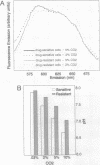
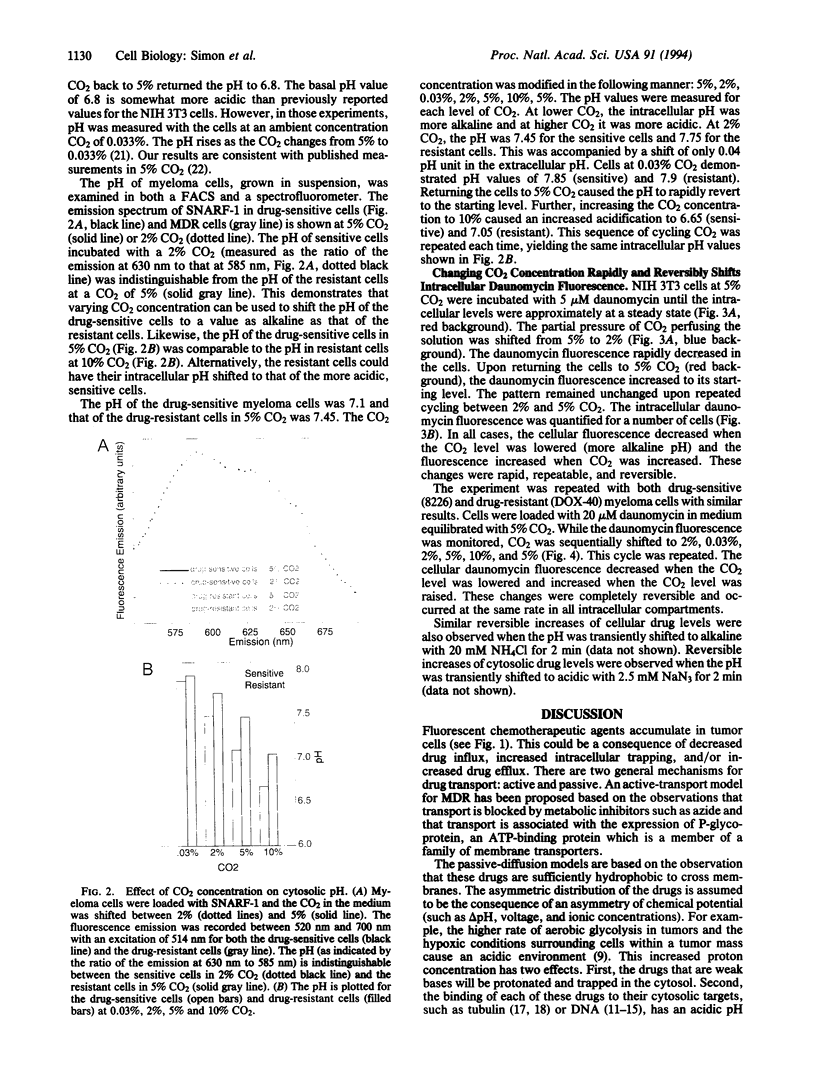
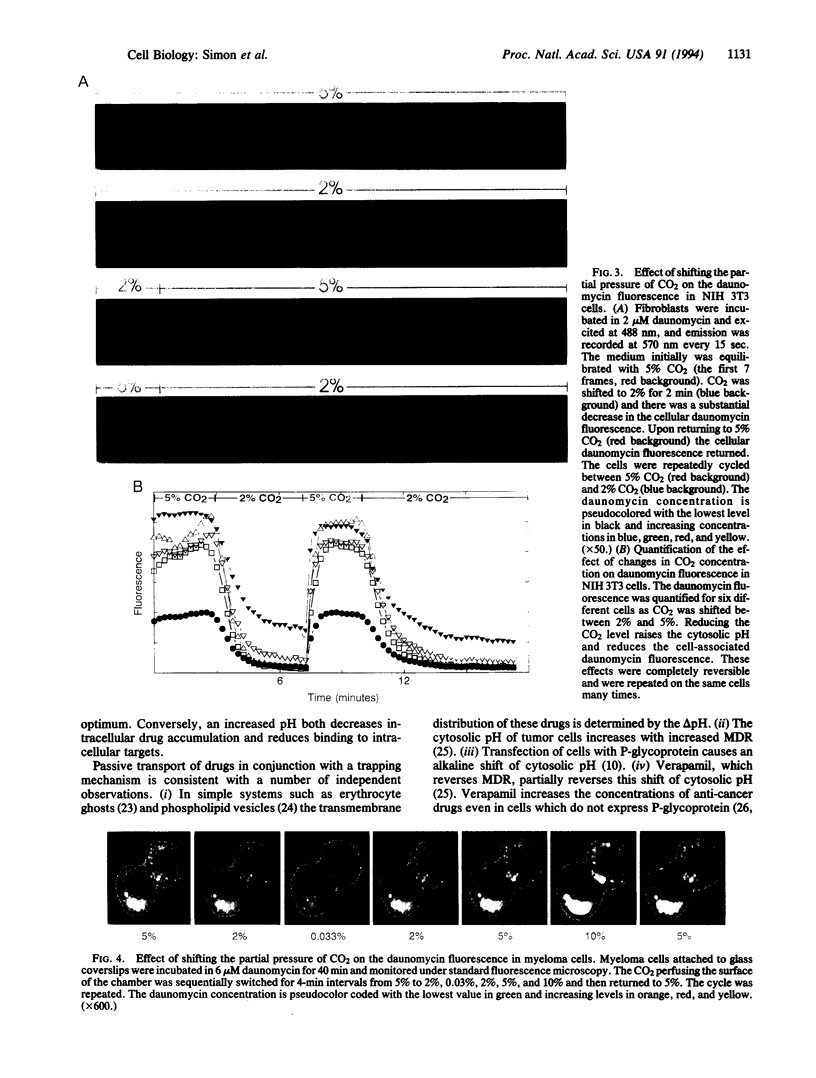
Abstract
Many anticancer drugs are classified as either weak bases or molecules whose binding to cellular structures is pH dependent. Accumulation of these drugs within tumor cells should be affected by transmembrane pH gradients. Indeed, development of multidrug resistance (MDR) in tumor cells has been correlated with an alkaline shift of cytosolic pH. To examine the role of pH in drug partitioning, the distribution of two drugs, doxorubicin and daunomycin, was monitored in fibroblasts and myeloma cells. In both cell types the drugs rapidly accumulated within the cells. The highest concentrations were measured in the most acidic compartments--e.g., lysosomes. Modifying the cellular pH in drug-sensitive cells to mimic reported shifts in MDR caused an immediate change in the cellular drug concentration. Drug accumulation was enhanced by acidic shifts and reversed by alkaline shifts. All of these effects were rapid and reversible. These results demonstrate that the alkaline shift observed in MDR is sufficient to prevent the accumulation of chemotherapeutic drugs independent of active drug efflux.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T., Cirtain M. C., Lefko J. L. Energy-dependent reduced drug binding as a mechanism of Vinca alkaloid resistance in human leukemic lymphoblasts. Mol Pharmacol. 1983 Nov;24(3):485–492. [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. doi: 10.1146/annurev.ph.48.030186.002113. [DOI] [PubMed] [Google Scholar]
- CALENDI E., DIMARCO A., REGGIANI M., SCARPINATO B., VALENTINI L. ON PHYSICO-CHEMICAL INTERACTIONS BETWEEN DAUNOMYCIN AND NUCLEIC ACIDS. Biochim Biophys Acta. 1965 May 11;103:25–49. doi: 10.1016/0005-2787(65)90539-3. [DOI] [PubMed] [Google Scholar]
- Carlsen S. A., Till J. E., Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta. 1976 Dec 14;455(3):900–912. doi: 10.1016/0005-2736(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Dalmark M., Storm H. H. A Fickian diffusion transport process with features of transport catalysis. Doxorubicin transport in human red blood cells. J Gen Physiol. 1981 Oct;78(4):349–364. doi: 10.1085/jgp.78.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Di Marco A., Casazza A. M., Dasdia T., Necco A., Pratesi G., Rivolta P., Velcich A., Zaccara A., Zunino F. Changes of activity of daunorubicin, adriamycin and stereoisomers following the introduction or removal of hydroxyl groups in the amino sugar moiety. Chem Biol Interact. 1977 Dec;19(3):291–302. doi: 10.1016/0009-2797(77)90052-7. [DOI] [PubMed] [Google Scholar]
- Di Marco A., Silvestrini R., Di Marco S., Dasdia T. Inhibiting effect of the new cytotoxic antibiotic daunomycin on nucleic acids and mitotic activity of HeLa cells. J Cell Biol. 1965 Dec;27(3):545–550. doi: 10.1083/jcb.27.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskocil J., Fric I. Complex formation of daunomycin with double-stranded RNA. FEBS Lett. 1973 Nov 15;37(1):55–58. doi: 10.1016/0014-5793(73)80425-9. [DOI] [PubMed] [Google Scholar]
- Epand R. F., Epand R. M., Gupta R. S., Cragoe E. J., Jr Reversal of intrinsic multidrug resistance in Chinese hamster ovary cells by amiloride analogs. Br J Cancer. 1991 Feb;63(2):247–251. doi: 10.1038/bjc.1991.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Gillies R. J., Martinez-Zaguilan R., Martinez G. M., Serrano R., Perona R. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7414–7418. doi: 10.1073/pnas.87.19.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gruber A., Briese B., Areström I., Vitols S., Björkholm M., Peterson C. Effect of verapamil on daunorubicin accumulation in human leukemic cells with different levels of MDR1 gene expression. Leuk Res. 1993 Apr;17(4):353–358. doi: 10.1016/0145-2126(93)90023-e. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Inaba M., Sakurai Y. Enhanced efflux of actinomycin D, vincristine, and vinblastine in adriamycin-resistant subline of P388 leukemia. Cancer Lett. 1979 Dec;8(2):111–115. doi: 10.1016/0304-3835(79)90003-x. [DOI] [PubMed] [Google Scholar]
- Keizer H. G., Joenje H. Increased cytosolic pH in multidrug-resistant human lung tumor cells: effect of verapamil. J Natl Cancer Inst. 1989 May 3;81(9):706–709. doi: 10.1093/jnci/81.9.706. [DOI] [PubMed] [Google Scholar]
- Lin P. Y., Ahluwalia M., Gruenstein E. An alkaline pH-activated Cl(-)-anion exchanger regulates pH homeostasis in fibroblasts. Am J Physiol. 1990 Jan;258(1 Pt 1):C132–C139. doi: 10.1152/ajpcell.1990.258.1.C132. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Ma L., Center M. S. The gene encoding vacuolar H(+)-ATPase subunit C is overexpressed in multidrug-resistant HL60 cells. Biochem Biophys Res Commun. 1992 Jan 31;182(2):675–681. doi: 10.1016/0006-291x(92)91785-o. [DOI] [PubMed] [Google Scholar]
- Marsh W., Sicheri D., Center M. S. Isolation and characterization of adriamycin-resistant HL-60 cells which are not defective in the initial intracellular accumulation of drug. Cancer Res. 1986 Aug;46(8):4053–4057. [PubMed] [Google Scholar]
- Mayer L. D., Bally M. B., Cullis P. R. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim Biophys Acta. 1986 May 9;857(1):123–126. doi: 10.1016/0005-2736(86)90105-7. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay K., Parrack P. K., Bhattacharyya B. The carboxy terminus of the alpha subunit of tubulin regulates its interaction with colchicine. Biochemistry. 1990 Jul 24;29(29):6845–6850. doi: 10.1021/bi00481a013. [DOI] [PubMed] [Google Scholar]
- Na C., Timasheff S. N. Physical-chemical study of daunomycin-tubulin interactions. Arch Biochem Biophys. 1977 Jul;182(1):147–154. doi: 10.1016/0003-9861(77)90293-4. [DOI] [PubMed] [Google Scholar]
- Nygren P., Larsson R., Gruber A., Peterson C., Bergh J. Doxorubicin selected multidrug-resistant small cell lung cancer cell lines characterised by elevated cytoplasmic Ca2+ and resistance modulation by verapamil in absence of P-glycoprotein overexpression. Br J Cancer. 1991 Dec;64(6):1011–1018. doi: 10.1038/bjc.1991.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owellen R. J., Donigian D. W., Hartke C. A., Hains F. O. Correlation of biologic data with physico-chemical properties among the vinca alkaloids and their congeners. Biochem Pharmacol. 1977 Jul 1;26(13):1213–1219. doi: 10.1016/0006-2952(77)90108-3. [DOI] [PubMed] [Google Scholar]
- Ramu A., Pollard H. B., Rosario L. M. Doxorubicin resistance in P388 leukemia--evidence for reduced drug influx. Int J Cancer. 1989 Sep 15;44(3):539–547. doi: 10.1002/ijc.2910440328. [DOI] [PubMed] [Google Scholar]
- Sehested M., Skovsgaard T., van Deurs B., Winther-Nielsen H. Increase in nonspecific adsorptive endocytosis in anthracycline- and vinca alkaloid-resistant Ehrlich ascites tumor cell lines. J Natl Cancer Inst. 1987 Jan;78(1):171–179. doi: 10.1093/jnci/78.1.171. [DOI] [PubMed] [Google Scholar]
- Sehested M., Skovsgaard T., van Deurs B., Winther-Nielsen H. Increased plasma membrane traffic in daunorubicin resistant P388 leukaemic cells. Effect of daunorubicin and verapamil. Br J Cancer. 1987 Dec;56(6):747–751. doi: 10.1038/bjc.1987.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Yang C. H., Mines L. S., Oribé E., Biedler J. L. Markedly altered membrane transport and intracellular binding of vincristine in multidrug-resistant Chinese hamster cells selected for resistance to vinca alkaloids. J Cell Physiol. 1986 Feb;126(2):266–274. doi: 10.1002/jcp.1041260217. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Mechanisms of resistance to daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Jun;38(6):1785–1791. [PubMed] [Google Scholar]
- Skovsgaard T. Transport and binding of daunorubicin, adriamycin, and rubidazone in Ehrlich ascites tumour cells. Biochem Pharmacol. 1977 Feb 1;26(3):215–222. doi: 10.1016/0006-2952(77)90306-9. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Currier S. J., Whitaker J., Haugland R. P., Gottesman M. M., Pastan I., Willingham M. C. Activity of the multidrug transporter results in alkalinization of the cytosol: measurement of cytosolic pH by microinjection of a pH-sensitive dye. J Histochem Cytochem. 1990 May;38(5):685–690. doi: 10.1177/38.5.1692055. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Weaver J. L., Pine P. S., Aszalos A., Schoenlein P. V., Currier S. J., Padmanabhan R., Gottesman M. M. Laser scanning and confocal microscopy of daunorubicin, doxorubicin, and rhodamine 123 in multidrug-resistant cells. Exp Cell Res. 1991 Oct;196(2):323–329. doi: 10.1016/0014-4827(91)90267-x. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Timasheff S. N. Aggregation of microtubule subunit protein. Effects of divalent cations, colchicine and vinblastine. Biochemistry. 1970 Oct 13;9(21):4110–4116. doi: 10.1021/bi00823a012. [DOI] [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]
- Zunino F., Di Marco A., Zaccara A. Molecular structural effects involved in the interaction of anthracyclines with DNA. Chem Biol Interact. 1979 Feb;24(2):217–225. doi: 10.1016/0009-2797(79)90010-3. [DOI] [PubMed] [Google Scholar]
- Zunino F., Gambetta R., Di Marco A., Velcich A., Zaccara A., Quadrifoglio F., Crescenzi V. The interaction of adriamycin and its beta anomer with DNA. Biochim Biophys Acta. 1977 May 3;476(1):38–46. doi: 10.1016/0005-2787(77)90283-0. [DOI] [PubMed] [Google Scholar]
- Zunino F., Gambetta R., Di Marco A., Zaccara A. Interaction of daunomycin and its derivatives with DNA. Biochim Biophys Acta. 1972 Sep 14;277(3):489–498. doi: 10.1016/0005-2787(72)90092-5. [DOI] [PubMed] [Google Scholar]
- van Adelsberg J., Al-Awqati Q. Regulation of cell pH by Ca+2-mediated exocytotic insertion of H+-ATPases. J Cell Biol. 1986 May;102(5):1638–1645. doi: 10.1083/jcb.102.5.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]