Abstract
Exposure to a prolonged restraint stressor disrupts the colonic microbiota community composition, and is associated with an elevated inflammatory response to colonic pathogen challenge. Since the stability of the microbiota has been implicated in the development and modulation of mucosal immune responses, we hypothesized that the disruptive effect of the stressor upon the microbiota composition directly contributed to the stressor-induced exacerbation of pathogen-induced colitis. In order to establish a causative role for stressor-induced changes in the microbiota, conventional mice were exposed to prolonged restraint to change the microbiota. Germfree mice were then colonized by microbiota from either stressor-exposed or non-stressed control mice. One day after colonization, mice were infected with the colonic pathogen, Citrobacterrodentium. At six days post-infection, mice that received microbiota from stress-orexposed animals had significant increases in colonic pathology and pro-inflammatory cytokine (e.g. IL-1β) and chemokine (e.g. CCL2) levels after C. rodentium infection in comparison with mice that received microbiota from non-stressed mice. 16S rRNA gene sequencing revealed that microbial communities from stressed mice did not have any detectable Bifidobacterium present, a stark contrast with the microbial communities from non-stressed mice, suggesting that stressor-induced alterations in commensal, immunomodulatory Bifidobacterium levels may predispose to an increased inflammatory response to pathogen challenge. This study demonstrates that the commensal microbiota directly contribute to excessive inflammatory responses to C. rodentium during stressor exposure, and may help to explain why gastrointestinal disorders are worsened during stressful experiences.
Introduction
The gastrointestinal (GI) tract is colonized by a consortium of microbes, including bacteria, fungi, viruses, and archaea, that are collectively termed the microbiota. Commensal bacteria that reside as part of the microbiota normally exhibit considerable stability under healthy host conditions. However, when disruption in the microbial communities that inhabit both the lumen and mucosa of the GI tract do occur (in a state known as dysbiosis), they can negatively feedback upon the host and lead to dysfunction in host physiology and immunology (Chen et al., 2015; De Minicis et al., 2014; Duboc et al., 2013; Qin et al., 2012). A number of external effectors can induce dysbiosis, including antibiotics and diet, leading to well-established changes in community structure and resultant feedback upon host health (Kim et al., 2012; Kim et al., 2014; Martinez-Medina et al., 2014). Perception of a psychological stressor has also been implicated in microbiota community structure alterations, but prior to this study it was not known whether stressor-induced changes in the microbiota also directly impact host health.
Previous studies have demonstrated that exposure to prolonged restraint stress significantly shifts the mucosal-associated microbiota and reduced the relative abundance of the immunomodulatory genus, Lactobacillus (Galley et al., 2014b). Similar findings have been extended to human and non-human primate hosts (Bailey and Coe, 1999; Knowles et al., 2008). Exposure to prolonged restraint stress also exacerbates the inflammatory response to enteric pathogen challenge in animal models (Bailey et al., 2010; Mackos et al., 2013) as evidenced by increases in inflammatory cytokine mRNA and colonic pathology. However, whether stressor-induced dysbiosis is directly linked to host physiological or immune function has not yet been elucidated. Thus, this study aimed to determine whether stressor-induced changes to the colonic microbiota leads to increased colonic inflammation upon challenge with the colonic pathogen Citrobacterrodentium .
Germ-free (GF) mice are commonly used for examining the effects the microbiota have upon host physiology and immunity. For example, through the use of GF models, the microbiota have been implicated in weight gain, wherein conventionalized mice had increased fat deposition compared to GF mice (Turnbaugh et al., 2006). Germfree mice have also been used to demonstrate that the microbiota contribute to the development of mucosal immune cell (e.g. Th17 cells, macrophages) maturation, abundance and activation (Ivanov et al., 2008; Niess and Adler, 2010; Souza et al., 2004). Additionally, transplanting the microbiota from donors with altered genotypes (e.g. NOD2−/− mice) or phenotypes (e.g. obese humans) into GF mice via fecal transplant has been used to analyze the complex interplay between host-mediated modeling of the microbiota compositional structure, and microbiota-mediated feedback on host function (Couturier-Maillard et al., 2013; Ridaura et al., 2013). We hypothesized that stressor-induced alterations to the microbiota were directly associated with a heightened inflammatory response to colonic pathogens, and we tested this hypothesis by colonizing GF mice with the microbiota from stressor-exposed mice and assessing the inflammatory response to challenge with C. rodentium . To our knowledge, this is the first study in which the microbiota from stressed mice were transplanted into naïve GF mice, and thus represents a significant step in the characterization of the effect of psychological stress upon the microbiota and the resultant impacts upon host physiology and immunity.
Materials and Methods
Mice
Conventional male CD-1 mice (aged 6–8 weeks) were ordered from Charles River Laboratories (Raleigh, NC). Mice were housed 3 per cage and acclimated for a week in the vivarium before stressor exposure. Healthy male germ-free (GF) Swiss Webster (aged 6–8 weeks) mice were kept in sterile cages within a fully decontaminated BSL-2 level biosafety cabinet for the duration of the study. Mice were handled under aseptic conditions with sterile gloves as we have previously reported (Allen et al., 2012). Germfree mice were monitored by periodic fecal Gram-staining, 16s PCR, and intestinal pathology tests performed by the Ohio State University Lab Animal Resource Department and by our own laboratory and were confirmed to be free of pathogens and other microbial contaminants. All mice were given sterilized food and water ad libitum and kept on a 12-hour light:dark cycle (Conventional mice- 0600 to 1800 lights on, Germ-free mice- 0700 to 1900 on). All stressors, infection, and sacrifice protocols were approved by the Ohio State University Animal Care and Use Committee.
Stressor
Restraint (RST) stress was administered to conventional mice as previously published (Galley et al., 2014b). Briefly, mice were placed in a 50-mL conical tube for 16 consecutive hours, beginning at 1700 and concluding at 0900 the following morning. The RST stressor was continued each night for a total of seven cycles. Two control groups were used: a food and water deprivation group that had food and water removed during the restraint period, but were not otherwise restrained (FWD), and a control group that was undisturbed and kept in a separate room (HCC).
Germ-Free Reconstitution Experiments
Immediately following the final cycle of the respective stressors, conventional mice were euthanized via CO2 asphyxiation. Colons were aseptically removed, and the tissue was bisected using sterile forceps and scissors. An equivalent amount of fecal pellets for each group was pooled in 3 mL of anaerobically pre-reduced PBS, in addition to a mucosal scraping from each colon. Each donor group was comprised of pooled fecal and mucosal slurry from three total mice per experiment. The fecal slurry was immediately placed in an anaerobic canister with a BD GasPak EZ until transplant into the GF mice. Fecal microbiota were transplanted to GF mice via oral gavage within two hours of conventional mouse sacrifice. GF mice were gavaged with 200 µL total fecal slurry, and then were food and water deprived for two hours. Germfree mice that received microbiota from stressor-exposed donors are labeled RST-GF (n=7), those receiving microbiota from non-stressed control donors are labeled HCC-GF (n=6), and those receiving microbiota from donors that were deprived of food and water during the periods that restrained animals were in the restraining tubes are labeled FWD-GF (n=6). The slurries were kept at −80°C until sequencing via Illumina at a later date.
Bacteria
Citrobacterrodentium strain DBS120 (pCRP1::Tn5) was grown overnight at 37°C in tryptic soy broth. After growth, the pathogen culture was brought up to 1×109 CFU/mL in sterile water and 100 µL was given to RST-GF, HCC-GF, or FWD-GF mice 24 hours after receiving the fecal microbiota transplant. Mice were monitored and sacrificed at day 6 post-infection. Total infectious burden was measured by plating shed fecal pellets from the GF mice, at days 3 through 6 post-infection, on MacConkey agar supplemented with 40 µg/mL kanamycin.
qRT-PCR
On Day 6 post-infection, colons were collected and bisected. Half was used for RNA isolation using the previously published TriZOL method (Mackos et al., 2013). Briefly, isolated RNA was normalized to 1 µg per sample and then reverse transcribed to cDNA via Promega Reverse Transcription System. Multiplex qRT-PCR was performed on the ABI Prism 7000 system using primers targeting mRNA for interleukin1β (Forward: GGCCTCAAAGGAAAGAATCTATACC; Reverse: GTATTGCTTGGGATCCACACTCT, probe: ATGAAAGACGGCACACCCACCCTG), CCL2 (Forward: TTGGCTCAGCCAGATGCA; Reverse CCTACTCATTGGGATCATCTTGC; Probe: AACGCCCCACTCACCTGCTGCTACT), and inducible nitric oxide synthase (iNOS, Forward: CAGCTGGGCTGTACAAACCTT; Reverse: TGAATGTGATGTTTGCTTCGG, Probe: CGGGCAGCCTGTGAGACCTTTGA). Murine 18S (Forward: CGGCTACCACATCCAAGGAA; Reverse: GCTGGAATTACCGCGGCT; Probe: TGCTGGCACCAGACTTGCCCTC) was used as a housekeeper gene. The comparative threshold cycle method was used for data analysis as previously described, with HCC-GF set as baseline (Bailey et al., 2010).
Histopathology
Half of the colon collected from RST-GF, HCC-GF, and FWD-GF mice was fixed in neutral buffered formalin until paraffin embedding and hematoxylin and eosin staining. Total colonic pathology was scored by a board-certified veterinary pathologist (N.M.P.), who was blind to experimental conditions. Each section was scored by the pathologist based upon hyperplasia, dysplasia, edema, epithelial defects, and inflammation. Every category had a scale of 0 to 4, with 0 indicating not present and 4 indicating severe. All categories were totaled for a maximum score of 20 (Berg et al., 1996).
Sequencing
DNA was isolated from the pooled fecal slurry of each group (FWD, RST, and HCC) using a QIAamp Fast DNA Stool Kit with modifications. Briefly, fecal slurries were incubated with lysozyme buffer (20 mg/mL lysozyme, 20mM Tris-HCl, 2mM EDTA, 1.2% Triton-X). After a 45 min incubation at 37°C, 300 mg of 0.1 mm zirconia beads were added, and the tube placed in a bead beater for 150 seconds. After bead beating, InhibitEX Buffer was added (5 µl/mg stool), and the tube vortexed for 1 min followed by incubation at 95°C for 5 min. After centrifugation, proteinase K and Buffer AL were added (30 µl), and the tubes vortexed for 15 s prior to incubating at 70°C for 10 min. After incubating, 100% ethanol was added to each sample and the sample added to a QiAmp spin column. The spin column was centrifuged (8000 g for 1 min), and then 500 µl of Buffer AW1 followed by 500 µl of AW2 were added. Finally, 100 µl of buffer ATE was added to elute the DNA. 20 µl of RNase A (20 mg/ml) was added to the eluate and incubated for 10 min at room temperature, followed by addition of 200 µl of 100% ethanol. The solution was transferred to a new QIAamp spin column that was centrifuged for 1 min. The column was washed with AW1 and AW2 as above, and DNA eluted with 100 µl of ATE buffer. DNA concentration and purity was assessed using a Qubit 2.0 flourometer and gel electrophoresis. Next, Illumina sequencing was performed by the Molecular and Cellular Imaging Center, located at the Ohio State University Ohio Agriculture Research and Development Center. 2×300bp paired end sequencing was achieved using Illumina MiSeq. Upon completion of sequencing, sequences were demultiplexed using Sabre (website:http://github.com/najoshi/sabre), and joined on Quantitative Insights Into Microbial Ecology (QIIME) 1.8.0 using fastq-join (Aronesty, 2011; Caporaso et al., 2010). Closed-reference OTU picking was performed against the GreenGenes 13_8 database using UCLUST, set at 97% identity, which added another quality filtering step (Edgar, 2010; McDonald et al., 2012). Quality filtering was performed at a qual score of 20, with 0 allowed N characters, 1.5 allowed barcode errors (G to T, A to C count as 1 error, all other mismatches are 0.5 errors), and 3 consecutive low quality bases allowed before sequence truncation. After OTU picking and taxonomic assignment with GG_13_8, an average of 2693 sequences/samples remained, in a total of 561 unique OTUs. Relative taxonomic abundances were calculated in QIIME.
Statistical Analyses
C. rodentium colonization. H&E pathology, and qPCR data were all analyzed using two-factor ANOVA. Pairwise comparisons were performed with the LSD post-hoc test on SPSS (v. 24).
Results
Bifidobacterium was not present in the Restraint Donor Slurry
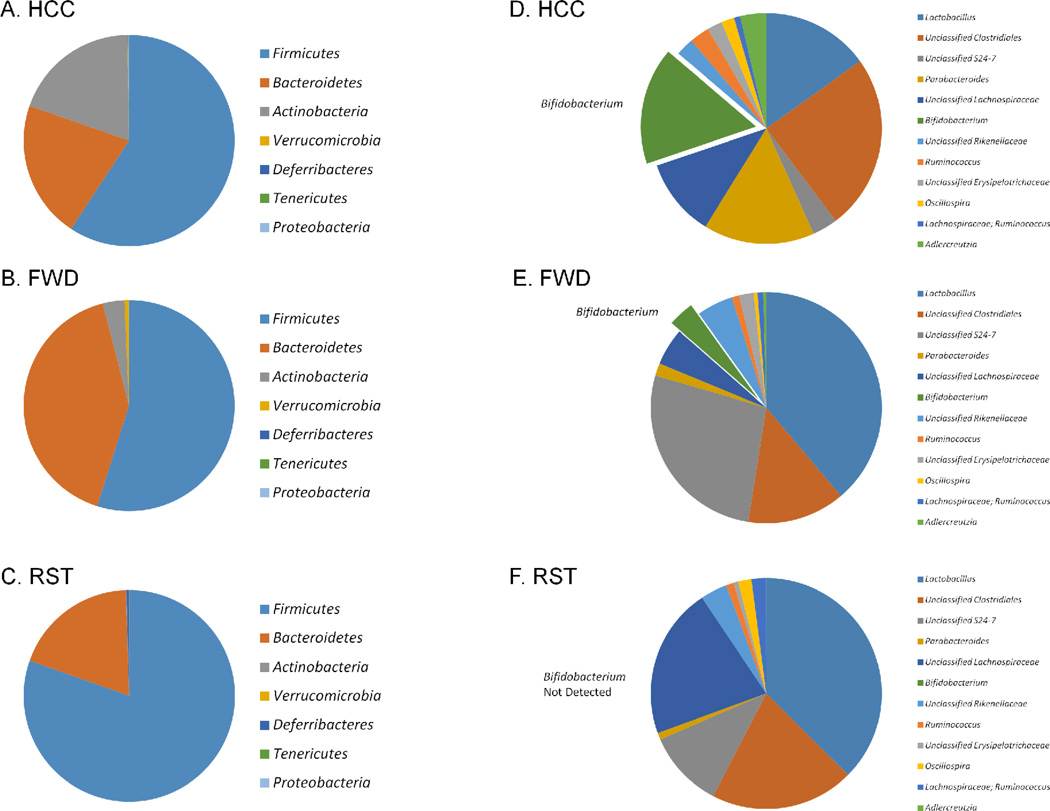
Donor microbiota transplant pools were pooled by experiment (the study consisted of replicate experiments), resulting in a total of two donor pools from mice exposed to restraint (RST), food and water deprivation (FWD) or non-stressed controls (HCC). The microbiota composition of each donor pool was analyzed in QIIME after Illumina sequencing. After quality filtering and closed reference OTU picking, a mean of 2693 sequences per experimental group remained, spread among 561 unique OTUs. Sequences from the stool/mucosal scraping slurry from non-stressed HCC mice were primarily derived from bacteria in the phylum Firmicutes (59.4%), Bacteroidetes (21.06%), and Actinobacteria (19.4%) with fewer sequences (less than 0.25% combined) derived from Verrucomicrobia, Deferribacteres, Tenericutes, and Proteobacteria (Fig. 1A). Firmicutes levels were higher in the RST Donor mice, while Actinobacteria were lower in these same mice.
Figure 1.
The RST Donor fecal slurry had marked differences in bacterial composition when compared with the HCC and FWD Donor slurries. A–C.) At the phylum level, the RST Donor slurry had a lower proportion of Actinobacteria , and an elevated proportion of Firmicutes in comparison to the HCC and FWD Donor slurries. D–F.) At the genus level, the RST Donor slurry did not contain any Bifidobacterium . The HCC Donor slurry was comprised of 15.9% Bifidobacterium , and the FWD Donor slurry had 3.00%. Data are the mean relative abundances of sequences from 2 pools of feces/mucosal scrapings from n=3 mice per group per pool.
Genus abundances varied as well between donor groups. The most abundant genera were Lactobacillus and an unclassified genus of Clostridiales which comprised 33 – 67% of total sequences (Fig. 1D–1F). Interestingly, Bifidobacterium , which was the sixth most abundant genus group, and comprised 15.9% of sequences from HCC samples, was not present in any of the restraint samples (Fig. 1F). All of the other top 6 most abundant genera were found in HCC, FWD, and RST samples.
Citrobacterrodentium burden was not increased in RST-GF mice
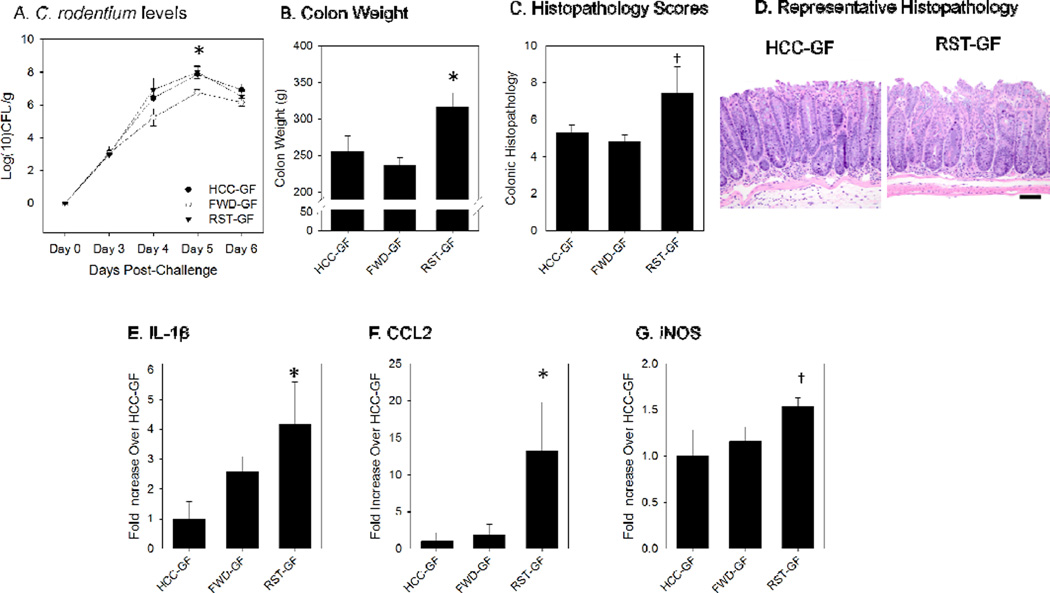
The level of Citrobacterrodentium burden within the colons was measured by fecal pellet culturing on MacConkey Agar. A two-factor ANOVA for days-post-infection (DPI) and group was performed. C. rodentium burden was significantly increased by both DPI (p<.05) and Group (p<.05). In order to evaluate how the RST microbiota or the FWD microbiota affected C. rodentium burden by DPI, post-hoc LSD tests were performed. Levels were not different in RST-GF, HCC-GF, or FWD-GF mice on d3, d4, or d6. However, C. rodentium levels were reduced in FWD-GF mice compared to RST-GF and HCC-GF mice on 5 DPI (p<.05) (Fig. 2A).
Figure 2.
Germ-free mice that received the RST Donor slurry exhibited an elevated inflammatory response to Citrobacterrodentium challenge in comparison with mice that received the microbiota slurry from un-stressed mice. A.) C. rodentium levels were not increased in the RST-GF group over the course of the 6 day study. Colonization levels are displayed in log(10) colony-forming units per gram of fecal pellet. * p<.05 vs. HCC-GF and RST-GF on Day 6 post-challenge. B.) Colon weight was significantly increased at 6 days-post-infection (DPI) in the RST-GF mice. Colon masses are presented in total grams. * p<.05 vs. HCC-GF and RST-GF. C.) Scoring of H&E stained slides for colonic pathology was increased in the RST-GF mice at 6 DPI. † p=.06 (one factor ANOVA). D.) A representative image of the histopathology for an HCC-GF mouse and an RST-GF mouse. E.) Colonic IL-1β mRNA levels were significantly increased in the RST-GF mice over HCC-GF, but not FWD-GF. * p <.05 vs. HCC-GF. F.) Colonic CCL2 mRNA was significantly increased in RST-GF in comparison with both FWD-GF and HCC-GF. * p <.05 vs. HCC-GF and FWD-GF. G.) Colonic iNOS mRNA showed a trend towards a significant increase in the RST-GF mice (p<0.10 in one factor ANOVA). Significance set at p<0.05. All mRNA data are fold increases over HCC-GF using the ∆∆ Ct method and are expressed as the mean +/− standard error.
Colonic pathology and colon mass was increased in RST-GF mice
Colonic tissue mass during C. rodentium infection is associated with the colonic hyperplasia induced by C. rodentium pathogen and is indicative of colonic hyperplasia, leukocyte infiltration, and edema. A one-factor ANOVA was used to compare RST-GF, HCC-GF, and FWD-GF. In addition, the LSD post-hoc test was used for pairwise comparisons. RST-GF mice had significantly increased colonic mass over both HCC-GF and FWD-GF at 6 DPI (p<0.05) (Fig. 2B). Due to the increased colonic mass, colonic pathology was examined using H&E staining. At 6 DPI, RST-GF mice showed a trend towards increased colonic pathology scores over both FWD-GF and HCC-GF groups (p=.06), indicating that transplant with the stress-exposed microbiota led to marginally increased colonic pathology (Fig. 2C–D).
Pro-inflammatory mRNA transcripts were increased in RST-GF mice
The mRNA levels of pro-inflammatory markers were examined in the GF mice that received the transplanted microbiota. IL-1β, a pro-inflammatory cytokine, was significantly increased in the RST-GF group over HCC-GF at 6 DPI (p<.05), but not over FWD-GF (Fig. 2E). CCL2, a pro-inflammatory chemokine that draws trafficking monocytes and macrophages from the bone marrow so they can migrate to the site of infection, was higher in the RST-GF group than in the HCC-GF and the FWD-GF groups (p<.05) (Fig. 2F). The mean level of iNOS mRNA was higher in RST-GF than in HCC-GF and FWD-GF, but this difference did not quite reach statistical significance (p=.10) (Fig. 2G).
Discussion
This study confirms our previous studies that have demonstrated that RST changes the composition of the colonic mucosal and luminal microbiotas (Galley et al., 2014b). Previous studies also demonstrated that exposure to the stressor increases the severity of infectious colitis upon challenge with C. rodentium (Bailey et al., 2010). However, whether the stressor-induced changes in the microbiota directly contribute to the stressor-induced exacerbation of mucosal inflammation in C. rodentium -challenged mice was not known. This study demonstrates that the mucosal immune response to C. rodentium is directly affected by commensal microbes from stressor-exposed mice. Mice given microbiota from RST-exposed donor mice had an elevated inflammatory response to C. rodentium (marked by significantly greater colon mass (a marker of pathogen-induced colonic hyperplasia) as well as significant increases in inflammatory cytokines) in comparison to mice that received microbiota from un-stress donors. The cytokines and chemokines assessed are necessary for the immune response to C. rodentium (Alipour et al., 2013; Kim et al., 2011; Vallance et al., 2002). However, when produced in excess, they can lead to tissue-damaging inflammatory responses. Indeed, mice given microbiota from RST-exposed donor mice had a tendency to have more severe colonic pathology after challenge with C. rodentium (although this difference was not quite statistically significant with a p=.06). Taken together, these data demonstrate that stressor effects on the microbiota have functional consequences and leave the male host more susceptible to tissue-damaging inflammatory responses. It is interesting to note that the FWD control condition was protective against C. rodentium colonization. The reasons for this protection are not yet known, but it is possible that the immunomodulatory effects of caloric restriction (Shibolet et al., 2002) are influenced by the commensal microbiota. Previous studies also indicate that the microbiota can have different effects on gastrointestinal inflammation in male and female mice (Lofgren, et al., 2011). Thus, there is a need to understand whether the effects of the stressor extend to females, or are limited to males.
To begin to understand which stressor-induced changes in the microbiota might contribute to tissue-damaging inflammatory responses, Illumina sequencing was performed on the RST (as well as HCC and FWD) donor stool and mucosal scraping slurry used in the oral gavage to the GF mice to characterize microbial communities in the samples. Sequencing did not identify differences between the RST slurry and the control group microbiotas in beta and alpha diversity, nor were there measured differences in Lactobacillus abundance (data not shown). This is in contrast to previous studies where we have demonstrated that exposure to the stressor significantly changes both measures of alpha and beta diversity in intestinal microbiota, and significantly decreased the relative abundance of the Lactobacillus genus (Bailey et al., 2010; Galley et al., 2014b). However, there are several differences that may account for the discrepancies. First, previous studies have assessed samples from individual animals (not pooled from multiple animals) and assessed luminal and mucosa-associated microbial communities separately. These previous studies found that microbial populations at these two niches are significantly different (Galley et al., 2014b). In this study, the decision was made to colonize with microbiota from both luminal and tissue-associated populations in an attempt to completely colonize the mice. It is possible that pooling samples from multiple animals and from two different niches within the intestines introduced too much variability for the effects of the stressor on overall community structure to be observed ex vivo. A second consideration is that in previous studies, 454 FLX Titanium Pyrosequencing of the V1–V3 variable regions of the 16s rRNA gene was used to characterize microbial communities, whereas this study involved the use of Illumina MiSeq sequencing of the V1–V3 16s rRNA gene region to characterize microbial communities. Further studies are needed to completely understand why differences in overall microbial community diversity were not observed in the current study.
Despite the lack of community-wide differences, there was an increase in Firmicutes and a decrease in Actinobacteria in samples from stressor-exposed mice. The Firmicutes are often associated with intestinal inflammation, and have been found to be elevated in mice with experimental colitis that are exposed to stress (Watanabe et al., 2016) as well as in humans with ulcerative colitis (Forbes et al., 2016). The Actinobacteria contain the beneficial bacterial genus Bifidobacterium , and interestingly the mean relative abundance of Bifidobacterium was different among the groups. Bifidobacterium was found in high abundance in samples from non-stressed HCC donors, but was not observed in the samples from stressor-exposed mice. Species in Bifidobacterium are associated with reductions in GI inflammation, and are involved with host physiology, particularly in obesity and insulin and glucose regulation (Cano et al., 2013; Le et al., 2014; Moya-Perez et al., 2014; O'Mahony et al., 2010; Philippe et al., 2011). Because Bifidobacterium were observed in microbiota from HCC and FWD donors, but not in RST donors, and because many species of Bifidobacterium can reduce mucosal inflammation, it is possible that the stressor-induced reduction in Bifidobacterium resulted in an environment conducive to an increased inflammatory response upon pathogen challenge. The role of Bifidobacterium in stressor-induced exacerbation of infectious colitis merits further investigation.
Fecal microbiota transplants are both a recent advent into gastrointestinal illness treatment and a hotly-debated methodology. Though patients with Clostridium difficile infection that have received fecal microbiota transplant from ‘healthy’ donors often report abrogation of symptoms (Jang et al., 2015; Youngster et al., 2014), the long-term effect of the transplanted microbiota upon the new host over time is not yet understood. Microbiota community structure has been associated with obesity and diabetes, as well as altered inflammatory responses (Ferrer et al., 2013; Larsen et al., 2010; Roberts et al., 2014). Although previous studies have demonstrated that psychological stress can impact microbiota profiles (Bailey et al., 2010; Galley et al., 2014a; Galley et al., 2014b), it was not known whether these stressor-induced changes to the microbiota impact host physiological and immunological functioning. Transplanting the microbiota from stressor-exposed conventional mice to GF mice provides evidence that stressor-induced changes in the microbiota directly affect the mucosal inflammatory response. These findings also have important implications for human patients. For example, stress has been associated with symptomatic flare-ups in patients with inflammatory bowel disease (IBD) (Bernstein et al., 2010). The reason for this association is unknown, but since patients with IBD often have altered microbiota structure (Frank et al., 2007; Machiels et al., 2013), and because the microbiota can affect host immune function, it is possible that stress-induced shifts in microbial populations of IBD patients could augment the inflammatory spikes observed in flare-ups. The results herein demonstrate how the microbiota can steer the inflammatory response within the GI tract, implicating the microbiota as key players in stressor-induced immunomodulation in the colon. Future studies must continue to characterize the particular community structures that associate with inflammation.
Highlights.
Germfree mice were colonized with microbiota from stressor-exposed or control mice.
Newly colonized mice were infected with the colonic pathogen Citrobacterrodentium .
Colonization with microbiota from stressed donors increased colonic inflammation.
Stressor-induced effects on the microbiota directly affect mucosal immunity.
Acknowledgments
This work was supported by grant NIH RO1AT006552 to MB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alipour M, Lou Y, Zimmerman D, Bording-Jorgensen MW, Sergi C, Liu JJ, Wine E. A balanced IL-1beta activity is required for host response to Citrobacterrodentium infection. PloS one. 2013;8:e80656. doi: 10.1371/journal.pone.0080656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RG, Lafuse WP, Galley JD, Ali MM, Ahmer BM, Bailey MT. The intestinal microbiota are necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain, behavior, and immunity. 2012;26:371–382. doi: 10.1016/j.bbi.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronesty E. ea-utils. pp. Command-line tools for processing biological sequencing data. 2011 [Google Scholar]
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Developmental psychobiology. 1999;35:146–155. [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacterrodentium. Infection and immunity. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in Interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. Journal of Clinical Investigation. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. The American journal of gastroenterology. 2010;105:1994–2002. doi: 10.1038/ajg.2010.140. [DOI] [PubMed] [Google Scholar]
- Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity. 2013;21:2310–2321. doi: 10.1002/oby.20330. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Nunez G, Chen G, Rosenstiel P, Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. The Journal of clinical investigation. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, Mingarelli E, Facinelli B, Magi G, Palmieri C, Marzioni M, Benedetti A, SvegliatiBaroni G. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, Thomas G, Barbu V, Humbert L, Despras G, Bridonneau C, Dumetz F, Grill JP, Masliah J, Beaugerie L, Cosnes J, Chazouilleres O, Poupon R, Wolf C, Mallet JM, Langella P, Trugnan G, Sokol H, Seksik P. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Ruiz A, Lanza F, Haange SB, Oberbach A, Till H, Bargiela R, Campoy C, Segura MT, Richter M, von Bergen M, Seifert J, Suarez A. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environmental microbiology. 2013;15:211–226. doi: 10.1111/j.1462-2920.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- Forbes JD, Van Domselaar G, Bernstein CN. Microbiome survey of the inflamed and noninflamed gut at different compartments within the gastrointestinal tract of inflammatory bowel disease patients. Inflammatory Bowel Diseases. 2016;22(4):817–825. doi: 10.1097/MIB.0000000000000684. [DOI] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC microbiology. 2014a;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, Bailey MT. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut microbes. 2014b;5:748–760. doi: 10.4161/19490976.2014.972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, FrutosRde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MO, An JH, Jung SI, Park KH. Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient. Intestinal research. 2015;13:80–84. doi: 10.5217/ir.2015.13.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PloS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Nunez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2) Cell host & microbe. 2014;15:95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biological psychology. 2008;77:132–137. doi: 10.1016/j.biopsycho.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sorensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS one. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TK, Hosaka T, Le TT, Nguyen TG, Tran QB, Le TH, Pham XD. Oral administration of Bifidobacterium spp. improves insulin resistance, induces adiponectin, and prevents inflammatory adipokine expressions. Biomedical research. 2014;35:303–310. doi: 10.2220/biomedres.35.303. [DOI] [PubMed] [Google Scholar]
- Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, Wang TC, Fox JG. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- Mackos AR, Eubank TD, Parry NM, Bailey MT. Probiotic Lactobacillus reuteri attenuates the stressor-enhanced severity of Citrobacter rodentium infection. Infection and immunity. 2013;81:3253–3263. doi: 10.1128/IAI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116–124. doi: 10.1136/gutjnl-2012-304119. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME Journal. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Perez A, Romo-Vaquero M, Tomas-Barberan F, Sanz Y, Garcia-Conesa MT. Hepatic molecular responses to Bifidobacterium pseudocatenulatum CECT 7765 in a mouse model of diet-induced obesity. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2014;24:57–64. doi: 10.1016/j.numecd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. Journal of Immunology. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- O'Mahony D, Murphy S, Boileau T, Park J, O'Brien F, Groeger D, Konieczna P, Ziegler M, Scully P, Shanahan F, Kiely B, O'Mahony L. Bifidobacterium animalis AHC7 protects against pathogen-induced NF-kappaB activation in vivo. BMC Immunology. 2010;11:63. doi: 10.1186/1471-2172-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe D, Favre L, Foata F, Adolfsson O, Perruisseau-Carrier G, Vidal K, Reuteler G, Dayer-Schneider J, Mueller C, Blum S. Bifidobacterium lactis attenuates onset of inflammation in a murine model of colitis. World journal of gastroenterology : WJG. 2011;17:459–469. doi: 10.3748/wjg.v17.i4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ME, Bishop JL, Fan X, Beer JL, Kum WW, Krebs DL, Huang M, Gill N, Priatel JJ, Finlay BB, Harder KW. Lyn deficiency leads to increased microbiota-dependent intestinal inflammation and susceptibility to enteric pathogens. Journal of immunology. 2014;193:5249–5263. doi: 10.4049/jimmunol.1302832. [DOI] [PubMed] [Google Scholar]
- Shibolet O, Alper R, Avraham Y, Berry EM, IIan Y. Immunomodulation of experimental colitis via caloric restriction: role of NK1.1 T cells. Clinical Immunology. 2002;105:48–56. doi: 10.1006/clim.2002.5260. [DOI] [PubMed] [Google Scholar]
- Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. Journal of immunology. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vallance BA, Deng W, De Grado M, Chan C, Jacobson K, Finlay BB. Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen citrobacterrodentium in infected mice. Infection and immunity. 2002;70:6424–6435. doi: 10.1128/IAI.70.11.6424-6435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Arase S, Nagaoka N, Kawai M, Matsumoto S. Chronic psychological stress disrupted the composition of the murine colonic microbiota and accelerated a murine model of inflammatory bowel disease. PloS One. 2016:0150559. doi: 10.1371/journal.pone.0150559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]