Abstract
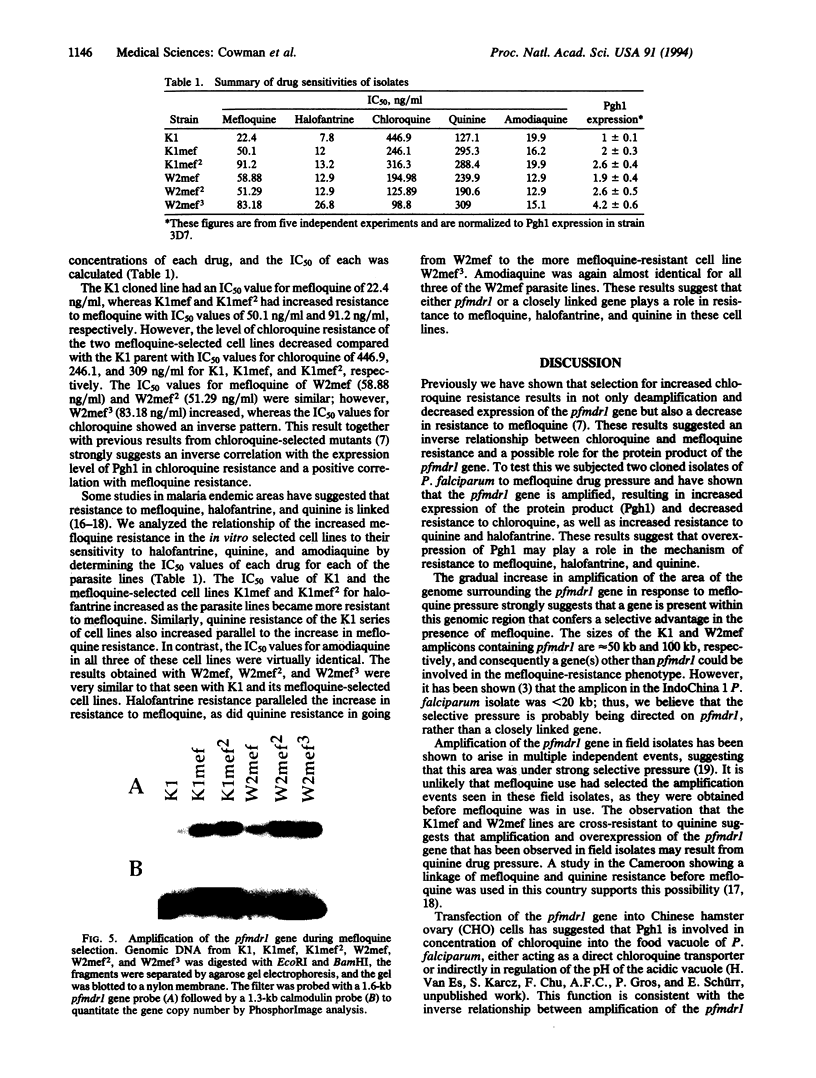
Two chloroquine-resistant cloned isolates of Plasmodium falciparum were subjected to mefloquine selection to test if this resulted in alterations in chloroquine sensitivity and amplification of the pfmdr1 gene. The mefloquine-resistant lines derived by this selection were shown to have amplified and overexpressed the pfmdr1 gene and its protein product (Pgh1). Macrorestriction maps of chromosome 5, where pfmdr1 is encoded, showed that this chromosome has increased in size in response to mefloquine selection, indicating the presence of a gene(s) in this area of the genome that confers a selective advantage in the presence of mefloquine. Concomitant with the increase in mefloquine resistance was a corresponding increase in the level of resistance to halofantrine and quinine, suggesting a true multidrug-resistance phenotype. The mefloquine-selected parasite lines also showed an inverse relationship between the level of chloroquine resistance and increased pfmdr1 gene copy number. These results have important implications for the derivation of amplified copies of the pfmdr1 gene in field isolates, as they suggest that quinine pressure may be involved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. A., Foote S. J., Galatis D., Kemp D. J., Cowman A. F. Selection for high-level chloroquine resistance results in deamplification of the pfmdr1 gene and increased sensitivity to mefloquine in Plasmodium falciparum. EMBO J. 1992 Aug;11(8):3067–3075. doi: 10.1002/j.1460-2075.1992.tb05378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. E., Battye F. L., Brown G. V. Plasmodium falciparum: rapid quantification of parasitemia in fixed malaria cultures by flow cytometry. Exp Parasitol. 1986 Oct;62(2):275–282. doi: 10.1016/0014-4894(86)90032-9. [DOI] [PubMed] [Google Scholar]
- Brasseur P., Kouamouo J., Druilhe P. Mefloquine-resistant malaria induced by inappropriate quinine regimens? J Infect Dis. 1991 Sep;164(3):625–626. doi: 10.1093/infdis/164.3.625. [DOI] [PubMed] [Google Scholar]
- Brasseur P., Kouamouo J., Moyou-Somo R., Druilhe P. Multi-drug resistant falciparum malaria in Cameroon in 1987-1988. I. Stable figures of prevalence of chloroquine- and quinine-resistant isolates in the original foci. Am J Trop Med Hyg. 1992 Jan;46(1):1–7. doi: 10.4269/ajtmh.1992.46.1. [DOI] [PubMed] [Google Scholar]
- Brasseur P., Kouamouo J., Moyou-Somo R., Druilhe P. Multi-drug resistant falciparum malaria in Cameroon in 1987-1988. II. Mefloquine resistance confirmed in vivo and in vitro and its correlation with quinine resistance. Am J Trop Med Hyg. 1992 Jan;46(1):8–14. doi: 10.4269/ajtmh.1992.46.8. [DOI] [PubMed] [Google Scholar]
- Bray P. G., Howells R. E., Ritchie G. Y., Ward S. A. Rapid chloroquine efflux phenotype in both chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. A correlation of chloroquine sensitivity with energy-dependent drug accumulation. Biochem Pharmacol. 1992 Oct 6;44(7):1317–1324. doi: 10.1016/0006-2952(92)90532-n. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Galatis D. Plasmodium falciparum: the calmodulin gene is not amplified or overexpressed in chloroquine resistant or sensitive isolates. Exp Parasitol. 1991 Oct;73(3):269–275. doi: 10.1016/0014-4894(91)90098-h. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Karcz S., Galatis D., Culvenor J. G. A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J Cell Biol. 1991 Jun;113(5):1033–1042. doi: 10.1083/jcb.113.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F., Lew A. M. Antifolate drug selection results in duplication and rearrangement of chromosome 7 in Plasmodium chabaudi. Mol Cell Biol. 1989 Nov;9(11):5182–5188. doi: 10.1128/mcb.9.11.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F. The P-glycoprotein homologues of Plasmodium falciparum: Are they involved in chloroquine resistance? Parasitol Today. 1991 Apr;7(4):70–76. doi: 10.1016/0169-4758(91)90197-v. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kyle D. E., Martin R. K., Oduola A. M., Forsyth K., Kemp D. J., Cowman A. F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990 May 17;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Limpaiboon T., Shirley M. W., Kemp D. J., Saul A. 7H8/6, a multicopy DNA probe for distinguishing isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1991 Aug;47(2):197–206. doi: 10.1016/0166-6851(91)90179-a. [DOI] [PubMed] [Google Scholar]
- Oduola A. M., Milhous W. K., Weatherly N. F., Bowdre J. H., Desjardins R. E. Plasmodium falciparum: induction of resistance to mefloquine in cloned strains by continuous drug exposure in vitro. Exp Parasitol. 1988 Dec;67(2):354–360. doi: 10.1016/0014-4894(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Trager W., Jenson J. B. Cultivation of malarial parasites. Nature. 1978 Jun 22;273(5664):621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]
- Triglia T., Foote S. J., Kemp D. J., Cowman A. F. Amplification of the multidrug resistance gene pfmdr1 in Plasmodium falciparum has arisen as multiple independent events. Mol Cell Biol. 1991 Oct;11(10):5244–5250. doi: 10.1128/mcb.11.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Volkman S. K., Thaithong S., Martin R. K., Kyle D. E., Milhous W. K., Wirth D. F. Amplification of pfmdr 1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol Biochem Parasitol. 1993 Jan;57(1):151–160. doi: 10.1016/0166-6851(93)90252-s. [DOI] [PubMed] [Google Scholar]