotic recombination in cultured Chinese hamster cell hybrids. Cell. 1984 Mar;36(3):697–707. doi: 10.1016/0092-8674(84)90350-7. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P. Detection of DNA sequence polymorphisms by enzymatic amplification and direct genomic sequencing. Am J Hum Genet. 1989 Oct;45(4):547–555. [PMC free article] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P., Little J. B. Somatic mutations at a heterozygous autosomal locus in human cells occur more frequently by allele loss than by intragenic structural alterations. Somat Cell Mol Genet. 1986 May;12(3):255–263. doi: 10.1007/BF01570784. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Little J. B. Chromosome 14 marker appearance in a human B lymphoblastoid cell line of nonmalignant origin. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):231–239. doi: 10.1016/0165-4608(86)90078-6. [DOI] [PubMed] [Google Scholar]