Abstract
Myelin basic protein (MBP) is an auto-antigen able to induce intractable pain from innocuous mechanical stimulation (mechanical allodynia). The mechanisms provoking this algesic MBP activity remain obscure. Our present study demonstrates that membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) releases the algesic MBP peptides from the damaged myelin, which then reciprocally enhance the expression of MT1-MMP in nerve to sustain a state of allodynia. Specifically, MT1-MMP expression and activity in rat sciatic nerve gradually increased starting at day 3 after chronic constriction injury (CCI). Inhibition of the MT1-MMP activity by intraneural injection of the function-blocking human DX2400 monoclonal antibody at day 3 post-CCI reduced mechanical allodynia and neuropathological signs of Wallerian degeneration, including axon demyelination, degeneration, edema and formation of myelin ovoids. Consistent with its role in allodynia, the MT1-MMP proteolysis of MBP generated the MBP69-86-containing epitope sequences in vitro. In agreement, the DX2400 therapy reduced the release of the MBP69-86 epitope in CCI nerve. Finally, intraneural injection of the algesic MBP69-86 and control MBP2-18 peptides differentially induced MT1-MMP and MMP-2 expression in the nerve. With these data we offer a novel, self-sustaining mechanism of persistent allodynia via the positive feedback loop between MT1-MMP and the algesic MBP peptides. Accordingly, short-term inhibition of MT1-MMP activity presents a feasible pharmacological approach to intervene in this molecular circuit and the development of neuropathic pain.
Keywords: neuropathic pain, MBP, MMP, peripheral nerve, myelin, allodynia
1. INTRODUCTION
Mechanosensation, a process of sensing and transducing mechanical stimuli into an electrochemical signal, depends on physical connectivity between extracellular matrix and cytoskeletal networks of the specialized cells. The mechanosensory networks, while ubiquitous in all living organisms, are especially complex in the human nervous system. In patients suffering from peripheral nervous system (PNS) damage due to trauma, cancer, diabetes, chemotherapy, viral pathogens and other causes, the network dysfunction may lead to a paradoxical state of mechanical allodynia [1]. Mechanical allodynia (pain from non-painful mechanical stimuli) is a devastating state of pain associated with daily activities, such as wearing clothing or using bedding sheets, which often lasts long after incitement of PNS injury and remains refractory to current analgesics [2].
Mechanoselective A-afferent fibers of the PNS, responsible for non-painful touch and vibration sense, are electrically insulated by myelin. By enwrapping their cell membranes around axons, Schwann cells form a multi-lamellar myelin sheath with structurally, functionally and molecularly defined domains (internode, juxtaparanode and paranode). In myelin gaps (nodes of Ranvier), voltage-gated sodium channel clustering warrants rapid, saltatory conduction [3]. Demyelination of A-afferents contributes to the pathogenesis of mechanical pain hypersensitivity via disruption in this precise molecular and structural signature, including ectopic insertion of ion channels [2, 4, 5]. Among such myelin-related changes contributing to mechanical allodynia we proposed is liberation of myelin auto-antigens, such as myelin basic protein (MBP), leading to the formation of the algesic complexes on A-afferents [6–8].
MBP is an intrinsically unstructured cationic protein, which interacts with anionic lipids and cytoskeletal proteins, including actin and tubulin, to regulate myelin compaction and structural assembly of the axon-glia unit [9]. As a putative auto-antigen, MBP exhibits the cryptic T cell epitopes that play an important role in the immunopathogenesis of autoimmune demyelinating conditions in the PNS [10]. According to our recent findings, a physical PNS injury also liberates these hidden MBP epitopes [4, 7], injection of the synthetic peptides encoding these epitopes into an intact sciatic nerve is sufficient to produce a robust and long-lasting allodynia [7, 8]. While the chronic phase of MBP-induced allodynia relies on T cell activity, as shown using athymic nude rats [7], the molecular events initiating the release of the algesic MBP peptides in the course of painful PNS injury have yet to be elucidated.
The matrix metalloproteinase (MMP) family of Zn2+-binding, Ca2+-dependent endopeptidases conduct pericellular and extracellular proteolysis and, as a result, regulate the functionality of multiple contractile, adhesion and ion channel proteins and proteoglycans within the biomechanical cell networks [11, 12]. Evidence exists that, via the activation of the inflammatory cytokines [13–16], ion channels [17, 18], and myelin sheath proteins, including MBP [7, 10, 31], MMP activity regulates the pro-nociceptive signaling associated with PNS injury. Several proteases among the fifteen MMP family members upregulated in the damaged PNS [18, 19] display a redundant ability to proteolyze MBP [20–25]. Based on these data, we proposed that a continuous release of the algesic MBP peptides in the damaged nerve relies on the catalytic activity of the several MMP family members [4, 6, 7].
Pericellular activity of membrane type MT1-MMP/MMP-14 controls both the cell biomechanical properties [12, 26] and the level of the MBP proteolytic degradation [24, 25]. Herein, using a model of painful mononeuropathy, we provide the first evidence that MT1- MMP proteolysis causes the release of the algesic MBP peptides in the damaged PNS. The algesic MBP peptides enhance the expression of MT1-MMP and other MMPs in the nerve. This maladaptive feedback loop feeding self-sustaining mechanical pain hypersensitivity is efficiently interrupted using a short-term, selective MT1-MMP therapy.
2. METHODS
2.1 Reagents
Routine reagents were purchased from Sigma. The function-blocking murine MT1-MMP monoclonal 9E8 antibody (mAb-9E8) [27] was described earlier [28, 29]; human MT1-MMP monoclonal DX2400 antibody (hAb-DX2400) was kindly provided by Kadmon (New York, NY) [30]. Human IgG1 was obtained from Abcam (ab184886). MBP69-86 (THYGSLPQKSQRTQDENPVV) and MBP2-18 (ASQKRPSQRSKYLATAS) peptides, derived from rat MBP sequence (GenBank, #CAA10804), protected N- and C-terminally by acetylation and amidation, respectively, were synthesized by GenScript. Human MBP (18.5 kDa isoform, GenBank #AAH08749) was purchased from Biodesign. The detection antibodies included mouse monoclonal (3G4/MAB1767) and rabbit polyclonal (AB8345) MT1-MMP antibody from EMD Millipore; mouse monoclonal MMP-2 antibody (MAB3308, Millipore); rabbit polyclonal S100B antibody (Z0311, Dako); rabbit polyclonal Iba1 antibody (019-19741, Wako); mouse monoclonal CD68 antibody (MCA341R, Serotec); and rabbit polyclonal antibody generated against the synthetic MBP peptide corresponding to amino acids 69–86 of the guinea pig protein (AB5864, Millipore).
2.2 Animal models and therapy
Sprague-Dawley rats (Envigo, 8–10-week-old, female) were housed in plastic cages in a temperature-controlled room with a 12-h light-dark cycle and free access to food and water. The procedure and testing were conducted during the light cycle. Under 4% isoflurane (Isothesia, Henry Schein) anesthesia, the common sciatic nerve was exposed unilaterally at the mid-thigh level through a gluteal muscle-splitting incision. Three loosely constrictive chromic gut ligatures were applied to produce the chronic constriction injury (CCI) model [31]. At day 3 after CCI, hAb-DX2400, mAb-9E8 or control IgG1 (1.0 mg/ml, each), diluted in PBS (Steris Labs) vehicle, were administered by intraneural (i.n.) injection into the nerve fascicle (the CCI site) in a 10 μl volume using a 33-gauge needle. In a separate set of naïve rats, a single bolus i.n. injection of MBP peptide (50 μg in 5 μl PBS) was administered into the sciatic nerve fascicle. Animals were sacrificed by intraperitoneal injection of Euthasol (100–150 mg/ml; Virbac Animal Health). All animal procedures were performed according to the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the protocols approved by the Institutional Animal Care and Use Committee at the Veterans Affairs San Diego Healthcare System, and complied with ethical guidelines of the International Association for the Study of Pain.
2.3 Von Frey testing
Sensitivity to non-noxious mechanical stimuli was measured by von Frey testing using the up-and-down method [32] by an investigator unaware of the animal groups. Rats were acclimated to being on a suspended 6-mm wire grid for 5 days. The plantar surface of the hind paw within the sciatic nerve innervation area was stimulated using calibrated von Frey filaments (Stoelting). Baseline measurements were done for 3 consecutive days before CCI or i.n. MBP peptide injection, and then up to daily thereafter, as specified. Stimuli were applied for 2 s with a 0.4–15.0 g buckling force to the mid paw plantar surface with ascending filament stiffness until a paw withdrawal response occurred. Stimuli were separated by several-second intervals or until the animal was calm with both hind paws placed on the grid. The consecutive way of applying filaments was continued until six responses were recorded. The 50% threshold was calculated as described [32].
2.4 Neuropathology
Sciatic nerve segments were excised and post-fixed for 48 h at 4°C using 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Specimens were washed using phosphate buffer, post-fixed with 1% osmic acid (Ted Pella), dehydrated in graded (30–100%) ethyl alcohol and propylene oxide, and embedded in Araldite resin (Ted Pella). One-μm-thick sections were cut using a diamond knife in an automated RM2065 microtome (Leica Microsystems) and stained using methylene blue/azure II [33]. Sections from n of 3 animals per group and 3 randomly selected areas per section were analyzed.
2.5 Immunohistochemistry
Sciatic nerves were isolated following transcardial perfusion in 4% paraformaldehyde in 0.2 M phosphate, post-fixed and embedded in paraffin. Transverse (10-μm-thick) sections were deparaffinized in xylenes and rehydrated in graded ethanol. Following the non-specific binding block (5% goat serum in 0.3% Triton X-100 for 30 min at room temperature), nerve sections were incubated with a primary antibody (16–18 h at 4°C), followed by the species-specific Alexa 488-conjugated secondary antibody (green, ThermoFisher Scientific, 2 h, room temperature), a second primary antibody (16–18 h at 4°C), and the species-specific Alexa 594-conjugated secondary antibody (red, ThermoFisher Scientific, 2 h, room temperature). PBS was used for rinsing. Slowfade Gold antifade reagent containing nuclear stain DAPI (4′,6-diamidino-2-phenylindole, ThermoFisher Scientific, blue) was used for mounting. Alternatively, the biotin-conjugated secondary antibodies (Jackson ImmunoResearch), followed by Vectastain Elite ABC System, 3′-3-diaminobenzidine substrate (brown, Vector Laboratories), and Methyl Green counterstain were used according to our protocols [15]. Staining specificity was confirmed by a primary antibody omission or replacement with a non-immune serum. The images were acquired using a Leica DMR microscope and Openlab 4.04 imaging software (Perkin Elmer), using equal parameters (digital gain, exposure time, maximum white and black levels) for all groups. Morphometry was done using Image J software in 3 randomly selected areas per section, 3 sections, over 90 μl apart, per animal, and n of 3 animals per group by an investigator blinded to experimental groups.
2.6 Gelatin zymography
Sciatic nerves were isolated, snap-frozen in liquid N2, and stored at −80°C. Proteins were extracted in 50 mM Tris–HCl, pH 7.4, containing 1% Triton-x 100, 150 mM NaCl, 10% glycerol, 0.1% SDS. Extract aliquots (10–70 μg total protein as determined by BCA Protein Assay, ThermoFisher Scientific) were analyzed using 10% acrylamide gels co-polymerized with 0.1% gelatin. After electrophoresis, gels were washed in 2% Triton X-100 for 30 to 60 min at ambient temperature, incubated for 16 to 18 h at 37°C in 50 mM Tris–HCl buffer, pH 7.4, containing 10 mM CaCl2 and 1 μM ZnCl2 and 0.2 mM sodium azide, and stained with Coomassie Blue R250 to visualize the clear gelatinolytic activity bands.
2.7 TaqMan qRT-PCR
The sciatic nerves were isolated and stored in RNAlater (ThermoFisher Scientific) at −20°C. Total RNA was extracted using TRIzol (ThermoFisher Scientific) and purified on an RNeasy mini column (Qiagen). The RNA purity was estimated by measuring the OD260/280 ratio. The integrity of the RNA samples was validated using an Experion automated electrophoresis system (Bio-Rad). The samples were treated with RNase-free DNAse I (Qiagen). cDNA was synthesized using a SuperScript first-strand RT-PCR kit (Invitrogen). Real-time RT-PCR was conducted using Mx3005P™ qPCR System (Agilent) in 25 μl reactions containing Taqman Universal PCR Master Mix (ThermoFisher Scientific), cDNA (50 ng), specific forward and reverse primers (900 nM each) and Taqman probes (200–300 nM) for rat MT1-MMP, MMP-2, MMP-9 and TIMP (tissue inhibitor of metalloproteinases)-2, using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as the least regulated normalizer gene after PNS injury [33–35] with a one-step program: 95°C, 10 min; 95°C, 30 sec; 60°C, 1 min for 50 cycles. Duplicate samples without cDNA (no template control) showed no contaminating DNA. Relative mRNA levels were quantified using the comparative ΔCt method [36]. The fold-change calculations of duplicate samples obtained from at least four animals were performed using MxPro qPCR software (Agilent).
2.8 MT1-MMP proteolysis of MBP in vitro
The catalytic domain of MT1-MMP was expressed in E. coli, purified from the inclusion bodies using metal-chelating chromatography and refolded to restore its native conformation [37]. The refolded protease was used immediately in activity assays. The concentration of the catalytically active MT1-MMP was measured using a fluorescent assay by titration against a standard GM6001 solution of known concentration (EMD Millipore). (7-methoxycoumarin-4-yl)Acetyl-Pro-Leu-Gly-Leu-(3-[2,4- dinitrophenyl]-L-2,3-diaminopropionyl)-Ala-Arg-NH2 (Bachem) was used as a fluorescent substrate. The steady-state rate of the substrate cleavage by MT1-MMP was plotted as a function of inhibitor concentration and fitted with the equation V = SA(E0 − 0.5{(E0 + I + Ki) − [(E0 + I + Ki)2 − 4E0I]0.5}), where V is the steady-state rate of substrate hydrolysis, SA is specific activity (rate per unit of enzyme concentration), E0 is enzyme concentration. I is inhibitor concentration, and Ki is the dissociation constant of the enzyme-inhibitor complex [38].
Human MBP (4 μg; ~11 μM) was co-incubated for 1 h at 37°C with MT1-MMP (10–100 nM; an enzyme-substrate ratio of 1:100 and 1:1,000) in 20 μl reactions containing 50 mM HEPES, pH 6.8, supplemented with 10 mM CaCl2 and 50 μM ZnCl2. Where indicated, GM6001 (2.5 μM) was added to the reactions to inhibit MT1-MMP. The cleavage reaction was stopped using a 5xSDS sample buffer. The digest samples were analyzed by SDS-PAGE and MALDI-TOF MS using an Autoflex II MALDI TOF/TOF instrument (Bruker Daltonics). For the MS analysis, the reactions were cooled on ice and then equal volumes (2 μl) of a sample and of a sinapic acid (20 mg/ml) in 50% acetonitrile-0.1% trifluoroacetic acid solution were mixed, spotted directly on a MALDI target plate, air-dried and co-crystallized for 10 min. Mass spectra were processed with FlexAnalysis 2.4 software (Bruker Daltonics). The singly charged cleavage products, observed in the cleavage reactions but not in the controls, were recorded and analyzed further.
2.9 Data analyses
Statistical analysis was performed using GraphPrism 6.0 software (Synergy Software) by analysis of variance (ANOVA) for repeated measures with Bonferroni or Tukey’s post-hoc test, or Student’s T-test, as detailed in each Figure legend. p≤0.05 was considered significant.
3. RESULTS
3.1 The MT1-MMP/MMP-2/TIMP-2 axis in a rat model of neuropathic pain
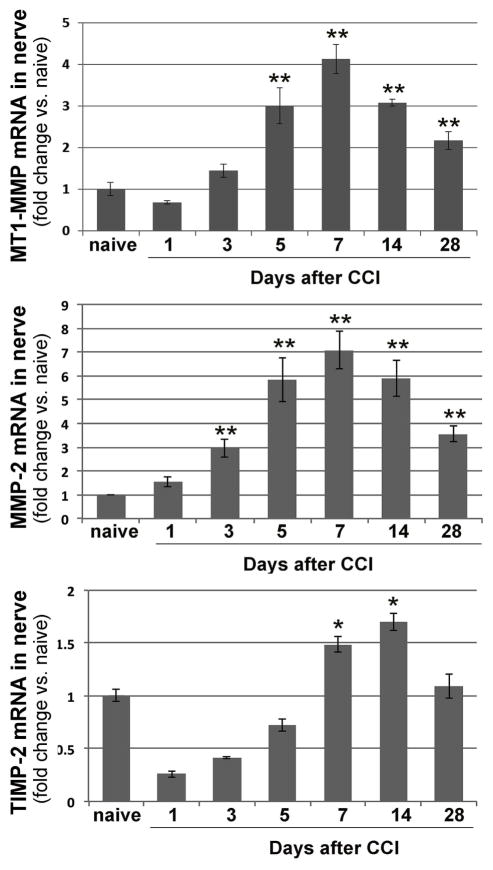
MT1–MMP is a key enzyme in the activation of latent MMP-2 [26]. TIMP-2 both assists MT1–MMP in MMP-2 activation and inhibits an excessive activity of both enzymes [26]. In naïve sciatic nerve, the MT1-MMP, MMP-2 and TIMP-2 transcripts are constitutively expressed [19, 34]. However, the ratio of the TIMP-2 expression level relative to those of the proteases is high, suggesting the repression of the MMP activity [34]. To determine whether there is a shift in the MT1-MMP, MMP-2 or TIMP-2 expression in the rat sciatic nerve over the course of painful mononeuropathy, we performed Taqman qRT-PCR of these genes at days 0 (naive), 1, 3, 5, 7, 14 and 28 after CCI (Fig. 1). Because females are prevalent sufferers of neuropathic pain, female rats were used [39]. At day 1 post-CCI, no change in MT1-MMP and MMP-2 expression was accompanied by a decline in TIMP-2 expression. Between days 3 and 7 post- CCI, the expression of all three genes was induced coordinately. At day 7 post-CCI, the expression of both proteases peaked, while TIMP-2 expression continued to increase until day 14. At day 28 post-CCI, TIMP-2 levels normalized, whereas the levels of both proteases remained elevated, relative to naïve nerve, and the TIMP-MMP ratio obviously shifted in favor of MMPs. These patterns of MT1-MMP, MMP-2 and TIMP-2 mRNA expression in CCI nerve are similar to their mRNA and protein expression in crushed nerve [34].
Figure 1. MT1-MMP, MMP-2 and TIMP-2 expression in CCI nerve.
Taqman qRT-PCR for MT1-MMP, MMP-2, and TIMP-2 in rat sciatic nerves at day 0 (naive) - day 28 after CCI. The mean relative mRNA ± SEM of 4–8 duplicate samples per group normalized to GAPDH and compared to naive nerve. *, p≤0.05, **, p≤0.005 by one-way ANOVA with Bonferroni corrections.
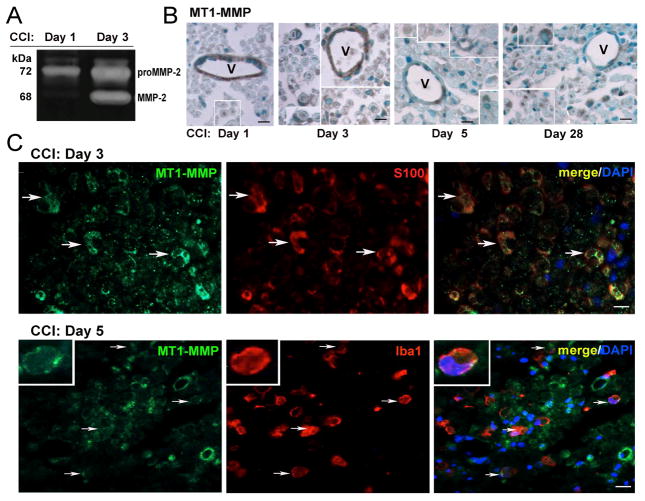
The proteolytic MT1-MMP activity in the nerve was measured by its ability to activate latent MMP-2. As determined by gelatin zymography (Fig. 2A), a significant portion of the latent MMP-2 proenzyme (72 kDa) observed at day 1 post-CCI was efficiently converted to the active (68 kDa) MMP-2 enzyme at day 3 post-CCI. The increasing levels of active MMP-2 sustained in nerve until at least day 7 post-CCI [7, 15, 40]. This zymography data suggests a significant increase of MT1-MMP activity at day 3 post-CCI.
Figure 2. MT1-MMP activity and distribution in CCI nerve.
A, Gelatin zymography of sciatic nerve extracts (70 μg protein/lane) post-CCI. The latent pro-MMP-2 and active MMP-2 species represented by the 72 and 68 kDa bands, respectively. Note the MT1-MMP-dependent MMP-2 activation between days 1 and 3 post-CCI. Representative of n=3/group. B, MT1-MMP immunostaining (brown) in nerve at days 0 (normal) - 28 post-CCI. Vessel (V) endothelial cells and crescent-shaped Schwann cells (insets) differentially produce MT1-MMP in normal and CCI nerves. C, Dual-immunofluorescence of MT1-MMP (green) and S100B (Schwann cells, red, top) or Iba1 (macrophages, red, bottom, inset) in nerve at days 3 and 5 post-CCI (arrows). DAPI, nuclear stain (blue). B–C: Representative of n=3–4/group. Scale bars, 25 μm.
Endothelial cells were the main endoneurial cell type producing MT1-MMP in normal nerve [34] and until day 3 post-CCI (Fig. 2B), similar to nerve crush [34]. Starting at days 3 post-CCI, MT1-MMP was also detected in crescent-shaped Schwann cells (confirmed by dual-labeling with S100B, Fig. 2C) and macrophages (confirmed by dual-labeling with Iba1, Fig. 2C). Axonal staining was noted at all time-points. The nerves remained immunoreactive for MT1-MMP until at least day 28 post-CCI (Fig. 2B).
3.2 Short-term MT1-MMP inhibition attenuates CCI-induced allodynia
To determine the functional role of MT1-MMP in CCI-induced allodynia, we used the two highly selective function-blocking monoclonal antibodies that do not cross-react with other MMPs: (1) a human monoclonal DX2400 antibody (hAb-DX2400) against the catalytic domain of MT1-MMP [30, 34]; and (2) the murine monoclonal 9E8 antibody (mAb-9E8) against MT1–MMP raised in an MT1-MMP null mouse [27]. Because it competes with the binding of TIMP-2 to the MT1-MMP catalytic domain, the unique mAb-9E8 antibody targets a single function of MT1-MMP: its ability to activate the MMP-2 proenzyme [28]. The human, rat, and murine catalytic domain sequences of MT1-MMP share 98% homology. In turn, hAb-DX2400 blocks the general proteolytic activity of MT1-MMP. The function-blocking antibodies were administered at day 3 post-CCI, the first manifestation of the increased MT1-MMP expression and activity (Figs. 1–2).
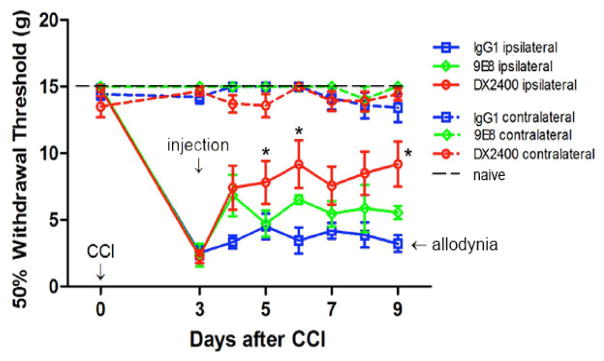
The rats were first confirmed to develop hypersensitivity to von Frey stimulation at day 3 post-CCI, as evident by reduced hind paw withdrawal threshold (Fig. 3). The rats then received a single bolus i.n. injection of hAb-DX2400 (n=11), mAb-9E8 (n=4), control IgG1 (n=9) or PBS vehicle (n=4) treatment into the CCI site. Within one day after treatment (day 4 post-CCI), the hAb-DX2400 treatment started to reduce the CCI-induced allodynia relative to control IgG1 and PBS (IgG1 only is shown) and its effect was significant at day 5 (p= 0.008 vs. PBS; 0.022 vs. IgG1), day 6 (p= 0.004 vs. PBS; 0.015 vs. IgG1) and day 9 post-CCI (p= 0.002 vs. PBS; 0.007 vs. IgG1), as determined using one-way ANOVA, with Bonferroni’s corrections. In turn, the mAb-9E8 treatment was briefly efficacious for one day but its effect was not significantly different from PBS or IgG1 treatment at any time-point. The contralateral to injury hind paws showed no change in hypersensitivity in the experimental or control groups.
Figure 3. Function-blocking MT1-MMP antibody (hAb-DX2400) attenuates CCI-induced allodynia.
von Frey testing after i.n. injection of the hAb- DX2400 (n=11), mAb-9E8 (n=4), or IgG1 (n=9), 1.0 mg/ml, each, at day 3 after CCI. von Frey testing was done in the hind paws ipsilateral and contralateral to injection. The mean withdrawal threshold (gram-force; g) ± SEM. Thresholds corresponding to naïve and allodynic animals are shown for reference. *, p≤0.01, hAb-DX2400 vs. IgG1 by repeated measures ANOVA with Bonferroni’s corrections.
3.3 Reduced myelin degeneration after short-term MT1-MMP inhibition
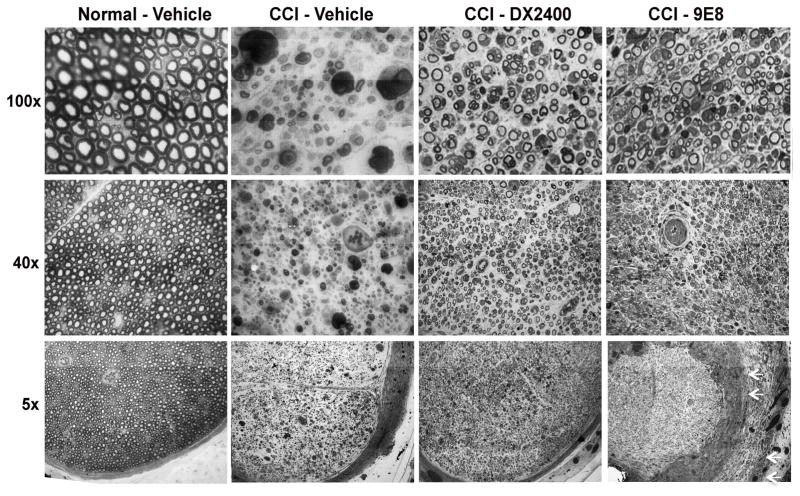
Upon completion of von Frey testing (Fig. 3), the neuropathological effects of MT1-MMP inhibition were assessed at day 10 after i.n. injection of hAb-DX2400, mAb-9E8 and vehicle, corresponding to day 13 post-CCI. Normal (contralateral) nerves displayed uniform morphology of intact axons, without visible neuropathological changes (Fig. 4). In contrast, CCI nerves exhibited features of Wallerian degeneration, including endoneurial edema, axon degeneration and myelin ovoids in the vehicle group. The CCI-induced degeneration and the formation of the myelin ovoids notably diminished after hAb-DX2400 and mAb-9E8 therapy. However, the mAb-9E8 tissues displayed significant subperineurial pathology (Fig. 4, 5x magnification). Due to these differences in the effects of hAb-DX2400 and mAb-9E8, we conclude that they target distinct substrates in the injured nerve. Consistent with the ability of mAb-9E8 to selectively target activation of MMP-2 [28], the subperineurial pathology relates to the unique localization of MMP-2 at the perineurial barrier (Suppl. Fig. S1) [15]. Conversely, the analgesic effect of hAb-DX2400 relates to other MT1-MMP substrates, such as MBP [24, 25].
Figure 4. Neuropathology of MT1-MMP inhibition in CCI nerve.
Methylene Blue Azure II staining in 1-μm-thick sciatic nerve sections after i.n. treatment with hAb-DX2400 (1.0 mg/ml), mAb-9E8 (1.0 mg/ml), or PBS (n=4) after completion of von Frey testing in Fig. 3, day 9 post-CCI. Representative micrographs of n=3/group, shown at 5x, 40x and 100x objective magnification. Normal (contralateral) nerve after vehicle injection showed intact nerve morphology. Features of Wallerian degeneration (axon degeneration, edema, myelin ovoids and immune cell infiltration) were observed in CCI nerves after IgG1 treatment. A greater number of uncompromised axons and fewer myelin ovoids were observed in CCI nerves treated with hAb- DX2400 or mAb-9E8. Note the disorganized subperineurial structures after mAb-9E8 treatment (arrows).
3.4 MT1-MMP liberates MBP69-89 in vitro and in CCI nerve in vivo
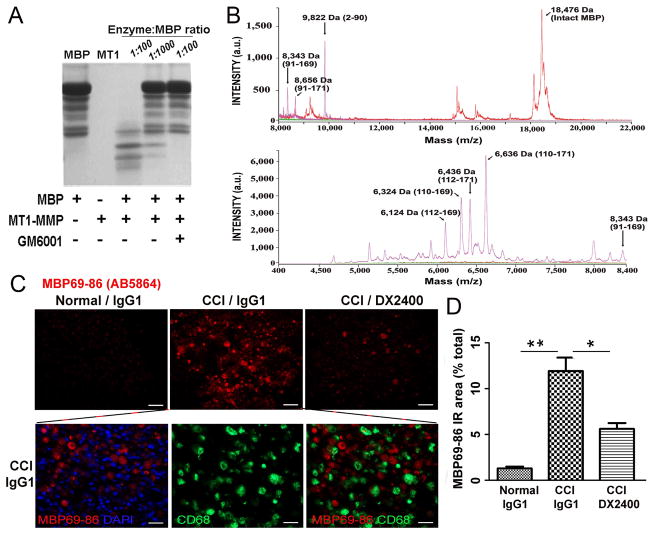
Based on our earlier [24, 25] and present findings, we hypothesized that the hAb-DX2400 therapy mitigated MBP degradation and the release of the algesic MBP69-96 peptide in CCI nerve. To confirm the ability of MT1-MMP to release the 69–86 region of MBP, we first co-incubated MBP (18.5 kDa) with the recombinant catalytic domain of MT1-MMP in vitro. A noticeable level of proteolysis was already observable in 1 h at a low, 1:1,000, MT1-MMP - MBP molar ratio (Fig. 5A). The degradation of MBP was accomplished at a molar ratio of 1:100. A broad-spectrum MMP inhibitor, GM6001, abolished MT1-MMP proteolysis of MBP. The MALDI-TOF MS analyses of the digest fragments confirmed that MT1-MMP released the MBP digest fragments containing the algesic 69–86 sequence (Fig. 5B).
Figure 5. MT1-MMP releases MBP69-86 peptides in vitro and in CCI nerve.
A, SDS-PAGE of MBP (11 μM) co-incubated for 60 min at 37°C with MT1-MMP (0.1 μM) at an enzyme-substrate ratio of 1:100 and 1:1,000. Where indicated, GM6001 was added to the reactions. B, Representative MALDI-TOF MS spectra of the resulting MBP peptide sample in A. The high and low molecular mass fragments are shown in the top and bottom panels, respectively. The intact and the digested MBP samples are in red and magenta, respectively. The buffer alone, green line. The numbers in the parentheses show the numbering of the peptide in the MBP sequence. a.u., arbitrary unit. C, MBP69-86 immunofluorescence (AB5864, Millipore, red) in nerve after i.n. hAb-DX2400 or IgG1 (1.0 mg/ml, each) injection at day 9 post-CCI alone (top panel) or with CD68 (green) in IgG1-treated CCI nerve (bottom panel); DAPI nuclear stain, blue. The algesic MBP69-86+ fragments are not phagocytosed by macrophages. Scale bar, 50 μm. D, The graph of the mean MBP69-86 immunoreactive (IR) as the percentage of total area of C. in n=3/group, 3 sections (≥90 μm apart) per n and 3 randomly selected areas per section at 400x magnification ± SEM, *, p≤0.05; **, p ≤0.0001, by one-way ANOVA with Tukey’s post-hoc test.
In CCI nerve, the MBP69-96 peptide was detected using the conformation-specific (AB5864) antibody to the 69–86 sequence of MBP (Fig. 5C–D). This antibody marks demyelinated lesions and, specifically, the degraded MBP69-86 rather than the intact MBP in the PNS [4, 7]. Upon completion of von Frey testing at day 9 post-CCI, the time-point of significant difference in mechanical sensitivity after hAb-DX2400 or IgG1 treatments (Fig. 3), MBP69-86 immunoreactivity showed a significant, 9.2-fold increase in MBP69-86 levels after IgG1 treatment in CCI nerve compared with the normal (contralateral to CCI) nerve. After hAb- DX2400 treatment, MBP69-86 levels increased only 4.4-fold, corresponding to ~2.1-fold reduction in MBP69-86 levels after hAb-DX2400 compared with IgG1. Importantly, the MBP69-86 immunoreactivity did not co-localize with CD68 at day 9 post-CCI (Fig. 5C), suggesting that the algesic MBP peptides are not phagocytized by macrophages during painful neuropathy. Further, hAb-DX2400 therapy did not affect macrophage numbers (Suppl. Fig. S2) or M1–M2 polarization (data not shown) in the nerve.
3.5 Exogenous MBP69-86 induces allodynia and MT1-MMP expression in nerve
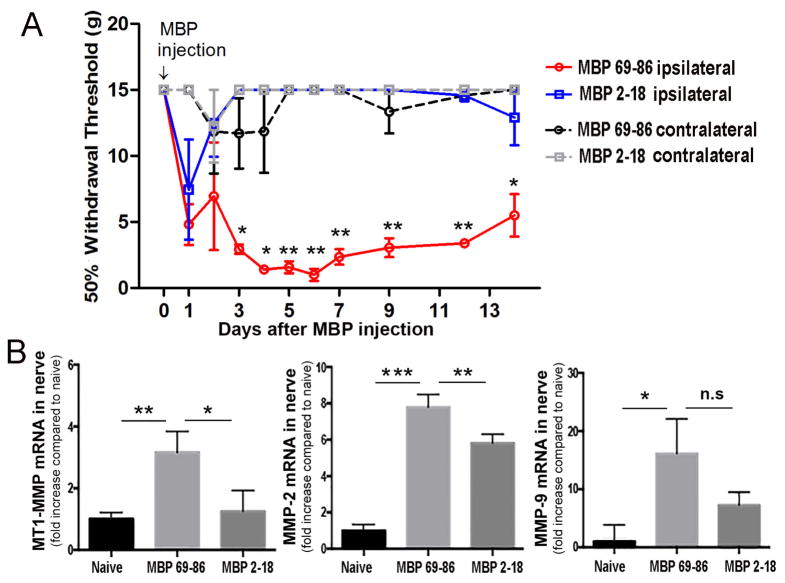
The inducers of MT1-MMP expression in the PNS are unknown [34], we tested the possibility that the CCI-induced release of the algesic MBP peptides by MMP-9 [7] and other proteases induced MT1-MMP expression at later time-points after CCI. To test this model, the pure rat MBP69-86 and MBP2-18 peptides (50 μg each) were administered by a single bolus i.n. injection into the rat intact sciatic nerve (Fig. 6). Within day 1 post-injection, a decline in the mechanical withdrawal threshold to von Frey stimulation was observed after both MBP69-86 and MBP2-18 peptide injections. MBP69-86 produced severe allodynia that lasted for the duration of the experiment (14 days). In contrast, the MBP2-18 group recovered within day 2 post-injection and maintained sensitivity comparable to that of the contralateral hind paws. This differential ability to induce allodynia was statistically significant at starting at day 3 and until day 14 was also observed with the MBP69-86 and MBP2-18 peptides encoding the human MBP sequences [7].
Figure 6. The algesic MBP69-86 peptides induce MMPs in nerve.
A, von Frey testing after single bolus i.n. injection of MBP69-86 and MBP2-18 peptides (50 μg in 5 μl, each) into a naïve sciatic nerve. A sustained decline in mechanical withdrawal thresholds was observed for two weeks after the MBP68-86 injection but not following the MBP2-18 injection. No change in thresholds was observed in the contralateral hind paws of either group. The mean withdrawal thresholds (gram force; g) ± SEM of n=6/group. *, p≤0.05, **, p≤0.01, by one-way ANOVA with Bonferroni’s corrections. B, Taqman qRT-PCR for MT1-MMP, MMP-2 and MMP-9 in nerve after A. (day 14 post-injection). The mean relative mRNA ± SEM of n=4–6 duplicate samples per group normalized to GAPDH and calibrated to naïve nerve. *, p≤0.05, **, p≤0.01, ***, p≤0.001, n.s, not significant, by one-way ANOVA with Tukey’s post-hoc test.
The nerves were analyzed using Taqman qRT-PCR for MT1-MMP, MMP-2 and MMP-9 mRNA expression, upon completion of von Frey testing, at day 14 after MBP69-86 and MBP2-18 peptide injection (Fig. 6B). Corresponding to allodynia, the expression of the MMP enzymes was induced significantly after MBP69-86 and to a lesser degree, after MBP2-18 peptide injection. These data imply the existence of the positive feedback loop between the MMP-mediated liberation of the algesic MBP peptides and increase in the MMP, including MT1-MMP, enzyme transcription in the nerve (Fig. 7).
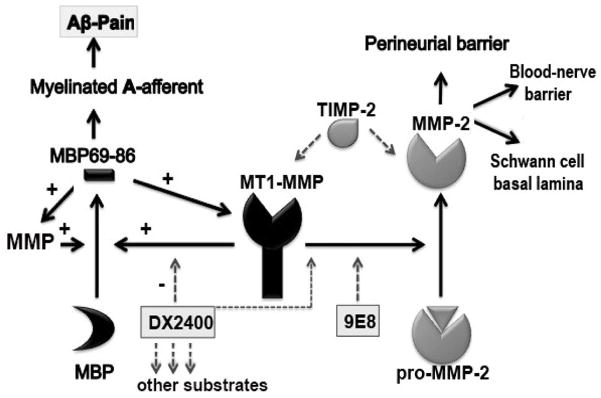
Figure 7. Neuropathic pain maintained via a positive feedback loop between MT1-MMP and MBP (a hypothesis diagram).
After nerve injury, MT1-MMP unencumbered by TIMP-2 releases the algesic MBP peptides (e.g., MBP69-86) from the intact MBP. The algesic MBP peptides enhance the expression of MT1-MMP and other MMPs and produce pain via the proposed mechanism [6]. Interference with this positive feedback loop, and the release of other pro-algesic substrates using the short-term and localized MT1-MMP function-blocking hAb-DX2400 therapy reduces allodynia. In addition, MT1-MMP activates latent MMP-2, involved in the maintenance of the perineurial barrier and axonal growth. Selective targeting of the MMP-2-activating function of MT1-MMP using mAb-9E8 adversely affects nerve repair and is not effective against allodynia. TIMP-2 is an endogenous MT1-MMP and MMP-2 inhibitor with an algesic action after nerve injury [16].
DISCUSSION
Mechanoselective A-afferent axons are electrically insulated by myelin, a multilamellar membrane that permits rapid propagation of low-threshold action potentials. Because myelin domains define both cytoarchitecture and molecular signature of A-afferents, demyelination causes electrical instability, ectopic insertion of ion channels, and ultimately, mechanical pain hypersensitivity [2, 5, 41, 42]. In addition to MMP-mediated demyelination, MMPs may regulate the pathogenesis of mechanical allodynia [4] via control of the structural and molecular assembly of myelinated axons. (1) By modulating Schwann cell signaling and number in the damaged nerve, MMPs control the length of myelin internodes and relative localization of nodal proteins [18, 43–45]. (2) By proteolysis of contractile, adhesive and ion channel proteins, including laminin, dystroglycan, sodium channels and NMDA receptor [17, 18, 34, 46–48], MMP may directly regulate the mechanosensitive machinery of A-afferents. (3) Because of the enhanced MMP proteolysis in the injured PNS, the liberated MBP peptides concentrate at the paranodal/nodal areas [4, 7], as part of pro-algesic immune complexes [6, 7]. Interestingly, membrane-tethered MT1-MMP partly translocates to paranodal/nodal regions after PNS injury [34]. The present study offers the first evidence that in a model of painful mononeuropathy: (1) MT1-MMP controls mechanical allodynia via release of the algesic MBP peptides; (2) the algesic MBP peptides induce the expression of MT1-MMP and other MMPs in nerve; and (3) short-term, localized and selective MT1-MMP inhibitor therapy blocks the release of the algesic MBP peptides and attenuates the development of mechanical allodynia.
Since first implicating MMPs in the pathogenesis of neuropathic pain [13, 15], we have identified MT1-MMP as the top-expressed, out of at least fifteen, MMP family members expressed in the normal or injured PNS [19]. Prior to injury, catalytic MT1-MMP activity in sciatic nerve is low due in part to exceedingly high TIMP-2 levels [19, 34]. Starting at day 3 post-CCI and crush [34], because of the increase in the MT1-MMP expression, excess MT1-MMP relative to TIMP-2 results in the presence of free MT1-MMP unencumbered by TIMP-2 and leads to a considerable level of activation of latent MMP-2. High levels of MMP-2 activity sustain for at least a week post-CCI [7, 34, 40]. To achieve high temporal and spatial specificity of blocking the excess MT1-MMP activity, the function-blocking MT1-MMP antibodies were administered once into the nerve fascicle at day 3 post-CCI, using the dose beneficial to sensory axon regrowth after sciatic nerve crush [34].
Human hAb-DX2400, but not murine mAb-9E8, attenuated CCI-induced mechanical allodynia. Both of these function-blocking antibodies are highly selective against MT1–MMP and effectively reduce Wallerian degeneration. However, only mAb-9E8 therapy produced subperineurial pathology. Our earlier work has established that by displacing TIMP-2 in the TIMP-2•MT1-MMP complex, mAb-9E8 represses selectively the MMP-2-activating capacity of MT1–MMP [28]. In contrast, hAb-DX2400 controls all proteolytic activities of MT1–MMP [30, 34], including the release the algesic MBP peptides or other substrates, as illustrated in Fig. 7. It is plausible to suggest that according to the unique MMP-2 deposition at the perineurial barrier and Schwann cell basal lamina [15], at comparable concentrations of both antibodies, mAb-9E8 has a more profound effect on blocking MMP-2 activity, including certain beneficial effects in the maintenance of the perineurial barrier or axonal growth [49, 50]. Long-term effects of either antibody remain to be determined. Despite the benefits of MT1-MMP/MMP-2 activity on axonal growth, cell migration or myelination [33, 34, 44, 49–53], broad-spectrum inhibition of the net catalytic MMP activity (using a hydroxamate derivative, GM6001) immediately or shortly after PNS injury facilitates nerve regrowth, limits myelin damage and attenuates pain [4, 7, 16, 18, 33, 54]. According to its analgesic effect, GM6001 inhibits MBP degradation both in the damaged nerve [4] and after MT1-MMP digestion in vitro.
The present data imply that the analgesic and MBP-degrading actions of the broad-spectrum MMP inhibition after PNS injury [4] are at least in part linked to inactivation of MT1-MMP. Specifically, MT1-MMP inhibition reduced the release of the algesic MBP69-86 peptides at the site of PNS damage, as determined using the AB5864 antibody. By selectively recognizing the cryptic 69–86 epitope, which is hidden in the intact, full-length MBP [4, 7], the use of the AB5864 antibody allows us to discern the effect of MT1-MMP proteolysis on MBP in nerve tissues. In addition, MMPs regulate Schwann cell maturation and myelination signaling [18, 44, 46, 54] and MMP inhibition results in reduced MBP mRNA levels in the injured nerve [54]. MT1-MMP proteolysis of MBP resulted in generation of peptides overlapping with the 69–86 region. Due to the well-documented MBP cleavage redundancy among the many MMP family members [20, 22–25] induced after PNS injury [19], we believe that several MMPs and potentially non-MMP enzymes work jointly to continuously release the algesic MBP residues over the course of PNS injury.
In addition to the algesic MBP release, inhibition of MMP activity protects neurovascular barriers from degradation, limits immune cell infiltration and glial activation [4, 55], although 15 the short-term and localized MT1-MMP inhibition here had no effect on macrophage number and M1-M2 polarization in the injured PNS. Other mechanisms of the nociceptive MT1-MMP activity may include the release of the algesic cytokines or regulation of proteolysis and function of adhesion molecules and ion channels at the PNS injury site, segmental ganglia or spinal cord [4, 13–16, 18, 34, 56]. If TIMP-2 compete with MT1-MMP antibody for the binding with MT1–MMP, the antibody excess may release free TIMP-2 to inhibit metalloproteinase activity or engage in trophic regulation [57] of nociceptive circuitry [16].
The algesic MBP peptides stimulated the nerve expression of MT1-MMP and other pronociceptive family members, MMP-2 and MMP-9 [4, 7, 13, 15, 16]. The degree of the MMP expression levels paralleled the pro-algesic properties of MBP69-86 and MBP2-18. While the specific cellular and molecular mechanisms of the algesic MBP action remain to be determined in future studies, full-length MBP is known to activate PI3K signaling via αMβ2 integrin/CD11b in microglia [58]. The differential ability of the central (68–104) but not the N-terminal (2–18) peptide sequences of MBP to promote allodynia observed presently and previously [8] support the notion that the cleavage of MBP are pivotal to initiation of its algesic action. Maintenance of the algesic MBP action depends on production of T cells, as shown using athymic nude rats [7]. Therapeutic immunization using the central MBP-derived mutant peptide ligand analogues impairs T cell function and alleviates CCI-induced allodynia [59]. These findings are consistent with the important role of Th1 and Th17 cells infiltrating the PNS injury site, segmental ganglia or spinal cord to promote the mechanical pain hypersensitivity [7, 60–66], especially in females [67].
From a clinical standpoint, mechanical allodynia is a common outcome of afferent/neuraxial lesions, neurodegenerative or neuroinflammatory states [1]. Because the algesic 68–104 sequence epitopes of MBP are detected in patients with Guillain-Barré syndrome and multiple sclerosis [9] and are released in animal models of painful peripheral neuropathy [4, 68], we believe they contribute to mechanical pain hypersensitivity associated with these clinical pain syndromes. The present study offers the first evidence that MT1-MMP controls mechanical allodynia via the release of the algesic MBP fragments from the intact myelin sheath, specifically in females, who are the prevalent sufferers of neuropathic pain [39]. A novel reciprocal mechanism of persistent mechanical allodynia has emerged via the MBP-induced expression of MT1-MMP and other MMP enzymes. Our data indicates that a short-term, localized and selective MT1-MMP monotherapy using human antibody is a promising and feasible means to intervene in the pathogenesis of neuropathic pain.
Supplementary Material
A, Illustration of sciatic nerve fascicle. Perineurial barrier (brown) separates the perineurium from endoneurium. B, MMP-2 immunoreactivity (brown, diaminobenzidine) in nerve at day 3 post-CCI at the perineurial barrier (arrows). Representative of n=4/group. Scale bar, 25 μm.
A, Macrophage CD68 (green) and MBP69-86 (red) dual-immunoreactivity (IR) in nerve at day 9 post-CCI. Single bolus i.n. injection of hAb-DX2400 or IgG1 (1.0 mg/ml each) was administered at day 3 post-CCI. MBP69-86 was not co-localized with macrophages and diminished after hAb-DX2400 treatment (also shown in Fig. 5A–B). B, Mean CD68 IR ± SEM of A. C, Mean DAPI+ cell counts ± SEM corresponding to A. B–C: quantified in n=3/group, 3 sections (≥90 μm apart) per n and 3 randomly selected areas per section at 400x magnification; n.s, not significant (p>0.05) by Student’s T-test.
MT1-MMP generates the algesic MBP peptides in vitro and the damaged PNS
The algesic MBP induces MT1-MMP expression in the PNS
Short-term MT1-MMP blockade attenuates allodynia and the algesic MBP release
Acknowledgments
NIH (to VIS, AYS and TLY) and the Department of Veterans Affairs Merit Review Award (to VIS) supported this study.
Footnotes
COMPETING INTERESTS: The authors declare no competing interests.
AUTHOR’S CONTRIBUTIONS: SHH, JD, WC and SH carried out animal procedures and behavioral analyses; SHH, JD and TN performed neuropathology, immunostaining, and zymography; MA, TN and SH performed qRT-PCR analyses; AR and SS performed mass spectrometry; VIS, SHH, AYS and TLY conceptualized the studies. VIS and SHH wrote the manuscript. All authors read, edited and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Treede RD, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–5. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 2.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–28. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 3.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4(12):968–80. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H, et al. MMPs initiate Schwann cell-mediated MBP degradation and mechanical nociception after nerve damage. Mol Cell Neurosci. 2008;39(4):619–27. doi: 10.1016/j.mcn.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry MA, et al. Sodium channel expression and localization at demyelinated sites in painful human dental pulp. J Pain. 2009;10(7):750–8. doi: 10.1016/j.jpain.2009.01.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shubayev VI, Strongin AY, Yaksh TL. Role of myelin auto-antigens in pain: a female connection. Neural Regen Res. 2016;11(6):890–1. doi: 10.4103/1673-5374.184452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, et al. Immunodominant fragments of myelin basic protein initiate T cell-dependent pain. Journal of neuroinflammation. 2012;9:119. doi: 10.1186/1742-2094-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko JS, et al. Spinal activity of IL-6 mediates mechanical allodynia induced by myelin basic protein. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.03.003. p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63(17):1945–61. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadlubowski M, Hughes RA. Identification of the neuritogen for experimental allergic neuritis. Nature. 1979;277(5692):140–1. doi: 10.1038/277140a0. [DOI] [PubMed] [Google Scholar]
- 11.Lu P, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrkonjic S, Destaing O, Albiges-Rizo C. Mechanotransduction pulls the strings of matrix degradation at invadosome. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006;11(1–2):8–20. doi: 10.1016/S1359-6446(05)03637-8. [DOI] [PubMed] [Google Scholar]
- 14.Dev R, et al. Therapeutic potential of matrix metalloprotease inhibitors in neuropathic pain. Expert Opin Investig Drugs. 2010;19(4):455–68. doi: 10.1517/13543781003643486. [DOI] [PubMed] [Google Scholar]
- 15.Shubayev VI, Myers RR. Endoneurial remodeling by TNFalpha- and TNFalpha-releasing proteases. A spatial and temporal co-localization study in painful neuropathy. J Peripher Nerv Syst. 2002;7(1):28–36. doi: 10.1046/j.1529-8027.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14(3):331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remacle AG, et al. MMP Proteolysis of the Extracellular Loop of Voltage-gated Sodium Channels and Potential Alterations in Pain Signaling. J Biol Chem. 2015 doi: 10.1074/jbc.C115.671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, et al. The MMP-9/TIMP-1 axis controls the status of differentiation and function of myelin-forming Schwann cells in nerve regeneration. PloS one. 2012;7(3):e33664. doi: 10.1371/journal.pone.0033664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernov AV, et al. Calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerve. The Journal of biological chemistry. 2015 doi: 10.1074/jbc.M114.622316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandler S, et al. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201(3):223–6. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 21.Gijbels K, et al. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36(4):432–40. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- 22.Proost P, Van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192(3):1175–81. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza CA, Moscarello MA. Differences in Susceptibility of MBP Charge Isomers to Digestion by Stromelysin-1 (MMP-3) and Release of an Immunodominant Epitope. Neurochem Res. 2006 doi: 10.1007/s11064-006-9116-9. [DOI] [PubMed] [Google Scholar]
- 24.Shiryaev SA, et al. Matrix metalloproteinase proteolysis of the myelin basic protein isoforms is a source of immunogenic peptides in autoimmune multiple sclerosis. PLoS One. 2009;4(3):e4952. doi: 10.1371/journal.pone.0004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiryaev SA, et al. Inflammatory proprotein convertase-matrix metalloproteinase proteolytic pathway in antigen-presenting cells as a step to autoimmune multiple sclerosis. J Biol Chem. 2009;284(44):30615–26. doi: 10.1074/jbc.M109.041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strongin AY, et al. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270(10):5331–8. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 27.Ingvarsen S, et al. Targeting a single function of the multifunctional matrix metalloprotease MT1-MMP: impact on lymphangiogenesis. The Journal of biological chemistry. 2013;288(15):10195–204. doi: 10.1074/jbc.M112.447169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiryaev SA, et al. A monoclonal antibody interferes with TIMP-2 binding and incapacitates the MMP-2-activating function of multifunctional, pro-tumorigenic MMP- 14/MT1-MMP. Oncogenesis. 2013;2:e80. doi: 10.1038/oncsis.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingvarsen S, et al. Targeting a single function of the multifunctional matrix metalloprotease MT1-MMP: impact on lymphangiogenesis. J Biol Chem. 2013;288(15):10195–204. doi: 10.1074/jbc.M112.447169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devy L, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer research. 2009;69(4):1517–26. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 31.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 32.Chaplan SR, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 33.Shubayev VI, et al. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31(3):407–15. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishihara T, et al. Matrix metalloproteinase-14 both sheds cell surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages and governs the response to peripheral nerve injury. The Journal of biological chemistry. 2015;290(6):3693–707. doi: 10.1074/jbc.M114.603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piller N, Decosterd I, Suter MR. Reverse transcription quantitative real-time polymerase chain reaction reference genes in the spared nerve injury model of neuropathic pain: validation and literature search. BMC Res Notes. 2013;6:266. doi: 10.1186/1756-0500-6-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Ratnikov B, et al. Determination of matrix metalloproteinase activity using biotinylated gelatin. Anal Biochem. 2000;286(1):149–55. doi: 10.1006/abio.2000.4798. [DOI] [PubMed] [Google Scholar]
- 38.Knight CG. Active-site titration of peptidases. Methods Enzymol. 1995;248:85–101. doi: 10.1016/0076-6879(95)48008-0. [DOI] [PubMed] [Google Scholar]
- 39.Fillingim RB, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855(1):83–9. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- 41.Wu G, et al. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;22(17):7746–53. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolf CJ, Doubell TP. The pathophysiology of chronic pain--increased sensitivity to low threshold A beta-fibre inputs. Current opinion in neurobiology. 1994;4(4):525–34. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann HC, et al. Matrix metalloproteinase-2 is involved in myelination of dorsal root ganglia neurons. Glia. 2009;57(5):479–89. doi: 10.1002/glia.20774. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay S, V, Shubayev I. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ErbB receptor-mediated activation of MEK/ERK pathway. Glia. 2009;57(12):1316–25. doi: 10.1002/glia.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner SR, Dotzlaf JE, Smith RC. MMP-28 as a regulator of myelination. BMC Neurosci. 2008;9:83. doi: 10.1186/1471-2202-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Court FA, et al. MMP2-9 cleavage of dystroglycan alters the size and molecular composition of Schwann cell domains. J Neurosci. 2011;31(34):12208–17. doi: 10.1523/JNEUROSCI.0141-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H, et al. Spinal Glia Division Contributes to Conditioning Lesion-Induced Axon Regeneration Into the Injured Spinal Cord: Potential Role of Cyclic AMP-Induced Tissue Inhibitor of Metalloproteinase-1. J Neuropathol Exp Neurol. 2015;74(6):500–11. doi: 10.1097/NEN.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, V, Shubayev I. Matrix metalloproteinase-9 controls proliferation of NG2+ progenitor cells immediately after spinal cord injury. Experimental neurology. 2011;231(2):236–46. doi: 10.1016/j.expneurol.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaublomme D, et al. Matrix metalloproteinase 2 and membrane type 1 matrix metalloproteinase co-regulate axonal outgrowth of mouse retinal ganglion cells. J Neurochem. 2014;129(6):966–79. doi: 10.1111/jnc.12703. [DOI] [PubMed] [Google Scholar]
- 50.Krekoski CA, et al. Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts. J Neurosci. 2002;22(23):10408–15. doi: 10.1523/JNEUROSCI.22-23-10408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo J, et al. Degradation of chondroitin sulfate proteoglycan enhances the neuritepromoting potential of spinal cord tissue. Exp Neurol. 1998;154(2):654–62. doi: 10.1006/exnr.1998.6951. [DOI] [PubMed] [Google Scholar]
- 52.Shubayev VI, Myers RR. Matrix metalloproteinase-9 promotes nerve growth factor-induced neurite elongation but not new sprout formation in vitro. J Neurosci Res. 2004;77(2):229–39. doi: 10.1002/jnr.20160. [DOI] [PubMed] [Google Scholar]
- 53.Muir D. Metalloproteinase-dependent neurite outgrowth within a synthetic extracellular matrix is induced by nerve growth factor. Exp Cell Res. 1994;210(2):243–52. doi: 10.1006/excr.1994.1036. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, et al. MMP inhibition enhances the rate of nerve regeneration in vivo by promoting de-differentiation and mitosis of supporting Schwann cells. J Neuropathol Exp Neurol. 2010;69(4):386–395. doi: 10.1097/NEN.0b013e3181d68d12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chattopadhyay S, et al. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun. 2007;21(5):561–8. doi: 10.1016/j.bbi.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remacle AG, et al. Matrix Metalloproteinase (MMP) Proteolysis of the Extracellular Loop of Voltage-gated Sodium Channels and Potential Alterations in Pain Signaling. J Biol Chem. 2015;290(38):22939–44. doi: 10.1074/jbc.C115.671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Science signaling. 2008;1(27):re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stapulionis R, et al. Structural insight into the function of myelin basic protein as a ligand for integrin alpha M beta 2. J Immunol. 2008;180(6):3946–56. doi: 10.4049/jimmunol.180.6.3946. [DOI] [PubMed] [Google Scholar]
- 59.Perera CJ, et al. Active immunization with myelin-derived altered peptide ligand reduces mechanical pain hypersensitivity following peripheral nerve injury. Journal of neuroinflammation. 2015;12(1):28. doi: 10.1186/s12974-015-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129(3):767–77. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 61.Draleau K, et al. Phenotypic Identification of Spinal Cord-Infiltrating CD4 T Lymphocytes in a Murine Model of Neuropathic Pain. J Pain Relief. 2014;(Suppl 3):003. doi: 10.4172/2167-0846.S3-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim CF, Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011;1405:95–108. doi: 10.1016/j.brainres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 63.Kim CF, Moalem-Taylor G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain. 2011;12(3):370–83. doi: 10.1016/j.jpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38(2):448–58. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweitzer SM, et al. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain. 2002;100(1–2):163–70. doi: 10.1016/s0304-3959(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 66.Costigan M, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29(46):14415–22. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–3. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H, et al. Immunodominant fragments of myelin basic protein initiate T cell-dependent pain. J Neuroinflammation. 2012;9:119. doi: 10.1186/1742-2094-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Illustration of sciatic nerve fascicle. Perineurial barrier (brown) separates the perineurium from endoneurium. B, MMP-2 immunoreactivity (brown, diaminobenzidine) in nerve at day 3 post-CCI at the perineurial barrier (arrows). Representative of n=4/group. Scale bar, 25 μm.
A, Macrophage CD68 (green) and MBP69-86 (red) dual-immunoreactivity (IR) in nerve at day 9 post-CCI. Single bolus i.n. injection of hAb-DX2400 or IgG1 (1.0 mg/ml each) was administered at day 3 post-CCI. MBP69-86 was not co-localized with macrophages and diminished after hAb-DX2400 treatment (also shown in Fig. 5A–B). B, Mean CD68 IR ± SEM of A. C, Mean DAPI+ cell counts ± SEM corresponding to A. B–C: quantified in n=3/group, 3 sections (≥90 μm apart) per n and 3 randomly selected areas per section at 400x magnification; n.s, not significant (p>0.05) by Student’s T-test.