Abstract
Background: Grilled, barbecued, and smoked meat intake, a prevalent dietary source of polycyclic aromatic hydrocarbon (PAH) carcinogens, may increase the risk of incident breast cancer. However, no studies have examined whether intake of this PAH source influences survival after breast cancer.
Methods: We interviewed a population-based cohort of 1508 women diagnosed with first primary invasive or in situ breast cancer in 1996 and 1997 at baseline and again approximately five years later to assess grilled/barbecued and smoked meat intake. After a median of 17.6 years of follow-up, 597 deaths, of which 237 were breast cancer related, were identified. Multivariable Cox regression was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality as related to prediagnosis intake, comparing high (above the median) to low intake, as well as postdiagnosis changes in intake, comparing every combination of pre-/postdiagnosis intake to low pre-/postdiagnosis intake. All statistical tests were two-sided.
Results: High prediagnosis grilled/barbecued and smoked meat intake was associated with increased risk of all-cause mortality (HR = 1.23, 95% CI = 1.03 to 1.46). Other associations were noted, but estimates were not statistically significant. These include high prediagnosis smoked beef/lamb/pork intake and increased all-cause (HR = 1.17, 95% CI = 0.99 to 1.38, Ptrend = .10) and breast cancer–specific (HR = 1.23, 95% CI = 0.95 to 1.60, Ptrend = .09) mortality. Also, among women with continued high grilled/barbecued and smoked meat intake after diagnosis, all-cause mortality risk was elevated 31% (HR = 1.31, 95% CI = 0.96 to 1.78). Further, breast cancer–specific mortality was decreased among women with any pre- and postdiagnosis intake of smoked poultry/fish (HR = 0.55, 95% CI = 0.31 to 0.97).
Conclusion: High intake of grilled/barbecued and smoked meat may increase mortality after breast cancer.
In the United States, there are over 3.1 million women who are survivors of breast cancer; these women represent approximately 40% of female cancer survivors (1). After a diagnosis of breast cancer, survivors are faced with making behavioral and dietary choices as they attempt to improve their long-term prognoses. Dietary changes are one area in which breast cancer survivors may choose to make more healthful changes. To aid in this decision-making, recommendations and guidelines are available for cancer survivors in general (2) and, more recently, for breast cancer survivors specifically (3). For example, the American Cancer Society, together with the American Society of Clinical Oncology, recently released their breast cancer survivorship care guidelines, which recommend that survivors be counseled to “achieve a dietary pattern that is high in vegetables, fruits, whole grains, and legumes, and limit alcohol intake to no more than one drink per day” (3). These recommendations are based on limited, but suggestive, evidence of improved survival among women with such diets (4,5). No recommendations exist for breast cancer survivors that specifically address intake of high-temperature cooked meat, including intake of grilled/barbecued and smoked meat, although, in relation to primary prevention of breast cancer incidence, it is recommended (6) that women limit intake of processed meats and high-temperature cooked meat because of the formation of polycyclic aromatic hydrocarbons (PAHs) and other carcinogenic chemicals during the cooking process (7).
Grilled/barbecued and smoked meat intake is a highly prevalent source of PAHs among US women (8) and has been associated with breast cancer incidence (9), but whether intake is related to survival after breast cancer is unknown. This study examined whether grilled, barbecued, and smoked meat intake prior to breast cancer diagnosis, as well as postdiagnosis changes in intake, is associated with long-term all-cause and breast cancer–specific mortality among a population-based sample of women with first primary breast cancer.
Methods
Study Population
Adult female residents of Nassau and Suffolk counties on Long Island, New York, with a first diagnosis of in situ or invasive breast cancer between August 1, 1996, and July 31, 1997, were identified for inclusion in the Long Island Breast Cancer Study Project (LIBCSP) (10). Identification of patients was done via active daily or weekly contact with local hospitals and confirmed by physicians and medical records. After providing written informed consent, the cohort of 1508 women with breast cancer was interviewed at home by trained interviewers via structured questionnaire at baseline, on average within three months of breast cancer diagnosis.
Approximately five years after the initial diagnosis of breast cancer, the 1414 women who at baseline consented to continued contact were recontacted for the follow-up interview. Of these, 143 refused, no proxy was identified for 96 women who were not alive at follow-up, and 55 could not be located, resulting in 1120 women providing consent and 1033 women completing the follow-up questionnaire (11). The follow-up interview was conducted over the telephone by trained interviewers using a structured questionnaire that assessed information similar to that obtained at baseline, but regarding the time period since the initial diagnosis of breast cancer. Institutional review board approval was obtained from all participating institutions.
Outcome Assessment
Date of death and cause of death were determined using the National Death Index (12). Indicators for death from any cause and those associated with breast cancer were created with breast cancer deaths identified using International Statistical Classification of Diseases codes 174.9 and C-50.9 listed on the death certificate. Follow-up for mortality occurred from the date of diagnosis in 1996 or 1997 until December 31, 2014. Among the 1508 case women, 597 deaths were identified, 237 (39.7%) of which were related to breast cancer, after a median duration of follow-up was 17.6 years (range = 0.2–18.4 years).
Grilled, Barbecued, and Smoked Meat Intake Assessment
As part of the main baseline questionnaire, participants were asked about their intake (number of times per week, month, or year) of four types of grilled/barbecued and smoked meats: 1) grilled/barbecued beef, lamb, and pork, 2) smoked beef, lamb, and pork, such as bacon or ham, 3) grilled/barbecued poultry and fish, and 4) smoked poultry and fish, such as smoked turkey or lox. The women were asked about their intake in each decade of life (<20 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, ≥60 years) and were asked to specify the seasons in which the foods were most frequently consumed (8,13). At baseline, intake during the decade prior to breast cancer diagnosis was used to represent the average intake before diagnosis; we also examined whether lifetime intake of grilled/barbecued and smoked meat was associated with mortality. At the five-year follow-up, participants responded to the same questions, which asked about the time period since the baseline questionnaire.
Responses given as per week or per month were first multiplied by 52 or by 12, respectively, and then multiplied by the proportion of the year that the foods were consumed (ie, 25% if they were consumed during one season, 50% if they were consumed during two seasons, etc.) to obtain measures of intake in number of times per year. The continuous measures were dichotomized at the median for each of the four meat types: grilled/barbecued beef/lamb/pork (low = 0–10 vs high = 11+ times/year prediagnosis; low = 0–8 vs high = 9+ times/year postdiagnosis), grilled/barbecued poultry/fish (low = 0–9 vs high = 10+ times/year prediagnosis; low = 0–6 vs high = 7+ times/year postdiagnosis), smoked barbecued beef/lamb/pork (low = 0–4 vs high = 5+ times/year prediagnosis and postdiagnosis), and smoked poultry/fish (none = 0 vs any intake = 1+ times/year prediagnosis and postdiagnosis), separately. Intake of the four meat types were also summed to create an overall measure of intake of grilled/barbecued and smoked meat (times/year), which was dichotomized at the median (low = 0–43 vs high = 44+ times/year prediagnosis; low = 0–35 vs high = 36+ times/year postdiagnosis). Lifetime intake of each of the four types of meat was dichotomized at the median as low = 0–4724 vs high = 4725+ times throughout the lifetime. In the analysis of postdiagnosis intake of grilled/barbecued and smoked meat, every combination of prediagnosis/postdiagnosis annual intake was examined (ie, low/low intake, low/high intake, etc.).
Covariate Assessment
Most covariates were assessed by interviewer-administered questionnaire. Potential confounders included age at diagnosis (years), menopausal status (premenopausal vs postmenopausal), annual household income (<$15 000–$24 999, $25 000–$49 999, and ≥$50 000), education (<high school/high school graduate, some college/college graduate, and postcollege), marital status (married or living as married vs not married, divorced, or widowed), body mass index (continuous, kg/m2), at-diagnosis physical activity (never, former, and current physical activity of least one hour per week for three months or more), at-diagnosis intake of alcoholic beverages such as beer, wine, or liquor (never, former, and current intake at least once a month for six months or more), at-diagnosis consumption of energy (kcal/day), at-diagnosis fruit and vegetable intake (servings/day), and at-diagnosis multivitamin supplement use (ever/never).
Other covariates, including estrogen receptor status and nodal involvement, were determined by medical record review, and tumor size was obtained from the New York State Cancer Registry. At baseline, women were interviewed after surgery but before initiation of most other components of the first course of treatment for the first primary breast cancer. Therefore, treatment received (radiation therapy, chemotherapy, or hormone therapy) was assessed by self-report at the follow-up questionnaire, which showed high agreement with medical record data (kappas ranged from 0.92 to 0.97) (14) but was more complete.
Statistical Analysis
Age-adjusted and multivariable-adjusted Cox proportional hazards models were fit for each of the four types of grilled and smoked meat intake, separately, and for the total measure of annual intake and for all-cause and breast cancer–specific mortality. The proportional hazards assumption was verified using exposure interactions with time. For analyses using breast cancer–specific mortality as the outcome, non–breast cancer deaths were censored at time of death. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between prediagnosis, lifetime and average annual intake, as well as postdiagnosis changes in grilled, barbecued, and/or smoked meat intake and all-cause and breast cancer–specific mortality. Tests for trend used continuous measures of grilled/barbecued and smoked intake in the proportional hazards models. Survival time began at the date of breast cancer diagnosis in the analyses of prediagnosis grilled/barbecued and smoked meat intake and at the date of the follow-up interview for the corresponding analyses on postdiagnosis intake. Survival time for all analyses ended at the date of death or, if alive, date of censoring. We also restricted the analysis to women with invasive cancer only, but results did not differ substantially from those among all women, so only the latter is shown. All analyses were done using the Cox Regression function in IBM SPSS Statistics Version 22.0 (IBM Corp., Armonk, NY).
In the analyses of postdiagnosis changes in grilled/barbecued, and smoked meat intake and survival, we employed multiple imputation to account for the missing exposure data after excluding 169 women who died within five years of diagnosis; 377 (28.2%) participants were lost to follow-up and thus were missing data on intake of grilled/barbecued and smoked meat. Missing values were imputed using SPSS, which employs a fully conditional specification algorithm, an iterative Markov Chain Monte Carlo procedure that sequentially imputes missing values starting from the first variable with missing values (15). SPSS applies linear regression to continuous scale variables, and logistic or multinomial logistic regression to categorical variables. We used 25 imputations with 1000 iterations and included demographics (age at diagnosis, menopausal status, income, education, marital status, BMI, physical activity, and alcohol intake, smoking status), prediagnosis and postdiagnosis grilled and smoked meat intake, disease characteristics (stage, tumor size, nodal involvement estrogen receptor status), treatment (radiation therapy, chemotherapy, and hormone therapy), and the outcome (the event indicator and the Nelson-Aalen estimator of the cumulative hazard) (16). As a sensitivity analysis, we also conducted a complete-case analysis, where the missing exposure data are ignored. This alternative approach is commonly employed in follow-up studies with multiple exposure assessments over time. However, the imputation approach is designed to reduce the bias associated with the complete case analysis (17). In analyses that used follow-up data, survival time began at the date of completion of the follow-up questionnaire to the date of death or December 31, 2014, if alive.
All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Participant demographic characteristics, as well as disease, tumor, and treatment characteristics, are presented in Table 1. Women with high intake of total grilled/barbecued and smoked meat were younger at diagnosis (56.7 years vs 60.9 years), and a higher proportion had an annual income of $50 000 or more (57.0% vs 40.4%) compared with women with low intake. Women with high intake were also more likely to be married (77.2% vs 60.4%). A higher proportion of women with high intake reported being current alcohol drinkers (51.9% vs 43.2%). Disease and treatment characteristics were similar across total intake of grilled/barbecued and smoked meat, except for chemotherapy; receipt of chemotherapy was reported by 45.8% of women with high intake compared with 36.7% of women with low intake.
Table 1.
Distribution of participant characteristics at diagnosis among the LIBCSP women diagnosed with first primary breast cancer in 1996 and 1997, overall and by grilled, barbecued, and smoked meat intake (n = 1508)*
| At-diagnosis characteristic | Prediagnosis grilled, barbecued, and smoked meat intake† |
||
|---|---|---|---|
| Total | Low | High | |
| (n = 1508) |
(n = 732) |
(n = 726) |
|
| No. (%) | No. (%) | No. (%) | |
| Age at diagnosis, y | |||
| <50 | 407 (27.0) | 160 (21.9) | 233 (32.1) |
| 50–64 | 582 (38.6) | 271(37.0) | 295 (40.6) |
| ≥65 | 519 (34.4) | 301 (41.1) | 198 (27.3) |
| Mean (SD) | 58.8 (12.7) | 60.9 (12.7) | 56.7 (12.3) |
| Menopausal status | |||
| Premenopausal | 472 (31.9) | 180 (25.1) | 276 (38.9) |
| Postmenopausal | 1006 (68.1) | 538 (74.9) | 434 (61.1) |
| Income | |||
| <$15 000–$24 999 | 286 (19.0) | 165 (22.7) | 110 (15.2) |
| $25 000–$49 999 | 488 (32.4) | 269 (36.9) | 202 (27.8) |
| ≥$50 000 | 730 (48.5) | 294 (40.4) | 414 (57.0) |
| Education | |||
| <HS/HS graduate | 721 (48.0) | 355 (48.8) | 338 (46.6) |
| Some college/college graduate | 551 (36.7) | 271 (37.3) | 264 (36.4) |
| Postcollege | 230 (15.3) | 101 (13.9) | 123 (17.0) |
| Marital atatus | |||
| Married or living as married | 1029 (68.3) | 442 (60.4) | 560 (77.2) |
| Not married | 478 (31.7) | 290 (39.6) | 165 (22.8) |
| BMI at diagnosis, kg/m2 | |||
| <25.0 | 683 (45.8) | 343 (47.4) | 321 (44.6) |
| 25–29.9 | 476 (31.9) | 237 (32.8) | 225 (31.3) |
| ≥30.0 | 332 (22.3) | 143 (19.8) | 174 (24.1) |
| Mean (SD) | 26.6 (5.7) | 26.3 (5.5) | 26.8 (5.8) |
| Physical activity‡ | |||
| Never | 334 (22.5) | 176 (24.4) | 140 (19.6) |
| Former | 253 (17.0) | 122 (16.9) | 124 (17.3) |
| Current | 900 (60.5) | 424 (58.7) | 452 (63.1) |
| Alcohol intake§ | |||
| Never | 588 (39.0) | 297 (40.6) | 263 (36.3) |
| Former | 212 (14.1) | 119 (16.3) | 86 (11.9) |
| Current | 707 (46.9) | 316 (43.2) | 376 (51.9) |
| Stage | |||
| Invasive | 1273 (84.4) | 608 (83.1) | 622 (85.7) |
| In situ | 235 (15.6) | 124 (16.9) | 104 (14.3) |
| Nodal involvement‖ | 622 (74.5) | 286 (73.0) | 320 (76.9) |
| Tumor size, cm‖ | |||
| ≤2.0 | 622 (75.5) | 299 (76.9) | 302 (73.5) |
| >2.0 | 202 (24.5) | 90 (23.1) | 109 (26.5) |
| Mean (SD) | 1.7 (1.6) | 1.7 (1.7) | 1.7 (1.5) |
| Estrogen receptor status‖ | |||
| Negative | 264 (26.7) | 129 (27.3) | 127 (25.9) |
| Positive | 726 (73.3) | 343 (72.7) | 363 (74.1) |
| Treatment received‖ | |||
| Radiation | 625 (60.9) | 295 (59.5) | 313 (62.2) |
| Chemotherapy | 423 (41.4) | 181 (36.7) | 230 (45.8) |
| Hormone therapy | 616 (61.1) | 292 (59.8) | 308 (62.2) |
*LIBCSP participants diagnosed with breast cancer between August 1, 1996, and July 31, 1997, followed-up for vital status through December 31, 2014. BMI = body mass index; HS = high school; LIBCSP = Long Island Breast Cancer Study Project.
†Low intake = 0–43 vs high intake = 44+ times/year in the most recent decade prior to diagnosis.
‡At-diagnosis recreational physical activity was defined as never, former, and current physical activity of least one hour per week for three months or more.
§At-diagnosis intake of alcoholic beverages was defined as never, former, and current intake of alcoholic beverages such as beer, wine, or liquor at least once a month for six months or more.
‖Variables with >2% missing values include (n missing): nodal involvement (n = 673), tumor size (n = 684), estrogen receptor status (n = 518), radiation treatment (n = 482), chemotherapy (n = 486), and hormone therapy (n = 499).
Prediagnosis Intake of Grilled/Barbecued and Smoked Meat
Table 2 shows the associations between prediagnosis annual intake of grilled/barbecued and smoked meat and mortality. Compared with low intake, high intake of grilled/barbecued and smoked meat prior to diagnosis was associated with a 23% increased hazard (HR = 1.23, 95% CI = 1.03 to 1.46, Ptrend = .02) of all-cause mortality. High vs low intake of smoked beef/lamb/pork intake was associated with a 17% increased hazard (HR = 1.17, 95% CI = 0.99 to 1.38, Ptrend = .10) of all-cause and a 23% increased hazard (HR = 1.23, 95% CI = 0.95 to 1.60, Ptrend = .09) of breast cancer–specific mortality, but the confidence intervals include the null value. Lifetime grilled/barbecued and smoked meat intake and prediagnosis annual intake of grilled/barbecued beef/lamb/pork and poultry/fish were not associated with mortality (Table 2).
Table 2.
Cox regression hazard ratios and 95% confidence intervals for the association between prediagnosis lifetime and annual intake of grilled, barbecued, and smoked meat and mortality in the LIBCSP women diagnosed with breast cancer in 1996 and 1997 and followed for 18+ years (n = 1508)*
| All-cause mortality (n = 597 deaths) |
Breast cancer–specific mortality (n = 237 deaths) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Type of meat intake | Age- adjusted |
Multivariable- adjusted† |
Age- adjusted |
Multivariable- adjusted† |
||||
| prediagnosis | Deaths | Censored | HR (95% CI) | HR (95% CI) | Deaths | Censored | HR (95% CI) | HR (95% CI) |
| Lifetime grilled, barbecued, and smoked meat intake‡ | ||||||||
| Low | 280 | 441 | 1 (ref.) | 1 (ref.) | 117 | 604 | 1 (ref.) | 1 (ref.) |
| High | 285 | 436 | 0.99 (0.84 to 1.17) | 1.02 (0.86 to 1.21) | 105 | 616 | 0.88 (0.68 to 1.15) | 0.89 (0.68 to 1.17) |
| Ptrend | .34 | .16 | .47 | .59 | ||||
| Annual grilled, barbecued, and smoked meat intake§ | ||||||||
| Low | 297 | 435 | 1 (ref.) | 1 (ref.) | 112 | 620 | 1 (ref.) | 1 (ref.) |
| High | 279 | 447 | 1.14 (0.96 to 1.34) | 1.23 (1.03 to 1.46) | 114 | 612 | 1.03 (0.79 to 1.34) | 1.11 (0.85 to 1.46) |
| Ptrend | .06 | .02 | .17 | .07 | ||||
| Annual grilled, barbecued beef, lamb, and pork intake‖ | ||||||||
| Low | 323 | 422 | 1 (ref.) | 1 (ref.) | 118 | 627 | 1 (ref.) | 1 (ref.) |
| High | 262 | 478 | 1.02 (0.86 to 1.21) | 1.09 (0.91 to 1.30) | 113 | 627 | 0.94 (0.72 to 1.22) | 1.04 (0.79 to 1.37) |
| Ptrend | .21 | .10 | .50 | .10 | ||||
| Annual smoked beef, lamb, and pork intake¶ | ||||||||
| Low | 288 | 453 | 1 (ref.) | 1 (ref.) | 106 | 635 | 1 (ref.) | 1 (ref.) |
| High | 302 | 441 | 1.13 (0.96 to 1.33) | 1.17 (0.99 to 1.38) | 127 | 616 | 1.20 (0.93 to 1.55) | 1.23 (0.95 to 1.60) |
| Ptrend | .06 | .10 | .10 | .09 | ||||
| Annual grilled, barbecued poultry, and fish intake# | ||||||||
| Low | 330 | 403 | 1 (ref.) | 1 (ref.) | 115 | 618 | 1 (ref.) | 1 (ref.) |
| High | 254 | 492 | 0.95 (0.80 to 1.12) | 1.06 (0.89 to 1.26) | 114 | 632 | 0.94 (0.72 to 1.23) | 1.07 (0.81 to 1.41) |
| Ptrend | .95 | .38 | .59 | .31 | ||||
| Annual smoked poultry and fish intake** | ||||||||
| None | 428 | 556 | 1 (ref.) | 1 (ref.) | 169 | 815 | 1 (ref.) | 1 (ref.) |
| Any | 161 | 341 | 0.80 (0.67 to 0.97) | 0.89 (0.74 to 1.08) | 66 | 436 | 0.72 (0.54 to 0.96) | 0.80 (0.59 to 1.07) |
| Ptrend | .43 | .09 | .99 | .63 | ||||
*Long Island Breast Cancer Study Project participants diagnosed with breast cancer between August 1, 1996, and July 31, 1997, followed-up for vital status through December 31, 2014. CI = confidence interval; HR = hazard ratio; LIBCSP = Long Island Breast Cancer Study Project.
†Adjusted for age at diagnosis, marital status, income, alcohol intake, body mass index, and physical activity.
‡Low intake = 0–4724 vs high intake = 4725+ times throughout the lifetime.
§Low intake = 0–43 vs high intake = 44+ times/year in the most recent decade prior to diagnosis.
‖Low intake = 0–10 vs high intake = 11+ times/year in the most recent decade prior to diagnosis.
¶Low intake = 0–4 vs high intake = 5+ times/year in the most recent decade prior to diagnosis.
#Low intake = 0–9 vs high intake 10+ times/year in the most recent decade prior to diagnosis.
**None = 0 vs any intake = 1+ times/year in the most recent decade prior to diagnosis.
Postdiagnosis Changes in Intake Grilled, Barbecued, and Smoked Meat
Table 3 shows the associations between postdiagnosis changes in annual intake of grilled/barbecued and smoked meat and mortality after imputation of missing covariates. Compared with women with low prediagnosis and low postdiagnosis intake of grilled/barbecued and smoked meat, continued high intake was associated with a 31% increased hazard (HR = 1.31, 95% CI = 0.96 to 1.78) of all-cause mortality. The increase in risk of death from any cause was similar in magnitude (HR = 1.28, 95% CI = 0.97 to 1.68) among women who reported high prediagnosis and low postdiagnosis intake of grilled/barbecued and smoked meat. Smoked beef/lamb/pork intake was positively associated with all-cause (HR = 1.36, 95% CI = 1.01 to 1.82) and breast cancer–specific mortality (HR = 1.71, 95% CI = 1.00 to 2.92) among women who had high intake at prediagnosis and low postdiagnosis intake, relative to low pre- and low postdiagnosis intake, but not among women with continued high postdiagnosis intake. Additionally, women who reported any pre- and postdiagnosis intake of smoked poultry and fish had a reduced risk of breast cancer mortality (HR = 0.55, 95% CI = 0.31 to 0.97) compared with no intake at pre- and postdiagnosis. Postdiagnosis changes in intake of grilled/barbecued poultry/fish were not associated with all-cause and breast cancer–specific mortality. Age-adjusted results from the complete-case analyses are presented in Supplementary Table 1 (available online), which are mostly similar to the imputation-based results, except for total grilled/barbecued and smoked meat intake, which are null in the complete-case analysis.
Table 3.
Cox regression hazard ratios and 95% confidence intervals for the association between prediagnosis/postdiagnosis annual intake of grilled, barbecued, and smoked meat and mortality in the LIBCSP women diagnosed with breast cancer in 1996 and 1997 and followed for 18+ years (n = 1339)*
| All-cause mortality (n = 428 deaths) |
Breast cancer–specific mortality (n = 126 deaths) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Type of meat intake | Age- adjusted |
Multivariable- adjusted† |
Age-adjusted |
Multivariable- adjusted† |
||||
| prediagnosis/postdiagnosis | Deaths | Censored | HR (95% CI) | HR (95% CI) | Deaths | Censored | HR (95% CI) | HR (95% CI) |
| Total grilled, barbecued, and smoked meat intake‡ | ||||||||
| Low/low | 160 | 295 | 1 (ref.) | 1 (ref.) | 42 | 413 | 1 (ref.) | 1 (ref.) |
| Low/high | 60 | 156 | 1.05 (0.73 to 1.50) | 1.10 (0.75 to 1.62) | 16 | 201 | 0.78 (0.38 to 1.63) | 0.77 (0.36 to 1.64) |
| High/low | 101 | 158 | 1.24 (0.96 to 1.62) | 1.28 (0.97 to 1.68) | 28 | 231 | 1.18 (0.71 to 1.98) | 1.14 (0.67 to 1.93) |
| High/high | 108 | 302 | 1.18 (0.88 to 1.58) | 1.31 (0.96 to 1.78) | 41 | 169 | 1.09 (0.66 to 1.80) | 1.08 (0.63 to 1.83) |
| Grilled, barbecued beef, lamb, and pork intake§ | ||||||||
| Low/low | 183 | 291 | 1 (ref.) | 1 (ref.) | 43 | 430 | 1 (ref.) | 1 (ref.) |
| Low/high | 52 | 137 | 0.98 (0.68 to 1.40) | 1.00 (0.69 to 1.45) | 16 | 174 | 0.93 (0.48 to 1.81) | 0.88 (0.45 to 1.75) |
| High/low | 81 | 156 | 1.06 (0.80 to 1.40) | 1.10 (0.83 to 1.46) | 19 | 219 | 0.86 (0.48 to 1.56) | 0.88 (0.48 to 1.61) |
| High/high | 112 | 327 | 1.10 (0.84 to 1.43) | 1.14 (0.87 to 1.51) | 48 | 390 | 1.25 (0.78 to 1.98) | 1.24 (0.76 to 2.03) |
| Smoked beef, lamb, and pork intake‖ | ||||||||
| Low/low | 142 | 326 | 1 (ref.) | 1 (ref.) | 36 | 432 | 1 (ref.) | 1 (ref.) |
| Low/high | 66 | 138 | 1.25 (0.87 to 1.80) | 1.18 (0.81 to 1.71) | 20 | 185 | 1.29 (0.65 to 2.58) | 1.22 (0.60 to 2.50) |
| High/low | 93 | 148 | 1.34 (1.01 to 1.77) | 1.36 (1.01 to 1.82) | 31 | 210 | 1.75 (1.04 to 2.94) | 1.71 (1.00 to 2.92) |
| High/high | 126 | 299 | 1.20 (0.92 to 1.56) | 1.20 (0.91 to 1.59) | 40 | 386 | 1.25 (0.76 to 2.04) | 1.19 (0.71 to 1.99) |
| Grilled, barbecued poultry, and fish intake¶ | ||||||||
| Low/low | 201 | 302 | 1 (ref.) | 1 (ref.) | 45 | 459 | 1 (ref.) | 1 (ref.) |
| Low/high | 41 | 110 | 1.01 (0.64 to 1.59) | 1.04 (0.65 to 1.65) | 13 | 138 | 0.97 (0.43 to 2.19) | 0.95 (0.41 to 2.19) |
| High/low | 93 | 180 | 1.01 (0.78 to 1.31) | 1.03 (0.78 to 1.34) | 29 | 244 | 1.21 (0.73 to 2.02) | 1.22 (0.72 to 2.05) |
| High/high | 93 | 318 | 0.98 (0.73 to 1.30) | 1.06 (0.79 to 1.43) | 39 | 373 | 1.06 (0.64 to 1.76) | 1.11 (0.66 to 1.88) |
| Smoked poultry and fish intake# | ||||||||
| None/none | 275 | 489 | 1 (ref.) | 1 (ref.) | 83 | 682 | 1 (ref.) | 1 (ref.) |
| None/any | 32 | 76 | 0.82 (0.52 to 1.30) | 0.84 (0.51 to 1.37) | 8 | 100 | 0.62 (0.27 to 1.45) | 0.56 (0.23 to 1.34) |
| Any/none | 51 | 122 | 0.89 (0.64 to 1.24) | 0.97 (0.69 to 1.35) | 19 | 154 | 0.96 (0.56 to 1.63) | 0.97 (0.57 to 1.67) |
| Any/any | 70 | 224 | 0.79 (0.59 to 1.06) | 0.88 (0.64 to 1.20) | 18 | 277 | 0.52 (0.30 to 0.92) | 0.55 (0.31 to 0.97) |
*Long Island Breast Cancer Study Project participants diagnosed with breast cancer between August 1, 1996, and July 31, 1997, followed-up for vital status through December 31, 2014. Missing data analyses exclude women who died within five years of breast cancer diagnosis (n = 169). CI = confidence interval; HR = hazard ratio; LIBCSP = Long Island Breast Cancer Study Project.
†Adjusted for age at diagnosis, marital status, income, alcohol intake, body mass index, physical activity, tumor size, lymph node involvement, and estrogen receptor status.
‡Low intake = 0–43 vs high intake = 44+ times/year prediagnosis in the most recent decade prior to diagnosis and low intake = 0–35 vs high intake = 36+ times/year postdiagnosis.
§Low intake = 0–10 vs high intake = 11+ times/year prediagnosis in the most recent decade prior to diagnosis and low intake = 0–8 vs high intake = 9+ times/year postdiagnosis.
‖Low intake = 0–4 vs high intake = 5+ times/year prediagnosis in the most recent decade prior to diagnosis and postdiagnosis.
¶Low intake = 0–9 vs high intake 10+ times/year prediagnosis in the most recent decade prior to diagnosis and low intake = 0–6 vs high intake = 7+ times/year postdiagnosis.
#None = 0 vs any intake = 1+ times/year in the most recent decade prior to diagnosis and postdiagnosis.
Discussion
In this population-based prospective study of grilled/barbecued and smoked meat intake and mortality among a cohort of women diagnosed with first primary breast cancer, high prediagnosis annual intake of total grilled/barbecued and smoked meat was statistically significantly associated with an elevated risk of all-cause mortality. Additionally, when considering postdiagnosis changes in intake, we observed that women who continued to consume a high amount of grilled/barbecued and smoked meat after diagnosis had a 31% increased risk of all-cause mortality. When each of the four types of grilled/barbecued and smoked meat were examined individually, some associations were noted, but the estimates were not statistically significant and include the following. Prediagnosis annual intake of smoked beef/lamb/pork was positively associated with all-cause and breast cancer–specific mortality. Postdiagnosis smoked beef/lamb/pork intake was also positively associated with all-cause and breast cancer mortality, with risk of mortality highest among women who reported high prediagnosis and low postdiagnosis intake. Risk of breast cancer–specific mortality was inversely associated with any pre- and postdiagnosis intake of smoked poultry/fish.
Grilled and smoked meat intake is a source of polycyclic aromatic hydrocarbons, including benzo[a]pyrene, chrysene, and fluoranthene, and is the primary route of PAH exposure among nonsmokers (18). PAHs, a group of over 100 different chemicals, are formed during the incomplete combustion or pyrolysis of organic substances (19). Specifically, during grilling and barbecuing, PAHs are formed when fat and juices from meat grilled directly over an open fire drip onto the fire, creating flames and smoke. The PAHs adhere to the surface of the meat upon contact (20). Wood smoke, which is used to cook and preserve foods, contains a large number of PAHs, which also contaminate the foods upon contact (21).
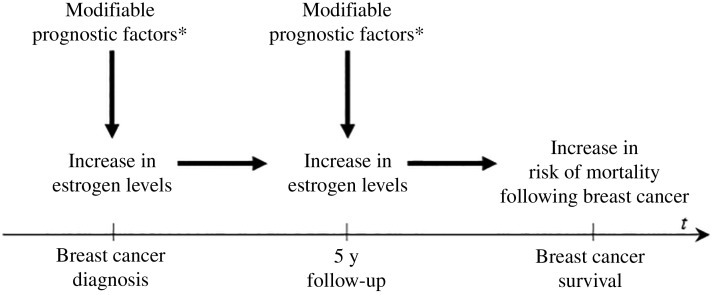
Although we could not definitively rule out chance as an explanation for some of our findings, a link between dietary sources of PAH and breast cancer prognosis is biologically plausible and epidemiologically consistent. First, these foods have been previously associated with increased risk of breast cancer incidence; effect estimates range from 1.5 to 2.2 when comparing the highest to the lowest quantiles of intake of well-done meat (8,22–25). Second, dietary PAH exposures are hypothesized to be etiologically related to breast carcinogenesis as PAHs are known to form DNA adducts, which can cause mutations during DNA replication and may alter promoter methylation or promoter binding, leading to inheritable abnormal gene expression, early steps in carcinogenesis (7). Third, PAHs are also likely to influence breast cancer development and prognosis through endocrine disruption (26). Several PAHs or derivatives including chrysene and fluoranthene show estrogenic activity in vitro, while others, such as benzo[k]fluoranthene, benzo[a]pyrene, and benz[a]anthracene, can be anti-estrogenic (27–29). Thus, as shown in Figure 1, our study hypothesis is that dietary PAH sources (and other modifiable factors) may influence breast cancer prognosis through an estrogen pathway.
Figure 1.
Conceptual model linking at-diagnosis and postdiagnosis changes in modifiable prognostic factors (indicated with an asterisk) including obesity, physical activity, fat intake, smoking, and intake of grilled/smoked foods and survival following breast cancer.
Our findings of a possible positive association with death and intake for smoked beef/lamb/pork may possibly be explained by the higher saturated fat content of these meats compared with poultry and fish. Though results are inconsistent, higher risk of mortality has been observed among women with high intake of total fat, saturated fat, and monounsaturated fat (30–32). Furthermore, higher fat content may also result in the formation of more PAHs (18). However, we did not observe the same elevated risk of mortality among women with continued postdiagnosis high intake and when we examined at-diagnosis intake of these meats cooked by grilling/barbecuing. The lack of association between mortality and intake of grilled/barbecued beef/lamb/pork may be due to method of preparation; marinating meat before grilling, as is often done, may inhibit the formation of PAHs (33). Our finding of an inverse association between smoked poultry/fish intake and mortality could also be related to the different fat composition of these meats. Moreover, it has been hypothesized that the amino acid content of white meat supports proper immune system function (34), while intake of fish, a source of omega-3 polyunsaturated fatty acids, could improve survival (30,35) by reducing pro-inflammatory derivatives (36). Nonetheless, we did not observe reductions in mortality risk associated with the intake of grilled/barbecued poultry/fish intake.
Ours is the first study to examine the associations between grilled/barbecued and smoked meat intake and mortality after breast cancer. Strengths of our study include the population-based cohort design, which utilized data collected shortly after diagnosis and again five years postdiagnosis. Women were followed for over 18 years using the National Death Index, which has accurate ascertainment of vital status (37).
However, this study also has several limitations. Women were asked to self-report their intake of grilled/barbecued and smoked meats. This could have resulted in nondifferential misclassification of the exposure, which would bias estimates towards the null (38). Given the prospective design, approximately 28% of women did not complete the follow-up assessment. Analyses using a complete-case approach could result in biased estimates (39); therefore, we used multiple imputation, a methodologically sound approach, to address the missing data. Additionally, given the missingness in the follow-up assessment, we were unable to consider time-varying covariates because of problems with model convergence. Lastly, given the complexity of diet, it is possible that our results are confounded by other correlated dietary factors; however, few dietary exposures have been consistently linked to breast cancer survival (32).
The results of our study indicate that grilled/barbecued and, particularly, smoked meat consumed prior to and after breast cancer diagnosis may influence survival. This study, with confirmation by future studies, may help to identify modifiable prognostic indicators for the more than 3 million women who are survivors of breast cancer (1).
Funding
The National Cancer Institute and/or the National Institute of Environmental Health Sciences (UO1 CA/ES66572, UO1 CA66572, 1K07 CA102640-01, R25 CA057726, T32 ES007018) and the American Institute for Cancer Research (AICR-03B091).
Notes
The funders had no role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts and Figures 2014-2015. American Cancer Society, Atlanta, GA; 2014. [Google Scholar]
- 2.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. [DOI] [PubMed] [Google Scholar]
- 3.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66(1):43–73. [DOI] [PubMed] [Google Scholar]
- 4.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. [DOI] [PubMed] [Google Scholar]
- 6.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. World Cancer Research Fund/American Institute for Cancer Research, Washington, DC; 2009. [Google Scholar]
- 7.Moorthy B, Chun C, Carlin DJ. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol Sci. 2015;145(1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steck SE, Gaudet MM, Eng SM, et al. Cooked meat and risk of breast cancer—lifetime vs recent dietary intake. Epidemiology. 2007;18(3):373–382. [DOI] [PubMed] [Google Scholar]
- 9.White AJ, Bradshaw PT, Herring AH, et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int. 2016;89:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gammon MD, Neugut AI, Santella RM, et al. The Long Island Breast Cancer Study Project: Description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat. 2002;74(3):235–254. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw PT, Ibrahim JG, Stevens J, et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology. 2012;23(2):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National death index. 2014. http://www.cdc.gov/nchs/ndi.htm. Accessed June 29, 2016.
- 13.Gammon MD, Santella RM, Neugut AI, et al. Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev. 2002;11(8):677–685. [PubMed] [Google Scholar]
- 14.Cleveland RJ, Eng SM, Abrahamson PE, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1803–1811. [DOI] [PubMed] [Google Scholar]
- 15.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 16.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338(b2393):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res Toxicol Environ Mutagen. 1999;443(1-2):139–147. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for polycyclic aromatic hydrocarbons. 1995. http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf. Accessed June 2, 2016. [PubMed]
- 20.Larsson BK. Formation of polycyclic aromatic hydrocarbons during the smoking and grilling of food. Prog Clin Biol Res. 1986;206:169–180. [PubMed] [Google Scholar]
- 21.Stumpe-Vīksna I, Bartkevičs V, Kukāre A, Morozovs A. Polycyclic aromatic hydrocarbons in meat smoked with different types of wood. Food Chem. 2008;110(3):794–797. [Google Scholar]
- 22.Dai Q, Shu XO, Jin F, Gao YT, Ruan ZX, Zheng W. Consumption of animal foods, cooking methods, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(9):801–808. [PubMed] [Google Scholar]
- 23.Zheng W, Gustafson DR, Sinha R, et al. Well-done meat intake and the risk of breast cancer. J Natl Cancer Inst. 1998;90(22):1724–1729. [DOI] [PubMed] [Google Scholar]
- 24.De Stefani E, Ronco A, Mendilaharsu M, Guidobono M, Deneo-Pellegrini H. Meat intake, heterocyclic amines, and risk of breast cancer: A case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev. 1997;6(8):573–581. [PubMed] [Google Scholar]
- 25.Knekt P, Steineck G, Jarvinen R, Hakulinen T, Romaa A. Intake of fried meat and risk of cancer: A follow-up study in Finland. Int J Cancer. 1994;59(6):756–760. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut. 2016;213:809–824. [DOI] [PubMed] [Google Scholar]
- 27.Arcaro K. Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology. 1999;133(2-3):115–127. [DOI] [PubMed] [Google Scholar]
- 28.Chaloupka K, Krishnan V, Safe S. Polynuclear aromatic hydrocarbon carcinogens as antiestrogens in MCF-7 human breast cancer cells: Role of the Ah receptor. Carcinogenesis. 1992;13(12):2233–2239. [DOI] [PubMed] [Google Scholar]
- 29.Fertuck KC, Kumar S, Sikka HC, Matthews JB, Zacharewski TR. Interaction of PAH-related compounds with the alpha and beta isoforms of the estrogen receptor. Toxicol Lett. 2001;121(3):167–177. [DOI] [PubMed] [Google Scholar]
- 30.Makarem N, Chandran U, Bandera E V, Parekh N. Dietary fat in breast cancer survival. Annu Rev Nutr. 2013;33:319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women’s Health Study. Cancer. 1995;76(2):275–283. [DOI] [PubMed] [Google Scholar]
- 32.Rock CL. Nutrition and survival after the diagnosis of breast cancer: A review of the evidence. J Clin Oncol. 2002;20(15):3302–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viegas O, Yebra-Pimentel I, Martínez-Carballo E, Simal-Gandara J, Ferreira IMPLVO. Effect of beer marinades on formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. J Agric Food Chem. 2014;62(12):2638–2643. [DOI] [PubMed] [Google Scholar]
- 34.Delfino RJ, Sinha R, Smith C, et al. Breast cancer, heterocyclic aromatic amines from meat and N-acetyltransferase 2 genotype. Carcinogenesis. 2000;21(4):607–615. [DOI] [PubMed] [Google Scholar]
- 35.Khankari NK, Bradshaw PT, Steck SE, et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer. 2015;121(13):2244–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabian CJ, Kimler BF, Hursting SD. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015;17(62):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–468. [DOI] [PubMed] [Google Scholar]
- 38.Wacholder S, Hartge P, Lubin JH, Dosemeci M. Non-differential misclassification and bias towards the null: A clarification. Occup Environ Med. 1995;52(8):557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim JG, Chu H, Chen M-H. Missing data in clinical studies: Issues and methods. J Clin Oncol. 2012;30(26):3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.