Abstract
Background
Roughly, 10% of elderly patients develop postoperative cognitive dysfunction. General anesthesia impairs spatial memory in aged rats, but the mechanism is not known. Hippocampal neurogenesis affects spatial learning and memory in rats, and isoflurane affects neurogenesis in neonatal and young adult rats. We tested the hypothesis that isoflurane impairs neurogenesis and hippocampal function in aged rats.
Methods
Isoflurane was administered to 16-month-old rats at one minimum alveolar concentration for 4 h. FluoroJade staining was performed to assess brain cell death 16 h after isoflurane administration. Dentate gyrus progenitor proliferation was assessed by bromodeoxyuridine injection 4 days after anesthesia and quantification of bromodeoxyuridine +cells 12 h later. Neuronal differentiation was studied by determining colocalization of bromodeoxyuridine with the immature neuronal marker NeuroD 5 days after anesthesia. New neuronal survival was assessed by quantifying cells coexpressing bromodeoxyuridine and the mature neuronal marker NeuN 5 weeks after anesthesia. Four months after anesthesia, associative learning was assessed by fear conditioning. Spatial reference memory acquisition and retention was tested in the Morris Water Maze.
Results
Cell death was sporadic and not different between groups. We did not detect any differences in hippocampal progenitor proliferation, neuronal differentiation, new neuronal survival, or in any of the tests of long-term hippocampal function.
Conclusion
In aged rats, isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome.
ROUGHLY, 10% of elderly patients suffer from postoperative cognitive dysfunction (POCD) 3 months after surgery.1,2 The mechanism of cognitive dysfunction is not well understood but does not involve hypoxemia or hypotension.1 General anesthesia has been implicated as a possible cause for POCD, because surgery in the elderly often involves general anesthesia. Furthermore, general anesthesia impairs spatial memory in aged rats.3–5 Spatial memory is considered a hippocampal cognitive domain, although other brain areas can contribute to spatial learning and memory as well.6 An important determinant of hippocampal function in animals is the degree to which new neurons are generated from stem cells in the subgranular zone of the dentate gyrus (DG) of the hippocampus.7–11 DG neurogenesis decreases progressively with age,12–14 but both a decrease in stress hormone levels12 and environmental enrichment15 can restore neurogenesis and improve spatial memory function in aged mice.15
We found that isoflurane impairs progenitor proliferation in the DG of 7-day-old rats and causes a cognitive deficit lasting for at least 8 months.16 Here, we tested the hypothesis that isoflurane impairs neurogenesis and hippocampal function in aged rats by assessing isoflurane-induced DG progenitor proliferation, neuronal differentiation, new neuronal survival, and long-term hippocampal function, using fear conditioning and Morris Water Maze tasks. We did not detect any effect of isoflurane on brain cell death, neurogenesis, or long-term hippocampal function in aged rats.
Materials and Methods
Rat Anesthesia
With approval from the Institutional Animal Care and Use Committee of the University of California San Francisco, San Francisco, CA, male 16-month-old rats (n = 37) were anesthetized in groups of 7–10 using isoflurane in 50% of oxygen–nitrogen. Each group contained one cardiorespiratory control animal (total n = 5), which had a 24-g polyethylene catheter inserted into the tail artery after induction of general anesthesia for hourly blood gas analysis and invasive blood pressure measurements. The cardiorespiratory control rats were not used for any other part of the study. Rats were placed in a preheated, humidified anesthetic chamber primed with 50% of oxygen–nitrogen containing 1.9–2.3% of isoflurane. The chamber was part of a semiclosed anesthetic circuit incorporating a fan recirculating waste gas via a carbon dioxide–absorbing canister filled with soda lime and a humidifier back into the anesthetic chamber. Fresh gas flow was 6 l/min. Gas composition within the anesthetic chamber was measured using a calibrated Datex Capnomac Ultima (Datex Instrumentarium Corp., Helsinki, Finland). The anesthetic concentration was titrated to 1 minimum alveolar concentration (MAC), the anesthetic concentration at which 50% of animals do not move in response to a painful stimulus. A supramaximal pain stimulus was generated by application of an alligator clamp to the rat’s tail for 30 s or until the rat moved. Movement was defined as any movement except breathing. Tail clamping was repeated every 15 min, starting 15 min after induction of general anesthesia. The anesthetic concentration was changed according to the empirically derived algorithm in table 1, which takes into account an overall tendency for the inspired isoflurane concentration to decrease during the initial 45–60 min, presumably because of incomplete equilibration of inspired and brain anesthetic concentrations. When less than 10 animals were anesthetized, the percentage of animals that moved was calculated, rounded to the nearest multiple of 10, and entered into the algorithm in table 1.
Table 1.
Algorithm for Changing Isoflurane Concentration in Response to Tail Clamping
| Number of Animals Moving in Response to Tail Clamping | Change in Isoflurane Concentration, %
|
|
|---|---|---|
| First Hour | Second–Fourth Hours | |
| 0 | −0.8 | −0.6 |
| 1 | −0.5 | −0.4 |
| 2 | −0.3 | −0.3 |
| 3 | −0.2 | −0.2 |
| 4 | −0.1 | −0.1 |
| 5 | No change | No change |
| 6 | No change | −0.1 |
| 7 | −0.1 | −0.2 |
| 8 | −0.2 | −0.3 |
| 9 | −0.4 | −0.4 |
| 10 | −0.6 | −0.6 |
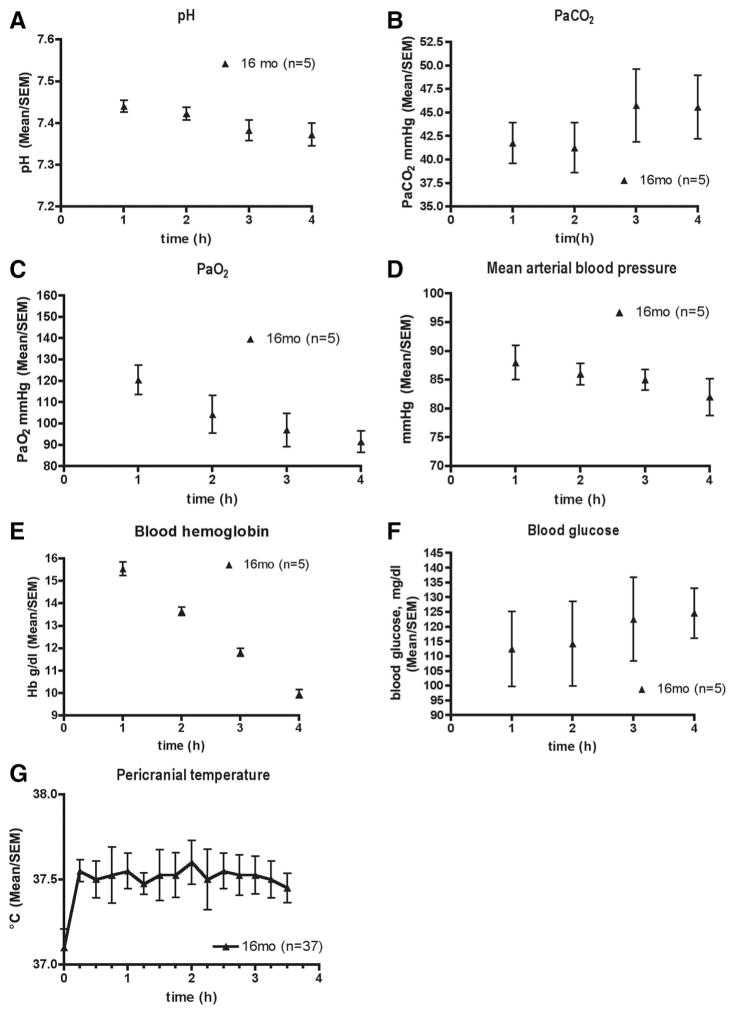
Hemoglobin oxygen saturation and heart rate were measured by application of a rodent transreflectance sensor (Nonin 2000T; Nonin Medical Inc., Plymouth, MN) to the ventral thoracic chest wall, where they are easily obtained and identical to hemoglobin oxygen saturation readings acquired by probe application to the head. The probe was attached to a Nonin V8600 pulse oximeter (Nonin Medical Inc.). At various intervals throughout the anesthetic pH, arterial carbon dioxide tension, arterial oxygen tension, base excess, blood hemoglobin, and blood glucose was analyzed by a blood gas analyzer (ABL 520, Radiometer, Copenhagen, Denmark). Blood (0.25 ml) was withdrawn every hour from the tail cannula of the designated homeostatic control rat, which did not take part in any other part of the study. The tail cannula was flushed with lactated Ringer’s solution.
Custom-made temperature probes were inserted into the temporalis muscle to individually control pericranial temperature at 37.5° ± 1°C using computer-controlled Pelitier heater or cooler plates integrated into the floor of the anesthesia box. After 4 h of isoflurane anesthesia and complete recovery, rats were returned to their home cages.
Sham Anesthesia
Control rats (n = 32) were placed in the warmed, humidified anesthesia box insufflated with oxygen–nitrogen at a fraction of inspired oxygen of 50% without anesthetic gas. After 4 h, rats were returned to their home cages.
Timing of Outcomes Assessment Relative to Anesthesia
Cell death was assessed 16 h after anesthesia. Progenitor proliferation was assessed 5 days after the anesthetic, namely, 12 h after the last of two bromodeoxyuridine injections given at a 12-h interval on day 4 after anesthesia, because this was previously found to be the peak of progenitor proliferation in 60-day-old rats.17 A detailed time course of progenitor proliferation after anesthesia did not seem warranted unless justified by behavioral deficits or altered net neurogenesis. Neuronal differentiation was determined on day 5 after the anesthetic, 12 h after the last of two bromodeoxyuridine injections administered on the fourth day after anesthesia. This is enough time for neural progenitors to express immature neuronal markers17 while the number of bromodeoxyuridine+ cells remains unaffected by programmed cell death.18 New neuronal survival was assessed 28 days after the last of eight bromodeoxyuridine injections on days 4–7 after anesthesia because new neurons present 4 weeks after bromodeoxyuridine labeling survive long term.18 Neurocognitive function was assessed 4 months after anesthesia to model long-term neurocognitive outcome of anesthesia in aged rats and for the purposes of comparability to similarly conducted studies in young adult and neonatal rats.17 The number of animals used for these experiments was six per group except for neurocognitive tests in which 14 animals per group entered the study.
Brain Cell Death
FluoroJade sensitively stains both apoptotic and necrotic cells.19 As a positive control, one rat underwent intraperitoneal injection of 10 mg/kg of kainic acid, which is known to cause cell death in the CA-3 and CA-1 of the hippocampus,20 16 h later that was followed by transcardiac perfusion, brain extraction, and sectioning to 40-μm coronal sections. Slices of the isoflurane-treated and control groups were mounted on glass slides next to one positive control slice on each slide. Slides were covered in 100% ethanol for 3 min, 70% ethanol for 1 min, and then deionized water (dH2O) for 1 min. Tissue was incubated with 0.06% potassium permanganate for 20 min on a shaker at room temperature (RT) followed by a 1-min wash with dH2O. Slides were then incubated with FluoroJade staining solution (4 ml FluoroJade [0.01%] in dH2O stock solution, 36 ml acetic acid [0.1%] in dH2O, 40 μl 4′,6-diamidino-2-phenylindole) for 30 min at RT followed by three washes with dH2O for 1 min each. After air drying, slides were briefly rinsed with xylene and coverslipped with Depex mounting solution (Electron Microscopy Sciences, Fort Washington, PA). FluoroJade+ cells in the entire brain slice were counted by immunofluorescent microscopy of every twelfth coronal brain section.
All brain structures were systematically analyzed, guided by Paxino’s Atlas of the rat brain,21 as described previously.22
Proliferation
Bromodeoxyuridine, 50 mg/kg (15 mg/ml; Sigma, St. Louis, MO), was injected twice intraperitoneally with a 12-h interval on the fourth day after isoflurane (n = 6) or sham anesthesia (controls, n = 6). Twelve hours later, animals were briefly and deeply anesthetized with isoflurane and then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4. The brains were removed, postfixed overnight in 4% paraformaldehyde or PBS, and placed in 20% sucrose until sunk. Forty micrometer coronal sections were cut on a microtome and stored in PBS. Incubation with 50% formamide in PBS for 2 h at 65°C preceded 2 N hydrochloric acid treatment for 30 min at 37°C and neutralization with 0.5 M boric acid (pH 8.5) for 10 min at RT and three washes with PBS for 10 min in between each step. Blocking of nonspecific epitopes with 2% serum and 0.1% bovine serum albumin in PBS with 0.3% Triton-X for 30 min at RT preceded incubation with the primary antibromodeoxyuridine antibody (Roche mouse-anti-BrdU, 1:400) in PBS and bovine serum albumin (0.2%) overnight at 4°C.
On the next day, incubation with a biotinylated antibody (Sheep antimouse-IgG,1:200; Amersham, Louisville, CO) was performed for 2 h at RT. Streptavidin–biotin treatment for 2 h at RT (Vectastain, ABC kit; Vector laboratories, Burlingame, CA) was followed by three thorough washes to eliminate residual peroxidase activity and by incubation with diaminobenzidine + urea (Fast DAB tablets; Sigma) with nickel chloride augmentation (7.5 μl of 8% stock in 25 ml) for 5 min. Slides were then mounted and a cover glass was applied with Depex mounting medium. The density of bromodeoxyuridine+ cells per DG was detected using brightfield microscopy with a 20× objective by an observer blinded to group assignment. The subgranular zone and the inner-to-mid granule cell layer of the DG were defined as the regions of interest. One random hemisphere per slice was used in which the area of interest was outlined using Stereo-Investigator software (MBF Bioscience, Williston, VT). The number of bromodeoxyuridine+ cells in the area of interest was counted and multiplied by 12, because every twelfth section was assessed.
Neuronal Differentiation
Isoflurane-treated (n = 6) and control (n = 6) rats received intraperitoneal injections of bromodeoxyuridine (50 mg/kg) twice at 12 hourly intervals on the fourth day after anesthesia. Twelve hours later, animals were transcardially perfused and coronal brain sections were prepared as described for assessment of proliferation. Immunohistochemical staining for bromodeoxyuridine and the immature neurononal marker NeuroD was performed using primary (rat monoclonal anti-BrdU, 1:200; Serotec, Raleigh, NC, goat polyclonal anti-NeuroD, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) and fluorescent secondary (AlexaFluor 488, A21202, IgG, 1:500 and AlexaFluor 594, A21209, IgG, 1:500, Invitrogen, Carlsbad, CA). Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (1:1000, Sigma) followed by mounting and coverslipping with an aqueous mounting medium. The granule cell layer and the adjacent subgranular zone were traced using StereoInvestigator software (MicroBrightField, Williston, VT). The proportion of bromodeoxyuridine+ cells that coexpressed the immature neuronal marker NeuroD as well as the density of these cells was assessed by an observer blinded to group assignment in every twelfth slice. Colocalization was confirmed using image stacks acquired by a laser scanning confocal microscope (Zeiss LSM 510, 63× NA 1.3) throughout the entire thickness of tissue containing bromodeoxyuridine+ cells.
New Neuronal Survival
Because cell division is a rare event in the subgranular zone of elderly rats and because many of these newborn cells do not survive, we were concerned that a short bromodeoxyuridine-labeling protocol, such as the one used to assess proliferation, would not yield a new neuronal number sufficient for a meaningful statistical analysis. Therefore, four times the amount of bromodeoxyuridine was administered, namely, 50 mg/kg eight times at 12-h intervals on days 4–7 after anesthesia. After the last of these injections, the animals survived for an additional 28 days before transcardiac perfusion at 17 months of age. Immunocytochemistry was performed as described earlier except that an antibody against the mature neuronal marker NeuN was used (1:200; Chemicon, Temecula, CA). The proportion of bromodeoxyuridine+ cells that coexpressed the mature neuronal marker NeuN as well as the density of these cells was assessed by an observer blinded to group assignment in every twelfth slice. Colocalization was confirmed using image stacks acquired by a laser scanning confocal microscope (Zeiss LSM 510, 63× NA 1.3) throughout the entire thickness of tissue containing bromodeoxyuridine+ cells. The granule cell layer and the adjacent subgranular zone were traced using StereoInvestigator software. The number of bromodeoxyuridine+ cells per slice was multiplied by 12 (every twelfth slice counted) to yield the total number of new neurons per DG that survived for at least 28 days.
Fear Conditioning
Four rats, counterbalanced for group assignment, were trained at a time. The chambers (32 cm long × 25 cm wide × 25 cm high) were constructed of clear acrylic. The grid floor used to deliver a foot shock was composed of 19 stainless steel bars, each 4 mm in diameter and spaced 16 mm center to center. These floors were connected to a shock delivery system (Med Associates, St. Albans, VT). The chambers were wiped with a pine-scented cleaner (5% Pine Scented Disinfectant; Midland, Inc., Sweetwater, TN) before and after each session. The room in which training took place was illuminated with overhead fluorescent bulbs, and a ventilation fan provided background noise (65 dB). The appearance, odor, and texture of the chambers and room comprised the training context.
After a 3-min baseline exploratory period in the chambers, rats received 3 tone (2000 Hertz, 90 dB)-shock (1 mA, 2 s) pairings, separated by 1 min. Freezing, the absence of all movement except that necessary for respiration, is an innate defensive fear response in rodents and a reliable measure of learned fear.23 Each animal’s behavior was scored every 8 s during the observation period and a percentage was calculated using the formula 100 × f/n, where f is the number of freezing events per rat and n is the total number of observations per rat.
The following day, rats were tested for fear to the training context and fear to tone. For the context test, each rat was once again placed in the chamber in which it was trained for a period of 8 min (in the absence of tone and shock). For the tone test, groups of rats were transported in separate plastic pots (14 cm high × 15.5 cm diameter) to a distinct context in a different room. The test chambers were triangular in shape with an acrylic floor (28 cm long × 25 cm wide) and two acrylic sidewalls (28 cm long × 22 cm wide) at a 45° angle. The chambers were equipped with a speaker and were wiped down with acetic acid (1%; Fisher Scientific, St. Louis, MO) before and after each session. The room appeared dark to the rats, being lit by a single 30-W red bulb. A different kind of white noise (65 dB) was used for background noise. Rats were given a 3-min exploratory period, followed by three 30-s tones (2000 Hz, 90 dB), separated by 60 s. Rats were removed from the chamber after an additional 30 s. The order of the context and tone tests was counterbalanced, such that half of each treatment group was tested to context first and tone second and vice versa. Freezing was scored by two observers blinded to the group assignment during the 3-min exploratory period, the training, and both tests.
Morris Water Maze Testing
Two cohorts of 20-month-old rats underwent testing of spatial reference memory acquisition and retention in the Morris water maze 4 months after 4 h of isoflurane administered at 16 months of age. In this test, the ability of rats to locate a submerged platform in a circular pool (180-cm diameter, 50-cm deep) filled with warm (24°C) opaque water was assessed. The rats were trained first to locate the round (4-in diameter) platform located 1 in below the surface of the water from which a flag emerged (cued trials) and then to locate the unmarked, hidden platform (place trials). In a subsequent trial, the platform was removed (probe trial) and memory retention was assessed by evaluating the proportion of time rats spent searching for the platform in the target quadrant versus the other quadrants. Rats received two training sessions per day for 6 consecutive days. The probe trial was performed on the seventh day. Each session consisted of three trials with a 60-s intertrial interval. The interval between the two daily sessions was 5 h. Once the rats located the platform, they were allowed to remain on it for 20 s. Time to reach the platform (latency), path length, and swimming speed were recorded with a video tracking system (Etho Vision; Noldus Information Technology, Wageningen, Holland) set to analyze 10 samples per second. To assign weight to proximity to the platform during the search, we chose as a primary outcome variable, the time-integrated distance to the platform.
Statistical Methods
Data were expressed as mean and 95% confidence interval of the mean after confirming that the data satisfied parametric assumptions using D’Agostino and Person’s omnibus normality test. A two-tailed Student t test was performed to determine differences between groups. Morris water maze data were analyzed by “mixed model regression” using SAS Version 9 (SAS Institute, Cary, NC) Proc MIXED. A model allowing quadratic trends and treatment by trend interactions was used first, from which nonstatistically significant terms were sequentially dropped, starting with nonstatistically significant random effects then dropping higher-order interactions. When all terms remaining in the model were considered statistically significant or required to be in for face validity (e.g., session number), the model was declared a “final” model. Terms remaining in the model at this point were group, session, and a quadratic session-by-session interaction. Dropped terms were the variance of the animal-specific slopes, a quadratic term-by-group interaction testing whether the curvature in the trend is the same for the two groups, and a session-by-group interaction. Post hoc tests were used to compare the least squares means and Fisher’s protected test was used to adjust for multiple comparisons. Probe trial data were analyzed using a Student t test. A P value of less than 0.05 was considered significant.
Sample Size Calculation
For immunocytochemistry, a minimal group size of six animals was required to detect a difference between means of 40% with an 80% power at a significance level of 0.05. This was based on the effect size of isoflurane on DG progenitor proliferation in young adult rats, which was 43% (bromodeoxyuridine+ cells/DG isoflurane 1499; control 871; SD isoflurane 569; control 589).17
For the behavioral tests, the sample size required to detect a 30% difference between groups at 80% power and a significance level of 0.05 was 12 animals per group. This was based on the effect size of isoflurane on the combined tone test and context test data 5 months after isoflurane in neonatal rats, which was 34% (SD of the outcome measure control 0.09; isoflurane 0.14).17 The rationale for not basing this portion of the sample size calculation on data from young adult rats as well is the fact that fear conditioning in young adult rats is not affected by isoflurane and spatial reference memory in fact improves after anesthesia in young adult rat.3,17 Given the animal data on neurocognitive outcome of anesthesia in aged rats (see Introduction section), it would have been unreasonable to hypothesize that isoflurane improves long-term neurocognitive outcome of aged rats. Therefore, the hypothesis that neurocognitive function would be adversely affected by isoflurane in aged rats required basing the expected effect size on data obtained from animals anesthetized as neonates in which isoflurane impairs long-term neurocognitive outcome. A group size of 14 was chosen to include a margin of error. SAS Version 9 Proc POWER was used for all sample size calculations.
Results
The inspired anesthetic concentration resulting in the movement of roughly 50% of animals is shown in figure 1 and is characterized by an initial decrease of more than 45–60 min followed by a stable plateau at roughly 1.5% isoflurane. Physiologic variables associated with this anesthetic are shown in figure 2. A total of four rats died, none during the anesthetic. One rat in the isoflurane group died after an intraperitoneal injection of bromodeoxyuridine. Three rats died while maturing to 20 months, two from abdominal tumors and one from unknown causes.
Fig. 1.
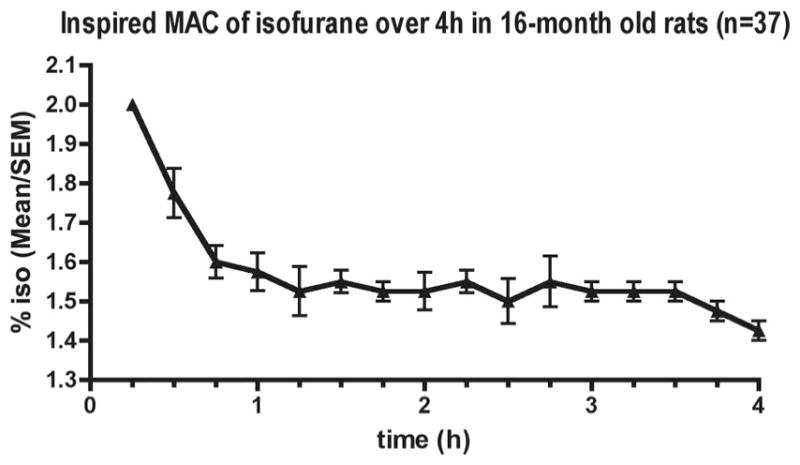
Minimum alveolar concentration (MAC) of isoflurane (iso) in 16-month-old rats. MAC was determined by tail clamping of simultaneously anesthetized rats (n = 7–10). Data are from five separate experiments.
Fig. 2.
Physiologic variables over 4 h of isoflurane anesthesia in 16-month-old rats. Data are pH (A), arterial carbon dioxide tension (PaCO2) (B), arterial oxygen tension (PaO2) (C), mean arterial blood pressure (D), blood hemoglobin (Hb) concentration (E), blood glucose concentration (F), and pericranial temperature (G) from five cardiorespiratory control animals that were not used for any other part of the study. The exception is pericranial temperature, which was computer-controlled and recorded in all animals individually (n = 37).
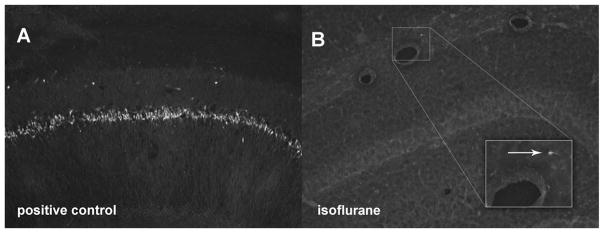
Only sporadic cell death was detected in the brain 16 h after anesthesia or sham anesthesia (fig. 3).
Fig. 3.
FluoroJade (FJ) stain of the hippocampus of 16-month-old rats 16 h after isoflurane (n = 6) or no isoflurane (control, n = 6). Extensive cell death in the CA-1 area of a positive control animal injected with kainic acid (10 mg/kg) 16 h before transcardiac perfusion (A). Almost complete absence of cell death in the hippocampus of a representative isoflurane-treated animal (B). Magnified view of one FJ-positive cell (arrow). Sham-anesthetized animals likewise had no significant detectable cell death (data not shown).
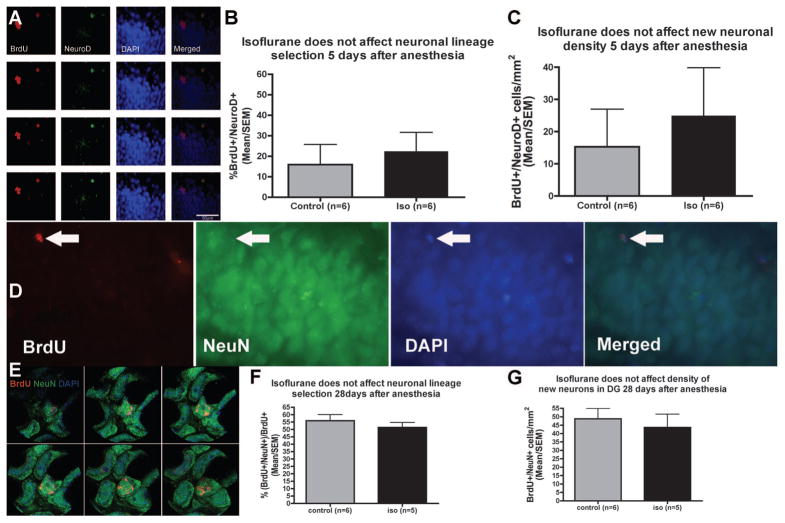
The mean number of bromodeoxyuridine+ cells/DG was 47 (95% CI, 27–66) in the isoflurane group, which is not significantly different from the mean in controls (60, 95% CI: 51–69, P = 0.24; fig. 4). The results of neuronal differentiation and new neuronal survival experiments are shown in figure 5. The proportion of cells expressing the early neuronal marker NeuroD 12 h after the last of the two bromodeoxyuridine injections on day 4 after anesthesia was 16% (95% CI, −15–47) in the control group and 22% (95% CI, −1–46) in the isoflurane group (P = 0.5; fig. 5B). The mean density of bromodeoxyuridine+ cells expressing the new neuronal marker NeuroD at this time was 15 cells/ mm2 (95% CI, −22–52) in controls and 25 cells/mm2 (95% CI, −13–63) in isofluraneanesthetized animals (P = 0.66; fig. 5C). The proportion of bromodeoxyuridine+ cells that expressed the mature neuronal maker NeuN 28 days after the last of the eight bromodeoxyuridine injections administered at 12-h intervals on days 4–7 after anesthesia was 56% (95% CI, 45–67%) in controls and 51% (95% CI, 42–61%) in isoflurane-treated animals (P = 0.62; fig. 5F). The density of bromodeoxyuridine+ cells expressing the mature neuronal marker NeuN at this time was 49 cells/mm2 (95% CI, 33–65) in controls and 44 cells/mm2 (95% CI, 22–65) in isoflurane-treated animals (P = 0.43; fig. 5G). There was no difference in the size of the dentate granule cell layer between groups (data not shown).
Fig. 4.
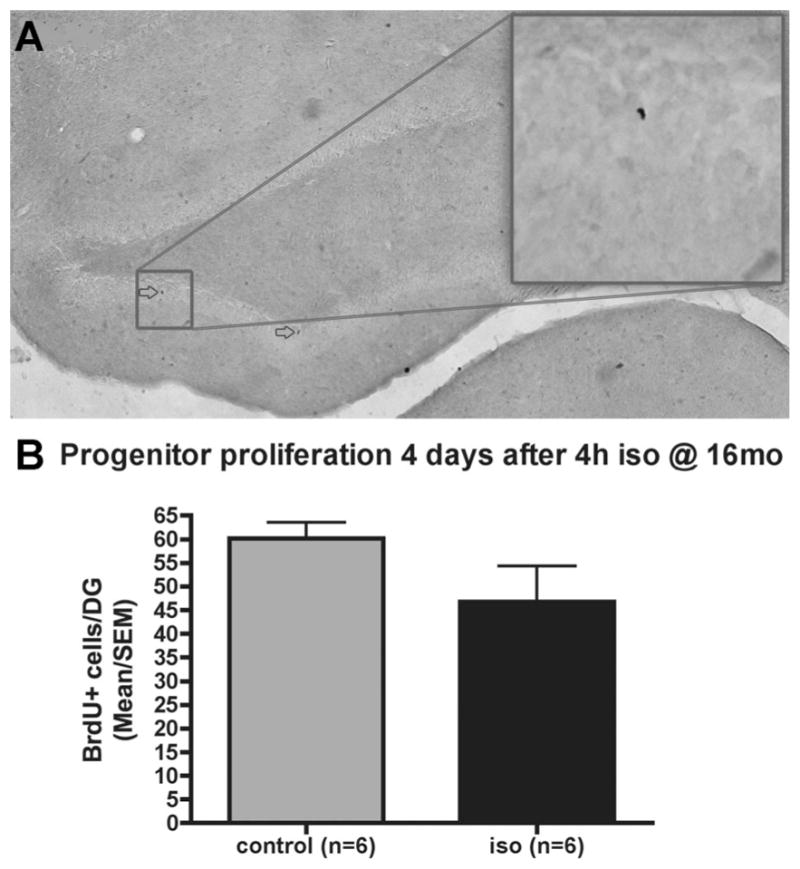
Progenitor proliferation in the dentate gyrus (DG) after 4 h of isoflurane (iso) in 16-month-old rats. Bromodeoxyuridine (BrdU) (50 mg/kg) was injected twice with a 12-h interval 4 days after 4 h of isoflurane (n = 6) or no isoflurane (control, n = 6). Immunocytochemical staining of bromodeoxyuridine+ cells (arrows in A, main image 4×, inset 20× objective) of every twelfth coronal section of the hippocampus and quantification of every bromodeoxyuridine+ cell (B) revealed no difference between groups (P > 0.2, Mann–Whitney U test).
Fig. 5.
Neuronal differentiation and new neuronal density are not affected by isoflurane (iso) in aged rats. Stack of microscopic images acquired 1 mm apart in the Z-plane (A) showing co-staining of the S-phase marker bromodeoxyuridine (BrdU) with the immature neuronal marker NeuroD 12 h after the last of two BrdU injections given intraperitoneally to aged rats on the fourth day after 4 h of isoflurane anesthesia. Neither the proportion of BrdU+ cells that coexpress NeuroD on day 5 after anesthesia (B) nor the density of BrdU+ /NeuroD+ cells (C) is affected by isoflurane. New neuronal survival 28 days after a 4-day pulse of bromodeoxyuridine (50 mg/kg × 8 at 12-hourly intervals) starting 4 days after 4 h of isoflurane anesthesia (D, G). Immunofluorescent image of a bromodeoxyuridine+ /NeuN+ cell (D, arrow) and an image stack acquired on a confocal microscope showing colocalization of two bromodeoxyuridine+ /NeuN+ cells (E). The proportion of bromodeoxyuridine+ cells that coexpress the mature neuronal marker NeuN 35 days after isoflurane (n = 5) or no isoflurane (control, n = 6) does not differ between groups (F). The density of bromodeoxyuridine+ /NeuN+ cells in the DG is not different between groups either (G). DAPI = 4′,6-diamidino-2-phenylindole.
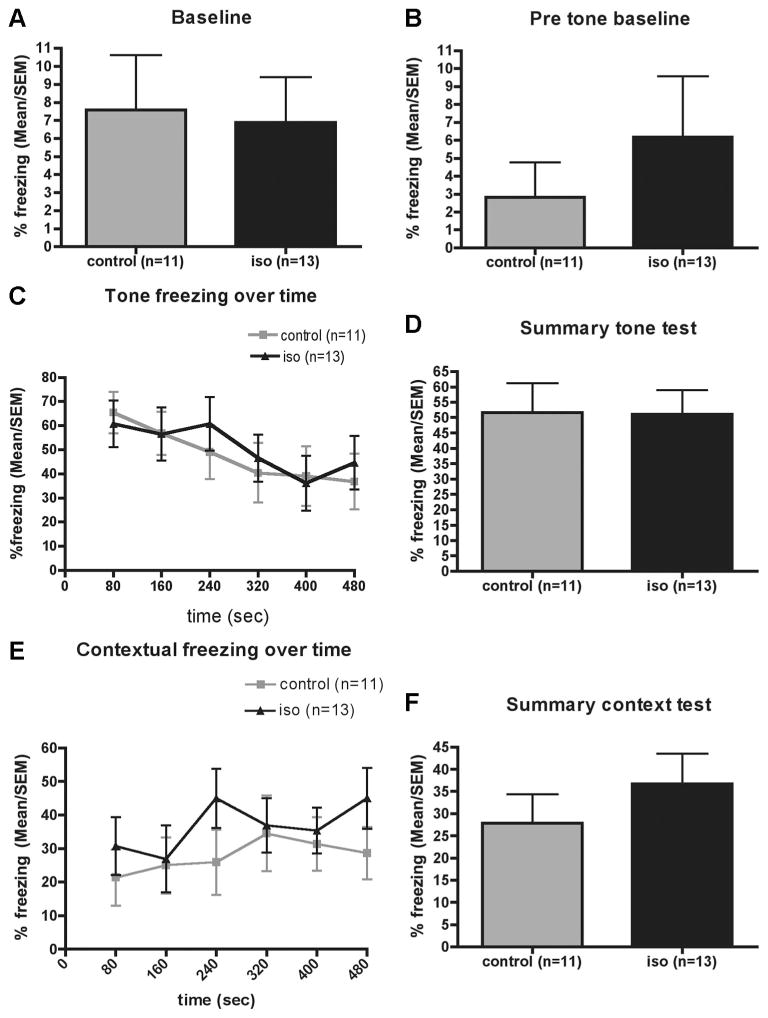
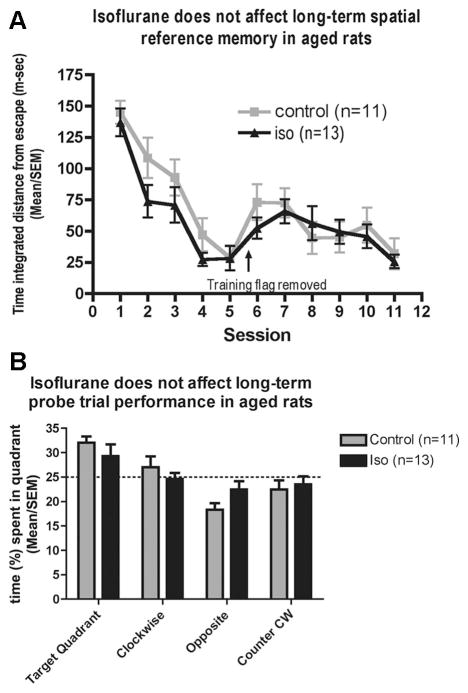
The level of unconditioned fear as determined by the amount of freezing was roughly 7% in either group at baseline (fig. 6A) and decreased further at the beginning of the second day to 3–6% with no difference between groups (fig. 6B). Isoflurane-anesthetized rats froze to a similar degree as control rats in both their response to exposure to the tone (figs. 6C and D) and the context (figs. 6E and F). In the Morris water maze, swim speed (data not shown), escape latency (data not shown), and time-integrated distance to the platform (fig. 7A) did not differ between groups. During the probe trial, isoflurane-anesthetized rats displayed a similar search pattern as control rats with both groups showing a marginal preference for the target quadrant (fig. 7B). At no point was the performance of isofluane-treated aged rats worse than that of controls.
Fig. 6.
Fear conditioning 4 months after 4 h of isoflurane (iso) anesthesia (n = 13) or sham anesthesia (control, n = 11) administered to 16-month-old rats. Unconditioned fear is measured by freeze scores at baseline before (A) and 24 h after exposure to the conditioned stimulus–unconditioned stimulus pairings (B). The time curve (C) and summary (D) of the tone test reveals no difference between groups in the degree to which the nonhippocampal-dependent association between the tone and the foot shock were formed. During the hippocampal-dependent context portion (E and F) of the test, there was also no difference in freeze scores between groups (P > 0.24, Mann–Whitney U test).
Fig. 7.
Morris water maze test 4 months after 4 h isoflurane (iso) anesthesia (n = 13) or sham anesthesia (control, n = 11) in 16-month-old rats. During the cued trials (data not shown), the submerged platform was marked with a flag, which was removed during the place trials (A), in which spatial memory is tested by measuring time-integrated distance to the platform allowing some credit to be assigned for searching in the general vicinity of the platform. The 90-s probe trial (platform removed) tests memory retention (B). The dotted line indicates the likelihood of time spent in quadrants by chance. No difference was found between groups in any of the measured parameters. CW = clockwise.
Discussion
The main findings of the study are that isoflurane does not cause neurodegeneration and does not affect hippocampal neurogenesis or long-term neurocongitive outcome in aged rats.
This is not in agreement with the findings of Culley et al.,3–5,24 who have consistently found memory deficits in aged rats up to 3 weeks after exposure to various types of anesthetic agents. In this study, no deficit was apparent 4 months after the anesthetic. This suggests that the deficit might be transient, which is consistent with the fact that Culley et al.24 were not able to demonstrate a neurocognitive deficit 8 weeks after 2 h of isoflurane–nitrous oxide (1.2%/ 70%), despite using the same methodology that showed a deficit before 5 weeks in the same rats. Despite obvious differences between rats and humans precluding extrapolation of rat data to the context of human clinical scenarios, this possible explanation is consistent with human data showing resolution of POCD over time. In the majority of patients diagnosed with POCD 1 week after surgery, there is no evidence of POCD 3 months later,1,2 and the majority of patients diagnosed with POCD 3 months after surgery do not suffer from POCD 1 or 2 yr after surgery.25
The rate of neurogenesis found in this study is consistent with previous reports of vastly reduced neurogenesis in old animals compared with young adults.15 It is noteworthy that the level of progenitor proliferation in old age can be restored to that of the young brain by environmental enrichment15 or reduction in stress hormone levels.12 It is possible that the low baseline levels of neurogenesis in aged rats made it more difficult to detect a potential effect of isoflurane on neurogenesis. It is therefore conceivable that augmentation of baseline neurogenesis by such measures as running or environmental enrichment may have unmasked a possible anesthetic effect on neurogenesis.
We did not detect any appreciable cell death 16 h after anesthesia. Both the timing and the cell death detection method used in this study (FluoroJade staining) should have allowed for detection of cell death if it had occurred. We base this conclusion on the following facts. Anesthesia-induced neurodegeneration has been well described in the developing rat brain.22,26–31 A triple anesthetic cocktail consisting of the N-methyl D-aspartate antagonist nitrous oxide as well as a γ-aminobutyric acid—A receptor-ergic agents isoflurane and midazolam caused massive cell death in the 7-day-old rat brain that is detectable by staining for caspase as early as 2 h after anesthesia and by silver staining as late as 18 h after the anesthetic.28 The full extent of neuronal cell death is evident 16 h after an apoptotic insult in 7-day-old rats.32 We conclude that the timing of FluoroJade staining should have allowed for detection of anesthesia-induced cell death. FluoroJade staining has been validated as a nonspecific but highly sensitive stain for dead brain cells.19 Using FluoroJade staining, ketamine-induced cell death is detected 24 h after anesthesia in rodents33 and after 24 h but not after 3 h of anesthesia in primates.34 Twelve hours after exposure to 1 MAC of isoflurane for 4 h, extensive cell death was demonstrated using FluoroJade staining in the brains of 7-day-old but not of 60-day-old rats.17,22 The absence of detectable cell death in this study at a postanesthesia interval that reliably detects cell death in various rodent models of anesthesia must therefore be interpreted as absence of cytotoxicity of isoflurane in the aged rat brain. This is not in agreement with in vitro findings. In pheochromcytoma cells,35,36 primary neurocortical cultures,36 and neuroglioma cell lines,37 isoflurane can cause apoptosis and, if sufficient amyloid β protein precursor is present, it may cause amyloid beta protein generation, which causes further apoptosis.37 This vicious cycle of apoptosis and altered amyloid β protein precursor processing represents a potential link between the pathoanatomic correlate of Alzheimer’s disease and POCD.37 However, it is unclear whether these in vitro findings also apply in vivo and whether histopathologic damage necessarily results in neurocognitive sequelae. In neonatal rats, anesthesia-induced cell death is indeed associated with neurocognitive decline, which can be demonstrated many months after anesthesia,17,28,26,27 although a causality between these two outcomes has been questioned.22 In contrast to the neurotoxicity of isoflurane in the 7-day-old rat brain, isoflurane is not only nontoxic, it is actually profoundly neuroprotective in the young adult rat brain.38,39 In adult rats, isoflurane, similar to other γ-aminobutyric acid-agonists, protects from the neurotoxic effects of the combination of the two N-methyl D-aspartate antagonists nitrous oxide and ketamine.38,39
Taken together, these findings indicate that depending on the experimental preparation and the age of the animal isoflurane can be either neurotoxic or neuroprotective.
In addition to not finding isoflurane-induced histopathology, we also did not detect an effect of isoflurane on neurogenesis or long-term neurocognitive outcome. We previously found an effect of isoflurane on DG progenitor proliferation and long-term neurocognitive outcome in both neonatal and young adult rats using a similar experimental design as used in this study.16,17,22 This comparability with studies with a known effect of isoflurane was the rationale for targeting progenitor proliferation 4 days after anesthesia and assessing neurocognitive function after 4 months. Future studies using different labeling protocols may clarify whether and how neurogenic events, such as progenitor proliferation or neuronal differentiation, are affected at other time points after isoflurane in aged rats.
The anesthetic dose used in this study was 1 inspired MAC, as guided by the response of a cohort of rats to a supramaximal pain stimulus (tail clamping). The inspired MAC of isoflurane decreases during the first hour of anesthesia and remains essentially stable thereafter. A similar initial decrease in inspired MAC was previously observed in young adult (2-month-old) rats.40 Once equilibration of the inspired and brain partial pressures of isoflurane is complete, MAC is stable in young adult rats40 as it was in these aged rats. Therefore, it seems reasonable to assume that the same relationship between inspired and brain partial pressures of isoflurane underlies the change of MAC over time in aged rats.
The density of new neurons seems to be greater 35 days after anesthesia (28 days after bromodeoxyuridine administered 4–7 days after anesthesia) than at 5 days after anesthesia (12 h after bromodeoxyuridine on fourth day after anesthesia). This apparent increase in new neuronal density is not in keeping with the fact that roughly 50% of neurons are eliminated between 1 and 4 weeks after they are born.18 Differences in bromodeoxyuridine-labeling protocols between the neuronal differentiation and new neuronal survival experiments may explain these inconsistencies. The 28-day survival groups received four times as much bromodeoxyuridine (50 mg/kg twice a day for 4 days) as the 12-h survival groups (50 mg/kg twice a day for 1 day), which resulted in a neuronal density that seems to be roughly twice as high as in the short-term labeling groups.
The fact that we were not able to detect a difference between isoflurane-treated and control animals in any of the measured outcomes does not allow to conclude that the two groups are equivalent. The study was not designed to show equivalence, which would have required a much larger sample size. However, because the 95% CIs of the mean overlap substantially between the two groups, that is, because isoflurane-treated animals showed no uniform trend toward poorer performance in the outcome measures, the chance that an effect of isoflurane exists that would have been revealed by a larger sample size seems remote.
In conclusion, we did not detect an effect of 4 h of isoflurane on cell death, neurogenesis, or long-term neurocognitive outcome in aged rats.
What We Already Know about This Topic
Postoperative cognitive dysfunction carries significant morbidity in the elderly
One possible mechanism for this problem is anesthetic-induced cell death or inhibition of neurogenesis
What This Article Tells Us That Is New
In aged rats, 4 h of exposure to isoflurane did not affect brain cell death or neurogenesis in the hippocampus, thus arguing against this as a mechanism of postoperative cognitive dysfunction
Acknowledgments
Supported by a Mentored Research Training Grant from the Foundation of Anesthesia Education and Research (FAER), Rochester, Minnesota.
The authors acknowledge the technical assistance of Angelo Encarnacio, B.S., and Nay Lui Saw, B.S., Research Assistants, Department of Anesthesia, University of California, San Francisco, San Francisco, California.
Footnotes
Presented at the Annual Meeting American Society of Anesthesiologists, Orlando, Florida, October 18–22, 2008.
Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue. Anesthesiology’s articles are made freely accessible to all readers, for personal use only, 6 months from the cover date of the issue.
References
- 1.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- 4.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99:1393–7. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- 5.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–14. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–44. [PubMed] [Google Scholar]
- 7.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 8.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–7. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 9.Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 11.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 12.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–82. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–43. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 16.Sall JW, Stratmann G, Leong J, McKleroy W, Mason D, Shenoy S, Pleasure SJ, Bickler PE. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–33. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 18.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 19.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: A novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 20.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–53. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Sydney: Academic Press; 2005. [Google Scholar]
- 22.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 24.Culley DJ, Raghavan SV, Waly M, Baxter MG, Yukhananov R, Deth RC, Crosby G. Nitrous oxide decreases cortical methionine synthase transiently but produces lasting memory impairment in aged rats. Anesth Analg. 2007;105:83–8. doi: 10.1213/01.ane.0000266491.53318.20. [DOI] [PubMed] [Google Scholar]
- 25.Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. ISPOCD group International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–51. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 26.Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153:367–76. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 28.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–8. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 30.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 31.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–27. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 32.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 33.Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–70. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 34.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 35.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–9. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–47. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–54. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jevtovic-Todorovic V, Beals J, Benshoff N, Olney JW. Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain. Neuroscience. 2003;122:609–16. doi: 10.1016/j.neuroscience.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Jevtovic-Todorovic V, Benshoff N, Olney JW. Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol. 2000;130:1692–8. doi: 10.1038/sj.bjp.0703479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stratmann G, Sall JW, Eger EI, II, Laster MJ, Bell JS, May LD, Eilers H, Krause M, Heusen F, Gonzalez HE. Increasing the duration of isoflurane anesthesia decreases the minimum alveolar anesthetic concentration in 7-day-old but not in 60-day-old rats. Anesth Analg. 2009;109:801–6. doi: 10.1213/ane.0b013e3181aff364. [DOI] [PubMed] [Google Scholar]