Abstract
Background
Preoperative transcatheter arterial chemoembolization (TACE) is administered to improve long-term outcome after surgical resection of hepatocellular carcinoma (HCC). However, the survival benefit of preoperative TACE is controversial. We conducted a retrospective case-control study to evaluate the effect of preoperative TACE on prognosis.
Methods
A total of 121 patients who underwent curative resection of HCC were divided into two groups according to whether they received preoperative TACE. We determined the control group (n = 34) and TACE group (n = 34) through propensity score matching. The primary endpoint of this study was overall survival, and the secondary endpoints were recurrence-free survival.
Results
The overall survival rate and the recurrence free survival rate were significantly lower in the TACE group than in the control group (P = 0.014 and P = 0.043, respectively). Furthermore, recurrence free survival within less than 2 years after resection was significantly worse in the TACE group than in the control group (P = 0.035).
Conclusion
Preoperative TACE seemed to worsen the long-term outcomes of the patients who underwent surgical resection for the treatment of resectable HCC. Therefore, preoperative TACE should not be considered as a standard therapy in patients with resectable HCC.
Keywords: surgical resection, survival, hepatectomy, neoadjuvant
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide.1 Hepatic resection is considered a curative treatment for HCC, and the current the Japan Society of Hepatology, European Association for Study of the Liver and the American Association for the Study of Liver Diseases guidelines recommend resection as one of the first-line treatments for early stage HCC.2, 3 However, survival of HCC patients after hepatic resection remains unsatisfactory. Tumor recurrence in the liver remnant complicates 70% of cases at 5 years after resection, reflecting either intrahepatic metastasis from the primary tumor or the development of de novo tumors.3–8 As a result, the 5-year overall survival rate after curative resection is reported to be 40%–50%.9 To counter this, several therapies have been administered prior to surgical resection, in an attempt to improve overall survival.10–12 Because the efficacy of preoperative therapy before curative resection remains unclear, there is no preoperative therapy that is currently recommended.
Transcatheter arterial chemoembolization (TACE) has been used since the beginning of the 1980s as a neoadjuvant therapy to improve long-term survival by preventing cancer cell dissemination and intrahepatic recurrence.13–15 Several reports, including four randomized controlled trials, failed to demonstrate an improvement in survival rate with the administration of preoperative TACE.13, 16–18 Other studies, however, presented conflicting results.19–21 Key reasons for the continuing debate are the considerable variation in background factors and radiological techniques involving HCC in previous studies and the various improvements in the TACE technique over the period of study. Because TACE has been shown to offer a survival advantage for patients with unresectable HCC,22, 23 in theory, preoperative TACE is considered to have positive effects on postoperative clinical course. Thus, the aim of the present study was to evaluate the survival benefit of preoperative TACE in patients who underwent resection of HCC.
MATERIALS AND METHODS
Patients
We used a propensity-based matching case-control design in this study. A series of 189 consecutive patients who underwent a curative hepatic resection of primary hepatic cancer at our hospital between January 2004 and December 2012 were included in the current study. All patients were diagnosed based on contrast-enhanced computed tomography (CT) or Gd ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI). Intrahepatic cholangiocarcinoma and combined HCC-cholangiocarcinoma tumors were excluded based on histological findings in the resected specimens. Patients with extrahepatic lesions, preoperatively diagnosed as vascular invasion, positive surgical margins or macroscopic residuals were also excluded. Patients who had undergone liver transplantation or surgical resection combined with ablation therapy were also excluded. On the basis of this exclusion criteria, 121 patients were eligible in this study. Then, patients were divided into two groups according to whether they underwent preoperative TACE or not (the TACE group and control group). Thirty-four patients who had highly vascularized HCCs so that were considered to have sensitivity for preoperative TACE by hepatologists assigned to the TACE group were administered preoperative TACE for two reasons: to improve long-term outcome (29 cases), to be completely cured (5 cases).
Written informed consent was obtained from all patients prior to TACE and surgery. Medical records were reviewed retrospectively after approval by the Institutional Review Board (IRB) of our institution in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments (IRB approval number: 1606A029).
Variables
Data including patient characteristics [age, sex, body mass index (BMI), past medical history, presence or absence of prior local treatment for HCC, cause of hepatitis, Child–Pugh score, serum creatinine, albumin, total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), cholinesterase, platelet count, prothrombin time, rate of indocyanine green disappearance 15 min after injection (ICG-R15)], tumor characteristics [number of tumors, maximum diameter of tumor, alpha-fetoprotein (AFP) level], intra-operative data (extent of resection, surgical procedure, duration of surgery, intraoperative hemorrhage volume, length of hospital stay after surgical resection, postoperative complications), tumor pathological findings (histotype and stage of fibrosis of nontumor-bearing liver according to the new Inuyama classification of chronic hepatitis), tumor recurrence and patient survival were collected from our database. Tumor recurrence was diagnosed based on the findings of either CT or MRI.
Propensity score matching was performed using R version 3.1.3 software; the grouping variable was preoperative TACE and the matching variables were age, presence or absence of prior treatment, cause of hepatitis, Child–Pugh score, comorbidity (cardio-vascular disease and diabetes mellitus), serum albumin, bilirubin, AFP level, platelet count, prothrombin time, ICG-R15, number of tumors, tumor size, and stage of fibrosis of nontumor-bearing liver. The stage of fibrosis was the only histological finding included in this list of variables because this stage was rarely affected by preoperative TACE,24 and played an important role in the development of de novo tumors in the remnant liver.25 Because histological findings concerning the primary tumor in the TACE group were believed to be affected by preoperative TACE, histological findings were not included in the list of variables to be matched.
Statistical analysis
We used R version 3.1.3 software for comparative statistical analysis. All continuous values are presented as the mean ± standard deviation and the median with inter quartile range. Statistical analysis was conducted using the chi-square test for categorical variables and Welch’s two-sample t test for continuous variables, with the exception of categorical variables containing factors less than 5, which were analyzed using Fisher’s exact test. Overall survival and recurrence free survival rates were calculated according to the Kaplan–Meier method and compared using the Gehan-Breslow-Wilcoxon test. All P values < 0.05 were considered significant. The primary endpoint of this study was overall survival, and the secondary endpoints were recurrence-free survival and postoperative complications.
RESULTS
Patient characteristics
Table 1 shows the characteristics of the 121 eligible patients before matching. Propensity score matching was carried out using 16 selected patient characteristics, tumor-related factors and surgical factors. Thirty-four patients were selected as the control group and included for further analysis. Tables 2 and 3 detail the clinicopathological characteristics and perioperative clinical outcomes, respectively, of the patients included in the current study. There were no significant differences in patient characteristics and perioperative outcomes between the control and TACE groups. To determine methodological homogeneity of preoperative TACE, survey period was divided into two terms. Details of preoperative TACE were shown separately depends on periods of study in Table 4. We also confirmed no significant difference of surgical outcomes between first- and second- half period of this study.
Table 1.
Patient background before propensity score matching
| Control group (87) | TACE (34) | P | ||||
| Mean, SD | Median [IQR] | Mean, SD | Median [IQR] | |||
| Age (y) | 67, s = 10.8 | 69 [62–74] | 71.0, s = 8.5 | 72.5 [66.8–77.0] | 0.036 | |
| Prior treatment (%) | 17 (20) | 8 (24) | 0.812 | |||
| Gender (male; %) | 73 (84) | 30 (88) | 0.777 | |||
| BMI (kg/m2) | 22.9, s = 3.0 | 22.8 [21.1–24.5] | 23.1, s = 2.8 | 24.2 [20.6–25.4] | 0.737 | |
| Cause of hepatitis (%) | NBNC | 15 (17) | 4 (11) | 0.098 | ||
| HBV | 39 (45) | 10 (29) | ||||
| HCV | 24 (28) | 14 (41) | ||||
| Alcohol | 9 (10) | 5 (15) | ||||
| Other | 1 (1) | 3 (9) | ||||
| Child-Pugh score (%) | 5 | 70 (80) | 26 (76) | 0.538 | ||
| 6 | 13 (15) | 8 (24) | ||||
| 7 | 2 (2) | 0 | ||||
| 8 | 2 (2) | 0 | ||||
| Comorbidity (%) | Cardio-vascular disease | 9 (10) | 5 (15) | 0.534 | ||
| Diabetes | 27 (31) | 11 (32) | 1.000 | |||
| Creatinine (mg/dL) | 17.6, s = 9.1 | 0.75 [0.66–0.88] | 0.78, s = 0.13 | 0.8 [0.70–0.87] | 0.321 | |
| Albumin (g/dL) | 3.9, s = 0.5 | 4 [3.6–4.3] | 3.9, s = 0.42 | 3.9 [3.7–4.2] | 0.504 | |
| Bilirubin (mg/dL) | 0.73, s = 0.27 | 0.7 [0.5–0.9] | 0.68, s = 0.22 | 0.7 [0.5–0.8] | 0.292 | |
| AST (IU/L) | 38.5, s = 21.9 | 31 [23.5–48] | 37.8, s = 21.9 | 33 [26.8–39.0] | 0.881 | |
| ALT(IU/L) | 41.2, s = 46.2 | 26 [19–46] | 36.3, s = 24.7 | 30 [23.3–39.8] | 0.451 | |
| Cholinesterase (IU/L) | 195.1, s = 74.3 | 196.5 [135.2–241.5] | 205.6, s = 80.0 | 191.5 [152.8–249.5] | 0.510 | |
| AFP (ng/mL) | 7645.4, s = 39707 | 9.8 [3.7–80.5] | 1251.4, s = 4445.4 | 10.1 [4.3–110] | 0.144 | |
| Platelet (/103μL) | 167, s = 63.2 | 161 [119.5–200.5] | 179.5, s = 80.7 | 172.5 [120.5–213] | 0.420 | |
| Prothrombin time (%) | 86.3, s = 18.0 | 87.7 [78.1–96.6] | 88.8, s = 9.8 | 90 [81–97.6] | 0.340 | |
| ICG-R15 (%) | 13.5, s = 6.9 | 13 [9–16] | 17.8, s = 13.9 | 15 [13–20] | 0.101 | |
| Extent of hepatic resection (%) | Non-anatomic | 38 (44) | 17 (50) | 0.851 | ||
| Segmentectomy | 14 (16) | 4 (12) | ||||
| Sectorectomy | 20 (23) | 7 (21) | ||||
| Lobe hepat-ectomy | 14 (16) | 8 (24) | ||||
| Hemi-hepat-ectomy | 11 (13) | 3 (9) | ||||
| Number of tumor (%) | 1 | 69 (79) | 25 (74) | 0.758 | ||
| 2 | 14 (16) | 8 (24) | ||||
| 3 | 2 (2) | 1 (3) | ||||
| ≥ 4 | 2 (2) | 0 | ||||
| Maximum diameter (mm) | 48.7, s = 41.5 | 30.5 [22–57.3] | 40.8, s = 30.0 | 30 [21.3–48.8] | 0.333 | |
| Histotype (%) | Well | 5 (6) | 3 (9) | 0.106 | ||
| Moderate | 77 (89) | 26 (76) | ||||
| Poor | 3 (3) | 4 (12) | ||||
| NA | 2 (2) | 1 (3) | ||||
| Fibrosis stage* (%) | 0 | 19 (22) | 1 (3) | 0.047 | ||
| 1 | 23 (26) | 7 (21) | ||||
| 2 | 10 (11) | 8 (24) | ||||
| 3 | 8 (9) | 5 (15) | ||||
| 4 | 24 (28) | 8 (24) | ||||
*New Inuyama classification of chronic hepatitis. AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; ICG-R15, 15-minute retention rates of indocyanine green; IQR, interquartile range; NA, not available; NBNC, non-HBV non-HCV hepatitis; TACE, transcatheter arterial chemoembolization; y, year(s).
Table 2.
Patient background after propensity score matching
| Control group (34) | TACE group (34) | P | ||||
| Mean, SD | Median [IQR] | Mean, SD | Median [IQR] | |||
| Age (y) | 71.8, s = 6.7 | 72.5 [68–75] | 71.0, s = 8.5 | 72.5 [66.8–77.0] | 0.647 | |
| Prior treatment (%) | 11 (32) | 8 (24) | 0.588 | |||
| Gender (male; %) | 25 (74) | 30 (88) | 0.217 | |||
| BMI (kg/m2) | 23.6, s = 2.4 | 23.8 [22.2–25.2] | 23.1, s = 2.8 | 24.2 [20.6–25.4] | 0.457 | |
| Cause of hepatitis (%) | NBNC | 8 (24) | 4 (11) | 0.171 | ||
| HBV | 15 (38) | 10 (29) | ||||
| HCV | 10 (29) | 14 (41) | ||||
| Alcohol | 1 (3) | 5 (15) | ||||
| Other | 1 (3) | 3 (9) | ||||
| Child-Pugh score (%) | 5 | 27 (79) | 26 (76) | 0.765 | ||
| 6 | 6 (18) | 8 (24) | ||||
| 8 | 1 (3) | 0 | ||||
| Comorbidity (%) | Cardio-vascular disease | 5 (15) | 5 (15) | 1.000 | ||
| Diabetes | 9 (26) | 11 (32) | 0.796 | |||
| Creatinine (mg/dL) | 0.80, s = 0.23 | 0.76 [0.68–0.92] | 0.78, s = 0.13 | 0.8 [0.70–0.87] | 0.626 | |
| Albumin (g/dL) | 3.9, s = 0.4 | 4.0 [3.7–4.2] | 3.9, s = 0.42 | 3.9 [3.7–4.2] | 0.815 | |
| Bilirubin (mg/dL) | 0.70, s = 0.28 | 0.60 [0.53–0.88] | 0.68, s = 0.22 | 0.7 [0.5–0.8] | 0.663 | |
| AST (IU/L) | 40.1, s = 25.2 | 32 [26–48.3] | 37.8, s = 21.9 | 33 [26.8–39.0] | 0.602 | |
| ALT(IU/L) | 34.4, s = 26.0 | 24 [20–40.1] | 36.3, s = 24.7 | 30 [23.3–39.8] | 0.753 | |
| Cholinesterase (IU/L) | 205.1, s = 68.5 | 203.5[146–250.5] | 205.6, s = 80.0 | 191.5 [152.8–249.5] | 0.979 | |
| AFP (ng/mL) | 3342, s = 19120 | 5.8 [2.9–21.5] | 1251.4, s = 4445.4 | 10.1 [4.3–110] | 0.540 | |
| Platelet (/103μL) | 181.1, s = 51.8 | 180.5 [143–217.8] | 179.5, s = 80.7 | 172.5 [120.5–213] | 0.923 | |
| Prothrombin time (%) | 91.5, s = 14.6 | 91.4 [80.6–102] | 88.8, s = 9.8 | 90 [81–97.6] | 0.396 | |
| ICG-R15 (%) | 16.4, s = 6.6 | 14 [12–19.5] | 17.8, s = 13.9 | 15 [13–20] | 0.596 | |
| Extent of hepatic resection (%) | Non-anatomic | 15 (44) | 17 (50) | 0.724 | ||
| Segmentectomy | 7 (21) | 4 (12) | ||||
| Sectorectomy | 6 (18) | 7 (21) | ||||
| Lobe hepat-ectomy | 5 (15) | 8 (24) | ||||
| Hemi-hepat-ectomy | 5 (15) | 3 (9) | ||||
| Number of tumor (%) | 1 | 26 (76) | 25 (74) | 0.803 | ||
| 2 | 6 (18) | 8 (24) | ||||
| 3 | 2 (6) | 1 (3) | ||||
| Maximum diameter (mm) | 42.3, s = 35.7 | 27 [22–50] | 40.8, s = 30.0 | 30 [21.3–48.8] | 0.851 | |
| Histotype (%) | Well | 1 (3) | 3 (9) | 0.138 | ||
| Moderate | 32 (94) | 26 (76) | ||||
| Poor | 0 | 4 (12) | ||||
| NA | 1 (3) | 1 (3) | ||||
| Necrosis rate (%) | ≥ 90% | 12 (35) | ||||
| 89–50% | 4 (12) | |||||
| < 50% | 18 (53) | |||||
| Fibrosis stage* (%) | 0 | 4 (12) | 1 (3) | 0.515 | ||
| 1 | 8 (24) | 7 (21) | ||||
| 2 | 5 (15) | 8 (24) | ||||
| 3 | 3 (9) | 5 (15) | ||||
| 4 | 11 (32) | 8 (24) | ||||
*New Inuyama classification of chronic hepatitis. AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; ICG-R15, 15-minute retention rates of indocyanine green; IQR, interquartile range; NA, not available; NBNC, non-HBV non-HCV hepatitis; TACE, transcatheter arterial chemoembolization; y, year(s).
Table 3.
Outcomes of clinical course
| Control group (34) | TACE (34) | P | ||||
| Mean, SD | Median [IQR] | Mean, SD | Median [IQR] | |||
| Time from diagnosis to surgical resection (d) | 66.2, s = 7.3 | 61.2 [33.8–75.5] | 83.9, s = 35.0 | 87.5 [62.5–94.5] | 0.134 | |
| Intraoperative hemorrhage volume (mL) | 946.7, s = 1135.5 | 502.5 [197.5–976.2] | 935.9, s = 1340.4 | 392.5 [258.8–995] | 0.975 | |
| Operative duration (min) | 437, s = 134.8 | 435 [324–515] | 391, s = 150.9 | 378 [316–457] | 0.197 | |
| Postoperative complication* | Any grade | 16 | 13 | 0.624 | ||
| Grade ≥ 3 | 5 | 8 | 0.537 | |||
| Hospital stay (d) | 24.2, s = 19.7 | 19.5 [16–22] | 20.5, s = 11.3 | 18 [14.3–22.3] | 0.350 | |
*According to Clavien-Dindo classification. d, day(s); IQR, interquartile range; TACE, transcatheter arterial chemoembolization.
Table 4.
Detail of methodology for preoperative TACE
| Prior term (17) | Latter term (17) | P | ||
| Selectivity of hepatic artery (%) | Lobe | 2 (12) | 3 (18) | 0.617 |
| Segment | 4 (24) | 5 (29) | ||
| Subsegment or more | 10 (59) | 9 (53) | ||
| Other (1st-branch of right inferior phrenic artery) | 1 (6) | 0 | ||
| Chemotherapeutic agent (%) | Epirubicin | 2 (12) | 3 (18) | 0.153 |
| Epirubicin plus mitomycin C | 5 (29) | 10 (64) | ||
| Cisplatin | 6 (35) | 2 (6) | ||
| Miliplatin | 0 | 1 (6) | ||
| NA | 4 | 1 | ||
| Embolic agent (%) | Gelatin sponge | 12 (71) | 14 (82) | NA |
| NA | 5 | 3 |
NA, not available; TACE, transcatheter arterial chemoembolization.
Overall and recurrence free survival
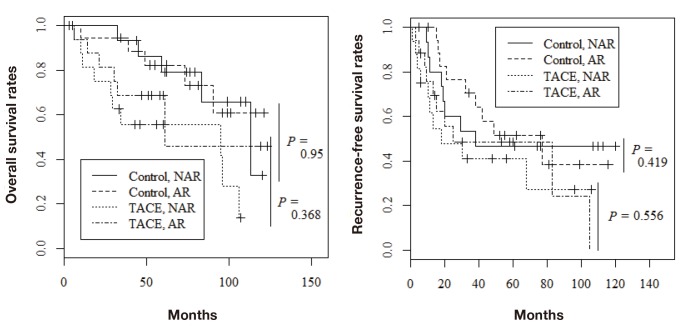
Among the 68 patients evaluated by propensity score matching, tumor recurrence occurred in 37 (54.4%) and death from all causes occurred in 26 (38.2%). Figure 1 shows the overall survival rates in the two groups. The 1-, 3-, and 5-year overall survival rates were 97.0%, 93.9%, and 80.5%, respectively, in the control group and 87.5%, 62.3%, and 62.3%, respectively, in the TACE group; the difference was statistically significant (P = 0.014). The 1-, 3-, and 5-year recurrence-free survival rates were 90.8%, 62.6%, and 49.4%, respectively, in the control group and 68.7%, 44.9%, and 44.9%, respectively, in the TACE group (Fig. 2; P = 0.043). Furthermore, recurrence free survival within less than 2 years was significantly worse in the TACE group than in the control group (Fig. 3; P = 0.035). With regard to type of surgical procedures, there were no significant difference between anatomical or non-anatomical resection among each groups (Fig. 4).
Fig. 1.
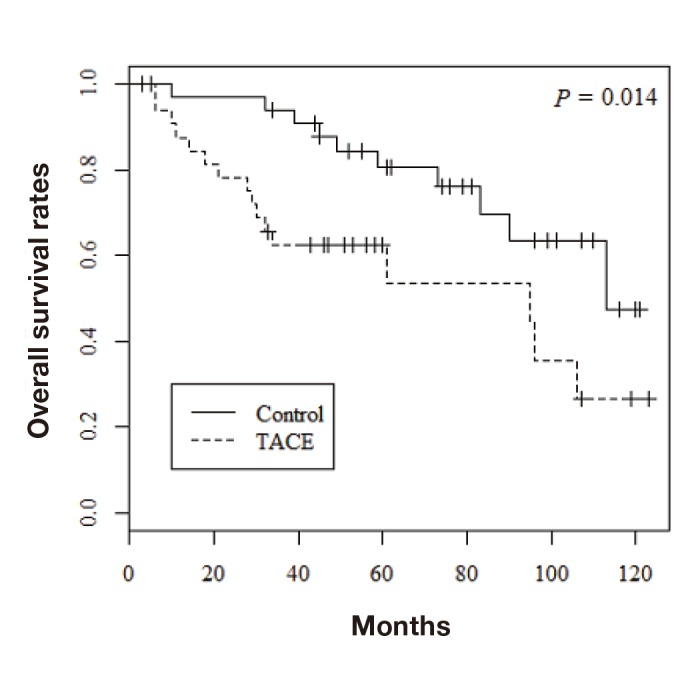
Overall survival rates of the TACE and control groups. TACE, transcatheter arterial chemoembolization.
Fig. 2.
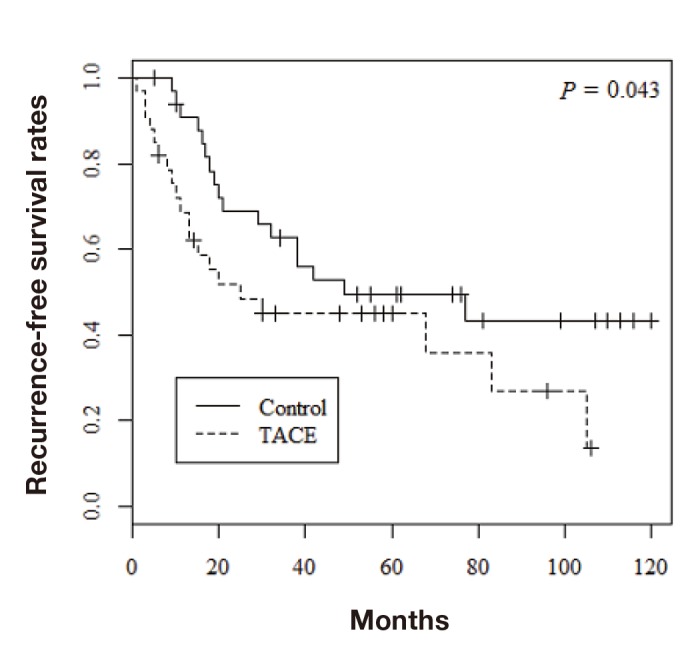
Recurrence-free survival rates of the TACE and control groups. TACE, transcatheter arterial chemoembolization.
Fig. 3.
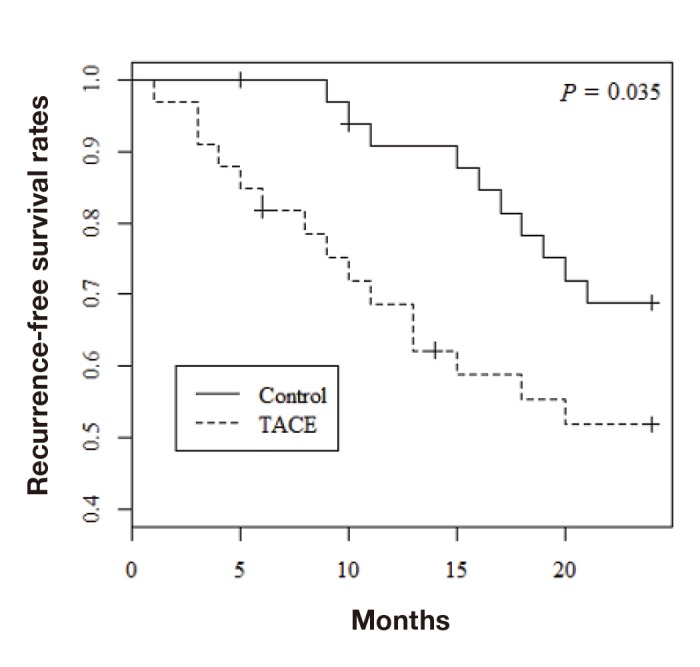
Recurrence-free survival rates within less than 2 years after resection. TACE, transcatheter arterial chemoembolization.
Fig. 4.
Long-term outcomes in both TACE and control group according to operative procedure. A: Overall survival rates. B: Recurrence-free survival rates. AR, anatomical resection; NAR, non-anatomical resection; TACE, transcatheter arterial chemoembolization.
DISCUSSION
Preoperative TACE has been used in the following ways: as neoadjuvant chemotherapy for resectable HCCs,13 as a conversion therapy for unresectable HCC through down-staging,21 to prepare for portal venous embolization to reduce the risk of rapid growth of HCC,26 and with curative intent prior to salvaging surgical resection. In the current study, we evaluated the efficacy of TACE as a neoadjuvant therapy by comparing the prognosis of patients who underwent preoperative TACE and those who did not. The overall 5-year survival rate and the recurrence-free survival rate were significantly lower in the TACE group than in the control group. These findings clearly indicate that preoperative TACE negatively affects the prognosis of patients with potentially resectable HCC. Similar findings have been reported in previous studies.18, 20, 27–31
Several disadvantages of preoperative TACE have been postulated. First, it is likely that treatment with TACE delays surgical resection and that preoperative TACE renders surgical resection more difficult and delays the operation time, resulting in intraoperative tumor feeding through collateral vessels.20, 27 Another possibility is that TACE mainly affects well-differentiated cells, without completely killing poorly differentiated cells that are related to poor prognosis.28 In the present study, however, such factors were unlikely to have influenced the poor prognosis observed in the TACE group because we performed statistical matching to reduce heterogeneity in patient profiles.
As previously mentioned, there are two types of intrahepatic recurrence after resection, namely metastasis of the primary tumor and secondary de novo tumor formation. Recurrence rates also peak twice after resection—recurrence in the early phase (< 2 years) is mainly the result of metastases, whereas recurrence in the late phase (≥ 2 years) is attributable to new lesions.2, 32 Several studies have demonstrated a higher recurrence rate and lower overall survival in patients who underwent preoperative TACE.18, 29–31 In the present study, recurrence free survival within less than 2 years was significantly worse in the TACE group than in the control group, indicating the possibility that intrahepatic metastasis occurred more frequently in the TACE group than in the control group. Therefore, it is possible that increased early recurrence is related to the poor overall survival observed in the TACE group in the current study.
Recent studies have revealed that preoperative TACE may enhance the expression of vascular endothelial growth factor protein, which encourages angiogenesis and results in metastasis.33 TACE may also increase the expression of a hypoxia-inducible factor that is related to hepatic damage, resulting in carcinogenesis.34–36 These molecular alterations might also be responsible for the low overall survival and high early-phase recurrence rate observed in the TACE group in the current study.
This study had several limitations. First, histopathological analysis could not be conducted prior to the interventions; therefore, the two groups could not be matched for histopathological characteristics, which may have been a confounding factor. Second, the study was retrospective in design, meaning that there were missing values for several variables. Finally, the sample size may have limited the statistical robustness of the results to some extent. However, consistent results were obtained in previous studies that support our current findings. With regard to survival rates in previous reports, 5-year overall and recurrence-free survival rates in patients with preoperative TACE were similar to our results.29 Moreover, Roayaie et al reported outcomes of treatments other than resection among patients who were candidate for resection.37 Comparing our result to their results, resection with preoperative TACE may be superior to radiofrequency ablation or TACE but inferior to resection, so that our result seem plausible.
In conclusion, our results indicated that preoperative TACE seems to adversely affect the long-term outcomes of patients who underwent surgical resection to treat resectable HCC. Ischemic stimulation of resectable HCC induced by preoperative TACE might worsen the postoperative clinical course, especially in the early phase. Therefore, preoperative TACE should not be considered as a standard therapy for patients with potentially resectable HCC.
The authors declare no conflict of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer Journal for Clinicians. 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2. Japan Society of Hepatology. Clinical practice guidelines for hepatocellular carcinoma. Tokyo: Kanehara; 2013. 223 p [Google Scholar]
- 3. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-43. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-40. [DOI] [PubMed] [Google Scholar]
- 5. Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified?. Ann Surg. 2002;236:602-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-16. [DOI] [PubMed] [Google Scholar]
- 8. Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. [DOI] [PubMed] [Google Scholar]
- 10. Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437-45. [DOI] [PubMed] [Google Scholar]
- 11. Huang SX, Wu YL, Tang CW, Feng WM, Xu YQ, Bao Y, et al. Prophylactic hepatic artery infusion chemotherapy improved survival after curative resection in patients with hepatocellular carcinoma. Hepatogastroenterology. 2015;62:122-5. [PubMed] [Google Scholar]
- 12. Kayashima H, Toshima T, Okano S, Taketomi A, Harada N, Yamashita Y, et al. Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2010;185:698-708. [DOI] [PubMed] [Google Scholar]
- 13. Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195-202. [DOI] [PubMed] [Google Scholar]
- 14. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y, et al. Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int J Oncol. 2013;42:151-60. [DOI] [PubMed] [Google Scholar]
- 15. Okamura J, Horikawa S, Fujiyama T, Monden M, Kambayashi J, Sikujara O, et al. An appraisal of transcatheter arterial embolization combined with transcatheter arterial infusion of chemotherapeutic agent for hepatic malignancies. World J Surg. 1982;6:352-7. [DOI] [PubMed] [Google Scholar]
- 16. Yamasaki S, Hasegawa H, Kinoshita H, Furukawa M, Imaoka S, Takasaki K, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaibori M, Tanigawa N, Kariya S, Ikeda H, Nakahashi Y, Hirohara J, et al. A prospective randomized controlled trial of preoperative whole-liver chemolipiodolization for hepatocellular carcinoma. Dig Dis Sci. 2012;57:1404-12. [DOI] [PubMed] [Google Scholar]
- 18. Adachi E, Matsumata T, Nishizaki T, Hashimoto H, Tsuneyoshi M, Sugimachi K, et al. Effects of preoperative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma. The relationship between postoperative course and tumor necrosis. Cancer. 1993;72:3593-8. [DOI] [PubMed] [Google Scholar]
- 19. Kang JY, Choi MS, Kim SJ, Kil JS, Lee JH, Koh KC, et al. Long-term outcome of preoperative transarterial chemoembolization and hepatic resection in patients with hepatocellular carcinoma. Korean J Hepatol. 2010;16:383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’Eng F K, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122-6. [DOI] [PubMed] [Google Scholar]
- 21. Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-9. [DOI] [PubMed] [Google Scholar]
- 23. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-71. [DOI] [PubMed] [Google Scholar]
- 24. Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123-8. [DOI] [PubMed] [Google Scholar]
- 25. Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-8. [DOI] [PubMed] [Google Scholar]
- 26. Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251-7. [DOI] [PubMed] [Google Scholar]
- 27. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ikeda K, Saitoh S, Tsubota A, Arase Y, Chayama K, Kumada H, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer. 1993;71:19-25. [DOI] [PubMed] [Google Scholar]
- 29. Ha TY, Hwang S, Lee YJ, Kim KH, Ko GY, Ii Gwon D, et al. Absence of Benefit of Transcatheter Arterial Chemoembolization (TACE) in Patients with Resectable Solitary Hepatocellular Carcinoma. World J Surg. 2016;40:1200-10. [DOI] [PubMed] [Google Scholar]
- 30. Sasaki A, Iwashita Y, Shibata K, Ohta M, Kitano S, Mori M, et al. Preoperative transcatheter arterial chemoembolization reduces long-term survival rate after hepatic resection for resectable hepatocellular carcinoma. Eur J Surg Oncol. 2006;32:773-9. [DOI] [PubMed] [Google Scholar]
- 31. Lee KT, Lu YW, Wang SN, Chen HY, Chuang SC, Chang WT, et al. The effect of preoperative transarterial chemoembolization of resectable hepatocellular carcinoma on clinical and economic outcomes. J Surg Oncol. 2009;99:343-50. [DOI] [PubMed] [Google Scholar]
- 32. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-7. [DOI] [PubMed] [Google Scholar]
- 33. Xiao EH, Guo D, Bian DJ. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:4582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Himoto T, Fujita K, Nomura T, Tani J, Miyoshi H, Morishita A, et al. Roles of Copper in Hepatocarcinogenesis via the Activation of Hypoxia-Inducible Factor-1alpha. Biol Trace Elem Res. 2016;174:58-64. DOI: 10.1007/s12011-016-0702-7 [DOI] [PubMed] [Google Scholar]
- 35. Herzog J, Ehrlich SM, Pfitzer L, Liebl J, Frohlich T, Arnold GJ, et al. Cyclin-dependent kinase 5 stabilizes hypoxia-inducible factor-1alpha: a novel approach for inhibiting angiogenesis in hepatocellular carcinoma. Oncotarget. 2016;7:27108-21. DOI: 10.18632/oncotarget.8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu W, Kwon JH, Moon YH, Kim YB, Yu YS, Lee N, et al. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1alpha pathway in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:1507-15. [DOI] [PubMed] [Google Scholar]
- 37. Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-51. [DOI] [PubMed] [Google Scholar]