ABSTRACT
Although CD4+CD8+ double positive (DP) T cells represent a small fraction of peripheral T lymphocytes in healthy human donors, their frequency is often increased under pathological conditions (in blood and targeted tissues). In solid cancers such as melanoma, we previously demonstrated an enrichment of tumor reactive CD4lowCD8highαβ DP T cells among tumor-infiltrating lymphocytes of unknown function. Similarly to their single positive (SP) CD8+ counterparts, intra-melanoma DP T cells recognized melanoma cell lines in an HLA-class-I restricted context. However, they presented a poor cytotoxic activity but a strong production of diverse Th1 and Th2 cytokines. The aim of this study was to clearly define the role of intra-melanoma CD4lowCD8highαβ DP T cells in the antitumor immune response. Based on a comparative transcriptome analysis between intra-melanoma SP CD4+, SP CD8+ and DP autologous melanoma-infiltrating T-cell compartments, we evidenced an overexpression of the CD40L co-stimulatory molecule on activated DP T cells. We showed that, like SP CD4+ T cells, and through CD40L involvement, DP T cells are able to induce both proliferation and differentiation of B lymphocytes and maturation of functional DCs able to efficiently prime cytotoxic melanoma-specific CD8 T-cell responses. Taken together, these results highlight the helper potential of atypical DP T cells and their role in potentiating antitumor response.
KEYWORDS: CD4+CD8+ double positive T cells, CD40L, helper function, melanoma, TIL
Abbreviations
- DP
double positive
- Mo-DC
monocyte-derived DC
- TIL
tumor-infiltrating lymphocyte
Introduction
It has long been admitted that the distribution of CD4+CD8+ double positive (DP) T cells was not limited to the thymus, and that mature DP T cells were also present in the peripheral blood of healthy humans as a distinct T lymphocyte population.1,2 This rare population, accounting for 1–2% of total peripheral T lymphocytes in healthy recipients,3,4 regroups at least three different subsets according to the surface expression level of the CD4+ and CD8+ co-receptors (CD4highCD8low, CD4highCD8high and CD4lowCD8high) and on the nature of the CD8+ dimer expressed (CD8αα or CD8αβ).5,6 Although not clearly established, these diverse phenotypes may reflect different lineage origins according to their thymic or peripheral differentiation.7-10 DP T cells were largely described in diverse pathological contexts such as infectious diseases,2,11-14 autoimmune syndromes,15-17 inflammatory disorders18-20 and cancer.21-25 In most cases, a peculiar DP T-cell subset was generally found enriched in the blood and/or in the target organ, suggesting their involvement in the pathological process. Functional analyses in a given context attributed at least three different functions for DP T cells from regulatory properties to helper or cytotoxic activity (for review, see Overgaard et al.26). In human cancer, DP T-cell subsets were found enriched at the tumor site in lymphomas (cutaneous T-cell lymphoma and nodular lymphocyte predominant Hodgkin lymphoma)21,24 and solid tumors (melanoma, colorectal cancer and breast cancer)22,23,25 and were described as a tumor-reactive population suggesting their role in the antitumor immune response. From one cancer setting to another, opposite results were obtained regarding DP T-cell function and phenotype; therefore, their study should be dealt on a case-by-case basis. An efficient cytolytic activity was attributed to the CD4highCD8low DP T-cell subset found in lymphoma.21 Conversely, in solid tumors, we reported at least two different phenotypic DP T-cell subsets, the CD4lowCD8high DP T-cell subpopulation in melanoma and breast cancer22,23 and the CD4highCD8high DP T-cell subpopulation in colorectal cancer,25 both sharing the CD8αβ dimer.
Because the frequency of these DP T cells was positively correlated with advanced cancer stage, we initially thought that intra-tumor DP T cells could exert regulatory functions. However, we excluded this hypothesis as DP T cells (i) did not expressed any regulatory markers nor secreted immunoregulatory cytokines and (ii) did not suppress proliferation and cytokine production by various T-cell subsets (personal unupublished results). In addition, even these DP T cells shared class-I restriction with single positive (SP) CD8+ T cells, they did not exhibit high cytotoxic activity ruling out the hypothesis that cytotoxicity may represent their main function. Interestingly, we previously showed that melanoma infiltrating DP T cells were able to secrete high level of diverse Th1 and Th2 cytokines mostly IL-13 and TNF-α.23 In addition, we demonstrated that DP T cells expressed high levels of IL-9R and that IL-9 both promotes their survival and their activation.27
In this study, we soughed out to clearly delineate the function of the melanoma-infiltrating CD4lowCD8highαβ DP T-cell subset (referred to as DP T cells for the rest of this manuscript) in order to define their role in the global antitumor immune response.
Starting from a comparative transcriptome analysis between autologous DP, SP CD4+ and SP CD8+ T-cell subsets sorted from melanoma-invaded lymph nodes, we identified a similar overexpression of the co-stimulatory molecule CD40L on activated DP and SP CD4+ T cells compared to SP CD8+ T cells. The expression of this key helper T-cell molecule by DP T cells led us to explore the potential helper function of these class-I restricted population. Results showed that CD40L allowed DP T cells to exert in vitro efficient helper activities on B cells and dendritic cells (DCs).
Results
CD40L overexpression is induced after activation of melanoma-infiltrating DP T cells
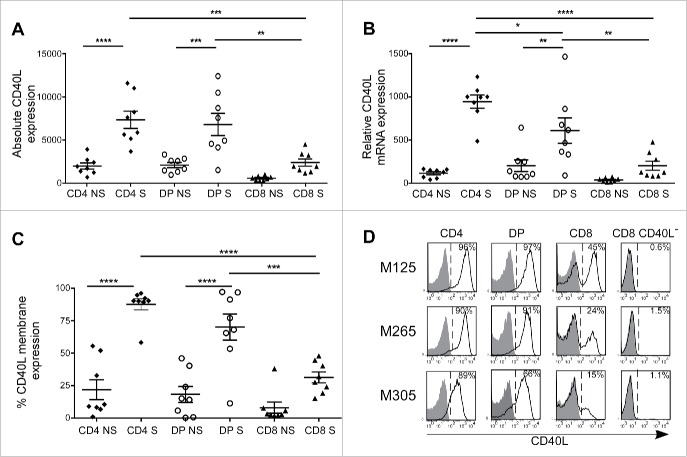
To decipher the role of the intra-melanoma DP T-cell population in melanoma, we initiated a comparative transcriptome analysis between autologous melanoma-infiltrating DP, SP CD4+ and SP CD8+ T lymphocytes at rest and upon anti-CD3 Ab activation. The three subpopulations were sorted from eight tumor-infiltrating lymphocytes (TIL) lines previously established from melanoma-invaded lymph nodes.27 This analysis showed that DP T cells shared with SP CD4+ T cells the ability to significantly induce the expression of CD40L mRNA upon activation (p < 0.01) (Fig. 1A), a key feature in CD4+ helper functions.28 This expression was similar between SP CD4+ and DP T cells and significantly elevated compared to SP CD8+ T cells (p < 0.01). These results were further confirmed by qPCR analysis (Fig. 1B). However, the expression profile of CD40L by activated DP T cells appeared more heterogeneous compared to SP CD4+ T cells. Flow cytometry identified at least three CD40L surface expression patterns on activated DP T cells: (i) some DP T-cell populations (3/8) expressed CD40L at a similar level than SP CD4+ T cells (>90 %), (ii) others (4/8) presented an intermediate expression level (50–80%) and (iii) one DP T-cell population displayed a poor expression (<10 %) (Fig. 1C). Although not significant, a non-negligible proportion (from 5% to 50%) of SP CD8+ T cells expressed CD40L. We also assessed the induction of CD40L expression by DP T cells in a more physiological context by using a tumor-reactive DP T-cell clone M314.13.2 that we have previously isolated from one melanoma TIL population.23 Following 6 h of co-culture with the autologous melanoma cell line M314, we observed a strong expression of the CD40L by the DP T-cell clone at a similar level to the one obtained upon non-specific anti-CD3 activation (Fig. S1). It is noteworthy that patients presenting the highest CD40L level on DP T cells were not necessarily the same as the ones expressing highest CD40L levels on CD4+ T cells. Since CD40L, through its interaction with its cognate receptor CD40, is a key element in T-cell help delivery, these data suggested that intra-tumor DP T cells could exert a helper function. To evaluate this hypothesis, we selected three representative DP T-cell populations for functional assays: two with a high CD40L expression (M125 and M265) and one with an intermediary expression level (M305) (Fig. 1D). As positive and negative controls, DP T cells were systematically compared to autologous SP CD4+ and SP CD8+ T cells. Since it was clearly demonstrated in the literature that CD40L-expressing CD8+ T cells can exert helper properties,29-31 and as a fraction of autologous SP CD8+ TILs expressed a non-negligible amount of CD40L, their use as a negative control was unsuitable. Therefore, sorted CD40L-negative (CD40L−) CD8+ T cells were used as a proper negative control (Fig. 1D).
Figure 1.
CD40L overexpression is induced on intra-melanoma DP T cells upon activation. CD40L expression of intra-melanoma SP CD4+ (black diamonds), DP (white circles) and SP CD8+ (black triangles) T-cell lines isolated from TILs, stimulated (S) or not (NS) with anti-CD3 mAb for 6 h was determined by (A) microarray analysis, (B) quantitative RT-PCR analysis and (C) flow cytometry (n = 8 melanoma patients). Results are expressed as mean ± SEM. Statistical analysis was performed using the one-way ANOVA analysis, followed by a Tukey multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (D) Representative flow cytometry staining of CD40L following anti-CD3 activation on the 3 autologous intra-melanoma SP CD4+, DP, SP CD8+ and SP CD8+ CD40L− TIL sub-populations (M125, M265 and M305) selected for further experiments. Cells were co-stained with CD4+, CD8α mAbs and with either the isotype control (filled histogram) or CD40L mAb (open histogram). Numbers indicated represent the percentages of CD40L+ cells.
Intra-tumor DP T cells induce memory B-cell proliferation and differentiation through the CD40L engagement
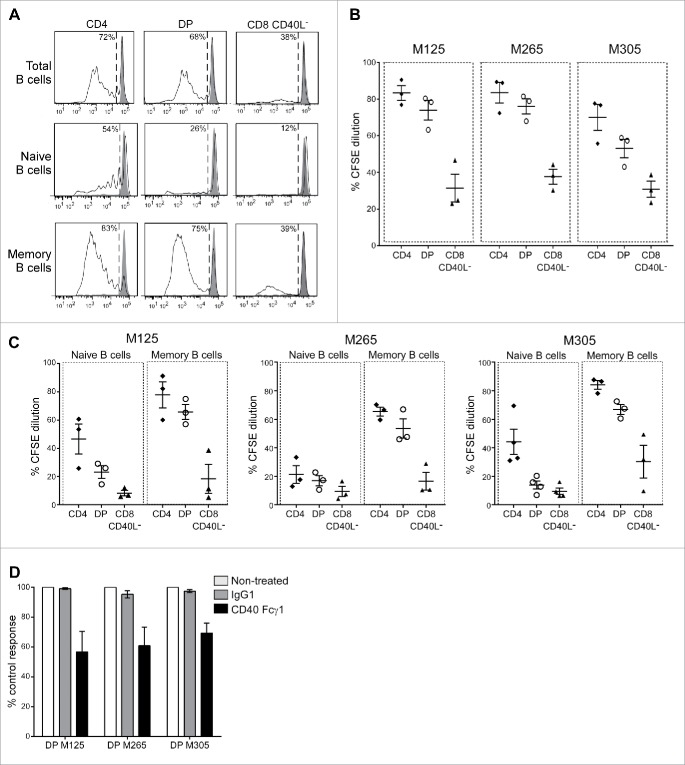
We started investigating CD40L functionality by looking at the ability of DP T cells to mediate B-cell help. Allogeneic CD19+ B cells were co-cultured with activated DP, SP CD4+ or SP CD40L− CD8+ T cells; B-cell proliferation was monitored 4 d later by CFSE dilution assay. Pre-activated SP CD4+ T cells and, to a lower extent, DP T cells induced B-cell proliferation (Fig. 2A and B). This induction was not achieved with resting SP CD4+ and DP T cells (data not shown). As expected,29 SP CD8+ CD40L− largely failed to induce B-cell proliferation (Fig. 2A and B). According to the DP T-cell population tested, the B-cell proliferation ranged from 50% to 80%, a result consistent with the CD40L expression levels on each DP T-cell subset. We further investigated whether the B-cell proliferation induced in our co-culture conditions was the result of naive or memory B-cell proliferation. These two B-cell subsets were isolated from CD19+ B cells on the basis of CD27 expression32 and individually co-cultured with each activated T-cell subset. SP CD4+ T cells and, to a lower extent, DP T cells led to a modest proliferation of naive B cells (Fig. 2A and C). Memory B-cell proliferation was induced by both DP T cells and SP CD4+ T cells with a comparable efficiency, whereas SP CD8+ CD40L− T cells were still inefficient. With the three DP T-cell populations tested, we obtained an average proliferation rate of naive and memory B cells up to 20% and 60%, respectively (Fig. 2C). To investigate the role of CD40–CD40L interaction in this process, the same experiments were performed in the presence of soluble CD40. Addition of a soluble CD40, added at the beginning of the co-culture, induced a clear decrease in memory B-cell proliferation up to 30% (Fig. 2D). This partial inhibition suggested the involvement of other co-stimulation pathways.
Figure 2.
Intra-melanoma DP T cells mediate memory B-cell proliferation through CD40L engagement. Intra-melanoma SP CD4+, DP and SP CD8+ CD40L− T-cell lines were co-cultured with CFSE-labeled B cells for 4 d at a 1:1 ratio on anti-CD3-coated microwells, and B-cell proliferation was evaluated by flow cytometry gated on CD19+ cells. (A) Representative CFSE dilution profiles of total (CD19+), naive (CD19+CD27−) and memory (CD19+CD27+) B-cell fractions after co-cultured with CD3-activated SP CD4+, DP and SP CD8+ CD40L− T cells. B cell proliferation was expressed as the percentage of CFSE dilution. Results of total (B), naive or memory (C) B-cell proliferation stimulated with each T-cell subpopulations derived from the three patients M125, M265 and M305 are gathered on a single graph (n = 3 independent experiments with three different B-cell donors). (D) Memory B-cell proliferation stimulated with DP T cells derived from M125, M265 and M305 patients in the presence of either recombinant human CD40 Fcγ1 (black bars), control IgG1 Fc (gray bars) or without treatment (white bars). Results are normalized to the non-treated control condition (n = 3 independent experiments with three different B-cell donors).
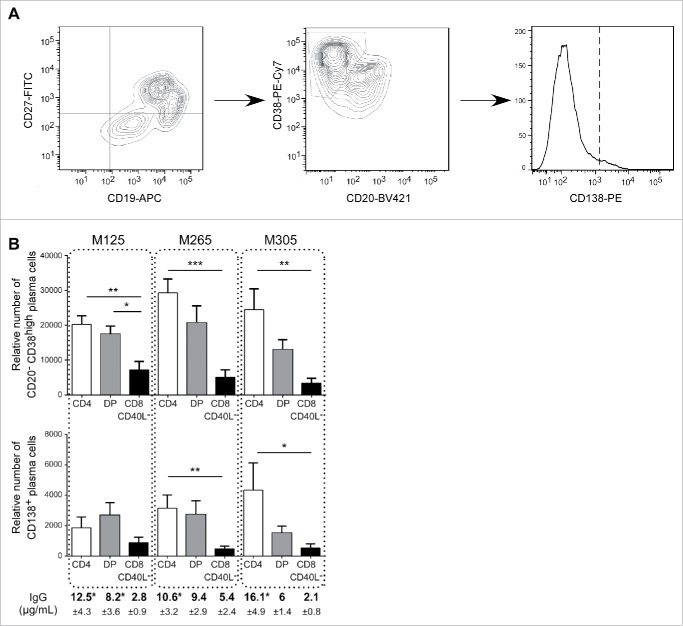
We then studied if, in addition to allow memory B-cell proliferation, DP T cells could support their differentiation into terminally differentiated B cells. Plasma cells (CD19+CD27+CD38highCD20lowCD138−) and terminally differentiated plasma cells (CD19+CD27+CD38highCD20lowCD138+) were counted 7 d after the initiation of the co-culture (Fig. 3A). Activated DP T cells induced the development of plasma cells from memory B cells at a level similar to SP CD4+ T cells (Fig. 3B). In addition, SP CD4+ cells and DP T cells were able to generate terminally differentiated plasma cells, more efficiently than SP CD8+ CD40L− T cells. Moreover, B-cell differentiation induced by DP and SP CD4+ T cells was associated with the production of IgG (Fig. 3B). Taken together, these results showed that intra-tumor DP T cells share a similar helper potential with intra-tumor SP CD4+ T cells to promote B-cell proliferation and differentiation and that this function was partially dependent on CD40L.
Figure 3.
Intra-melanoma DP T cells mediate B-cell differentiation. The differentiation status of B cells was determined by flow cytometry after 7 d of co-culture of sorted memory B cells at a 1:1 ratio with anti-CD3 activated DP, SP CD4+ or SP CD8+ CD40L−. (A) Gating strategy performed on viable cells to discriminate plasma cells (CD19+CD27+CD38highCD20lowCD138−) from highly differentiated plasma cells (CD19+CD27+CD38highCD20lowCD138+). (B) The relative number of plasma cells and highly differentiated plasma cells was determined using counting beads at the end of the 7 d of memory B cells co-cultured with SP CD4+ (white bars), DP (gray bars) or SP CD8+ CD40L− (black bars) T-cell subpopulations derived from M125, M265 and M305 melanoma patients (n = 7 independent experiments with seven B-cell donors). Supernatants from the same co-cultures were analyzed at day 7 by ELISA for secretion of IgG. Results are expressed as mean ± SEM. Statistical analysis was performed using the non-parametric Friedman test, followed by Dunn's multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001.
Intra-tumor DP T cells allow the maturation of functional DCs able to prime antitumor CD8+ T-cell responses
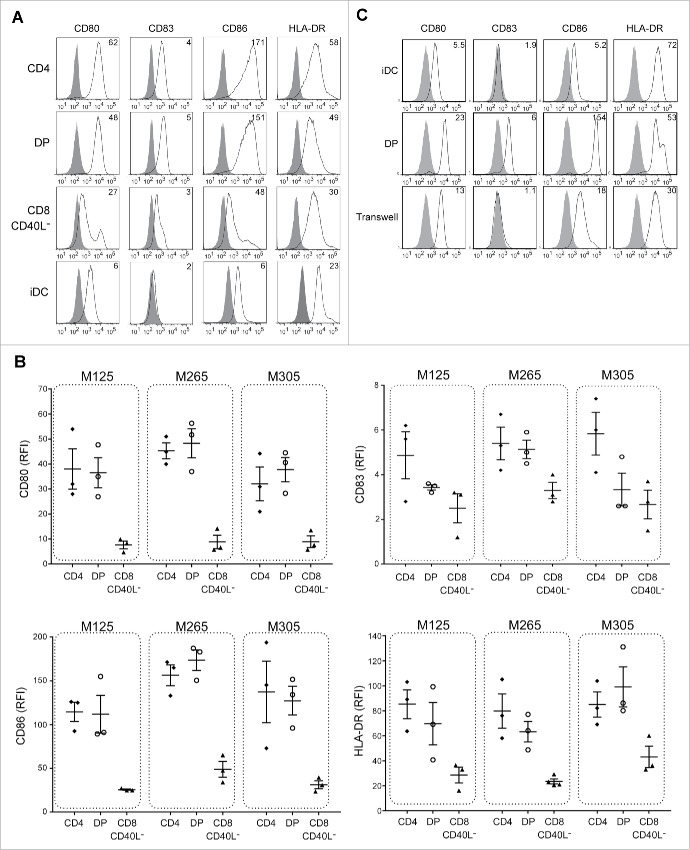
We then evaluated the ability of DP T cells to induce DC activation and efficient cross-priming of antitumor CD8+ T cells. Immature DCs differentiated from healthy blood monocytes (Mo-DCs) were co-cultured with activated DP, SP CD4+ or SP CD8+ T cells for 36 h before analyzing the expression of maturation markers by flow cytometry. Activated DP T cells strongly induced DC maturation from immature Mo-DCs as illustrated by an increased expression of CD83, CD80, CD86 and HLA-DR compared to immature Mo-DCs alone, whereas SP CD8+ CD40L− T cells were poorly effective (Fig. 4A and B). For all DP T-cell populations tested, CD80, CD86 and HLA-DR expression levels were comparable to those of activated SP CD4+ T cells. CD83 expression was controversial between the three DP T-cell populations tested with two of them failing in inducing its expression, suggesting that DP T cells induced a less matured DC phenotype than SP CD4+ T cells.
Figure 4.
Intra-melanoma DP T cells induce DC maturation in a contact-dependent manner. iDCs were co-cultured for 36 h with intra-melanoma SP CD4+, DP and SP CD8+ CD40L− T-cell subpopulations pre-stimulated for 6 h with OKT3 at ratio of 1:2, and the expression of maturation markers (CD80, CD83, CD86 and HLA-DR) was determined by flow cytometry. (A) Representative flow cytometry analysis of cell surface expression of different maturation markers. DCs were stained with either the isotype control mAb (filled histrogram) or with the indicated maturation marker mAb (open histograms). Results are expressed as the relative fluorescence intensity (RFI). (B) RFI of CD83, CD80, CD86 and HLA-DR expression on DCs after maturation with either SP CD4+ (black diamonds), DP (white circles) and SP CD8+ CD40L− (black triangles) T-cell subpopulations derived from TIL of M125, M265 and M305 melanoma patients (n = 3 independent experiments with three different DC donors). Results are expressed as mean ± SEM. (C) Representative flow cytometry analysis of DCs maturation profile after co-culture of intra-melanoma DP T cells with iDCs respectively seeded in the upper chamber and in the lower chamber of a Transwell plate at a 1:2 ratio for 36 h. DCs were stained with either the isotype control (filled histograms) or with CD80, CD83, HLA-DR or CD86 mAbs (open histograms). Data are expressed as the RFI. Control experiments included the standard co-culture of Mo-DCs with or without DP T cells.
We next asked whether direct contact between DP T cells and immature DCs was required to induce DC maturation. Using Transwell plates, we did not observe any phenotypic maturation of the DCs as assessed by the lack of CD83 expression and the low increase of CD80, CD86 and HLA-DR expressions, when immature DCs and activated DP T cells were cultured in separate chambers (Fig. 4C). These data demonstrated that the maturation of DC induced by DP T-cells was dependent on cell–cell contacts, in support of the role of CD40/CD40L interaction.
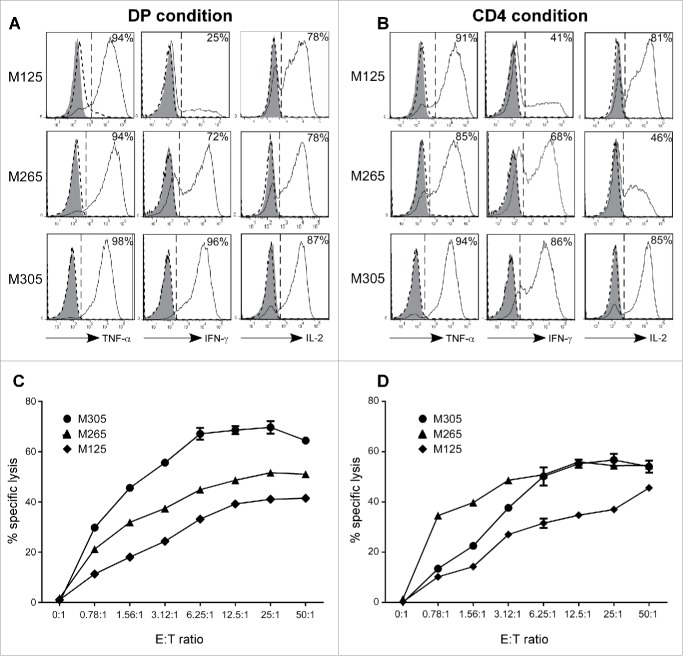
To determine whether DP T-cell-matured DCs were functional, we investigated their ability to prime Melan-A-specific CD8+ T cells. Peripheral blood T lymphocytes from HLA-A2 donors were stimulated with autologous Melan-A16-40L-pulsed-DCs matured with either activated intra-tumor DP or SP CD4+ T cells. Melan-A-specific CD8+ T cells were monitored 15 d after the stimulation by Melan-A/A2 tetramer staining. DCs matured in the presence of DP T cells were able to generate Melan-A-specific CD8+ T cells with a fraction of positive microcultures ranging from 2% to 19% according to TIL populations (Table 1). However, and in agreement with a less mature phenotype, the priming by DP-matured DCs was less efficient than the one induced by SP CD4+ T cells (fraction of positive microcultures ranging from 5% to 32%). Nonetheless, among the positive microcultures, the mean percentage of specific T cells obtained in CD4+ and DP conditions was quite similar (1.01% and 0.8%, respectively). Both DP and SP CD4+ T cells from M305 population generated fewer positive microcultures, a result consistent with a weaker CD40L expression (Fig. 1D). Finally, we evaluated the in vitro tumor reactivity of sorted Melan-A-specific CD8+ T cells generated with SP CD4+-matured-DCs or DP T cell-matured-DCs. In response to Melan-A/HLA-A2-positive melanoma cell line, Melan-A-specific CD8+ T cells induced with DP-matured DCs were able to secrete TNF-α, IFNγ and IL-2 (Fig. 5A) and exhibited an efficient cytotoxic activity as measured by 51Cr release assay (Fig. 5C). These responses were not observed against Melan-A-negative tumor cell lines (data not shown). Similar cytokine profile (Fig. 5B) and cytotoxic activity (Fig. 5D) were observed with Melan-A-specific CD8+ T cells induced with SP CD4+-matured DCs. These results demonstrate the efficient helper activity of the DP T cells in priming an antitumor CD8+ T-cell response.
Table 1.
Distribution of Melan-A-specific T lymphocytes in microcultures of healthy donor T cells stimulated with SP CD4+- or DP-matured HLA-A2+ DCs.
| CD4+ | DP | |
|---|---|---|
| M125 | ||
| Fraction of positive microcultures (%) | 28/96a (29.2%) | 15/96 (15.6%) |
| Mean percentage of specific T cells (% max) | 1.37%b (9.3%) | 1.08% (3.7%) |
| M265 | ||
| Fraction of positive microcultures (%) | 36/96 (32.3%) | 18/96 (18.7%) |
| Mean percentage of specific T cells (% max) | 0.87% (7%) | 0.87% (6.4%) |
| M305 | ||
| Fraction of positive microcultures (%) | 5/96 (5.2%) | 2/96 (2.1%) |
| Mean percentage of specific T cells (% max) | 0.80% (1.3%) | 0.50% (0.5%) |
CD3 T cells from healthy donors PBMCs were stimulated with Melan-A16-40 A27L peptide loaded on SP CD4+- or DP-matured autologous HLA-A2+ Mo-DCs. Intra-melanoma SP CD4+ or DP T-cell subpopulations isolated from TIL of M125, M265 and M305 melanoma patients. Microcultures positive from Melan-A specific CD8+ T cells were identified by flow cytometry after staining with Melan-A/A2 tetramer.
Ratio of positive to total microcultures.
Mean percentage of specific CD8+ T lymphocytes stained by tetramer among the positive microcultures.
Figure 5.
DCs matured with intra-melanoma DP T cells prime antitumor CD8+ T-cell responses as efficiently as conventional SP CD4+ T cells. CD3 T cells from healthy donors PBMCs were stimulated with Melan-A16-40 A27L peptide loaded HLA-A2+ DCs matured with intra-melanoma SP CD4+ (A, C) or DP (B, D) T cells derived from M125, M265 and M305 melanoma patients. After two weeks, Melan-A-specific CD8+ T cells were sorted and evaluated for their tumor reactivity. (A, B) Production of TNF-α, IFN-γ and IL-2 was determined by flow cytometry on Melan-A-specific CD8+ T cells at rest (filled histograms) or stimulated against a Melan-A-negative HLA-A2-positive melanoma cell line (dashed histogram) or a Melan-A/HLA-A2-positive melanoma cell line (solid line histogram). Results are expressed as the percentages of cytokine-positive T cells. (C, D) Cytolytic activity of Melan-A specific CD8+ T cells was measured by a 51Cr release assay at various effector/target ratios against a Melan-A/HLA-A2-positive melanoma-cell line. Results are expressed as the mean percentage of specific lysis ± SEM in triplicate cultures.
Discussion
According to the traditional dichotomy, the CD8+ and CD4+ co-receptors expression delineates respectively the MHC class-I-restricted cytotoxic and the MHC class-II-restricted helper T-cell lineages. This scheme became outdated with the inclusion of CD4+ Treg cells, CD4+/−/CD8+/− NKT cells and non-conventional CD8+ innate T cells as independent lineages33 as well as the description in the periphery of CD4+ cytotoxic T cells34 and CD8+ helper T cells.29 With this study, we add an additional degree of complexity by ascribing a helper potential to class-I restricted melanoma-infiltrating CD4lowCD8highαβ DP T cells involving the CD40L co-stimulatory molecule.
The CD40L co-stimulatory molecule through its interaction with its cognate receptor CD40 plays a key role in governing both the humoral and the adaptive immune responses. CD40 is constitutively expressed on all antigen-presenting cells and is also found on T cells as well as non-hematopoietic cells.35,36 Overall, CD40 signaling of B cells promotes their activation, proliferation, isotype switching and differentiation into antibody-secreting plasma cells.28 In addition, CD40 ligation triggers DC licensing by promoting their maturation and secretion of the pro-inflammatory cytokine IL-12p70 and thus, allowing an efficient CTL priming. In this context, CD40L is also required to generate memory CTL responses by a direct engagement of CD40 on CD8+ T cells.35 Thus, CD40L is a characteristic feature of helper T-cell function and as such, its expression was initially thought to be restricted to T cells from the class-II-restricted helper CD4+ lineage. Since then, CD40L expression was described on a larger cell panel following activation or under inflammatory conditions and includes, among others, CD8+ T cells, NKT cells, mast cells, activated B cells and DCs.28
Using an in vitro allogeneic co-culture model dedicated to the evaluation of DP T-cell-mediated B-cell help, we described the ability of activated DP T cells to stimulate the proliferation and differentiation of memory B cells at a level comparable to SP CD4+ T cells. Unlike memory B cells that have a reduced threshold of activation and can be activated without BCR signaling, naive B cells require additional signals including BCR and TLR stimulation for their complete activation.37 Therefore, consistently with our co-culture model, we did not observed a major proliferation of naive B cells upon DP T-cell stimulation, which does not exclude this possibility in vivo in the presence of adequate additional signals. The use of a CD40 antagonist demonstrated that memory B-cell proliferation was dependent on CD40L engagement. This inhibition was however partial, suggesting that other mechanisms related to the activation status of DP T cells might be involved. Among others molecules, the cytokines IL-2,38,39 IL-4,40,41 IL-10,42,43 IL-1344 or IL-2145,46 were shown to be required for B-cell proliferation and/or differentiation. We previously showed that melanoma-infiltrating activated DP T cells produced, upon activation, IL-2, TNF-α, IFNγ and GM-CSF and were distinguishable from SP CD4+ and CD8+ T cells by a cytokine expression profile biased toward IL-13, IL-4 and IL-5 expression.23 Therefore, IL-2, IL-13 and IL-4 could probably be involved in B-cell proliferation in addition to CD40L engagement as well as an allogeneic BCR recognition. DP T cells also induced B-cell differentiation into IgG-secreting plasma cells with a fraction of them expressing CD138, a marker of terminally differentiated plasma cells.47
In the tumor microenvironment, B cells are versatile and can exert both antitumor and regulatory functions.48 In melanoma, an immunosuppressive activity was recently attributed to IgG4-positive B cells since IgG4 serum level is associated to a negative prognosis factor.49,50 Since DP T cells distinguished themselves from SP T cells by their production of IL-4 and IL-13, and since these cytokines are described to promote IgG4 class switching in B cells,51,52 one can argue that DP T cells could promote the expression of IgG4 by B cells and therefore could negatively impact the antitumor immune response. In our co-culture system of naive B cells and activated DP T cells, we neither detect any IgG4 membrane expression (flow cytometry) nor soluble IgG4 production (ELISA), therefore, excluding this hypothesis (data not shown). Regulatory B cells (Bregs) constitute another immunosuppressive B cell subtype found in the infiltrate of several solid tumors and generally associated with an increase tumor progression.53 As the CD40/CD40L interaction is one of the mechanisms described in the induction of B-cell regulatory functions,54 we addressed this point through co-culture of naive B cells with activated DP cells. The results did not reveal any B cells expressing a regulatory phenotype (CD19+CD24highCD38high) (flow cytometry) no more than the secretion of the immunosuppressive cytokine IL-10 (ELISA) (data not shown). Overall, our results did not support the ability of DP T cells to induce a pro-tumor B-cell phenotype and are in favor of an antitumor function for DP T cells. The activation, proliferation and differentiation of B cells are a specific feature of follicular helper CD4+ T cells (Tfh) found in germinal center.55 Although DP T cells did not share some Tfh characteristics, such as CXCR5 expression and IL-21 production (data not shown), and are probably less efficient in activating B cells than Tfh, they still present a significant helper activity that can be valuable in situ to mediate an antitumor immune response.
In addition, we reported that melanoma-infiltrating DP T cells induced the maturation of DCs able to efficiently prime CD8+ anti-melanoma specific responses. Although the phenotypic maturation of DCs was clearly observed (as evidenced by an increased expression of CD83, CD80, CD86 and HLA-DR increased expression), we did not detect any IL-12p70 secretion by DCs matured in the presence of DP T cells and either with our SP CD4+ T cells. However, both populations produced IFN-γ that in combination with CD40L/CD40 engagement was described as necessary for IL-12p70 synthesis.56 This could be explained by the lack of additional signal(s), such as TLR signaling that has been reported to contribute to IL-12p70 production.57,58 Furthermore, we demonstrated, using a Transwell assay, that the maturation of DC by DP T cells was dependent on cell-contact, in support of the role of CD40/CD40L interaction.
Although CD40L expression is mainly associated to helper CD4+ T cells, early in vitro studies described that a subset of CD8+ T cells can also induce, upon activation, the expression of CD40L able both to induce the differentiation of B cells into IgG-secreting plasma cells30 and to induce DC maturation.58 Recent in vivo studies confirmed the existence of such CD4+ helper-independent CTL59,60 that are characterized in human as a memory T-cell population displaying a cytokine expression profile similar to conventional helper CD4+ T cells.29 It has been hypothesized that CD40L expression by CD8+ T cells might result from a strong TCR avidity for its peptide–MHC complex in combination with the presence of pro-inflammatory cytokines, such as IL-12.61 It was recently described in vitro and in vivo that some highly immunogenic CD8+ T-cell peptides could act as helper epitopes.60 Whether CD40L expression by DP T cells is an original feature or results from DP T-cell activation with such epitopes in the periphery remains to be established.
In conclusion, intra-melanoma DP T-cell population could supplement the conventional CD4+ helper T-cell function and actively participate in shaping antitumor immunity. First, it will contribute to the maturation of DCs directly in the tumor microenvironment, which would lead to the promotion of antitumor immune T-cell responses. Second, it will enhance B-cell infiltration and activation, which by antibody secretion, antigen presentation, tertiary lymphoid structure formation or direct killing of tumor cells could potentiate the antitumor immune response.62 This scheme is consistent with the view that tumor infiltration by activated B cells has been reported to be associated with a better prognosis, especially when combined with CD4+ or CD8+ tumor infiltration.63-65 Moreover, as we previously reported that IL-9 promotes the function of intra-melanoma DP T cells,27 this cytokine may also foster DP helper functions. In support, we observed that IL-9 enhances CD40L expression level on DP T cells (data not shown). Finally, it should be pointed out that due to their HLA class I restriction, CD4lowCD8highαβ DP T cells could fulfill their helper function even in HLA class II-negative tumors.
Materials and methods
PBMCs and cell lines
PBMCs were isolated from HLA-A2-positive healthy donors (Etablissement Français du Sang (EFS), Nantes, France), and PBL and monocytes fractions were isolated by elutriation (DTC facility, Nantes).
All melanoma cell lines were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum (FSC) (Gibco, 31870-025), 2 nM l-glutamine (Gibco, 25030-024), 100 U/mL penicillin and 0.1 mg/mL streptomycin (Gibco, 15140-122). They have been established in the GMP Unit of Cellular Therapy (PCU892-NL biocollection, CHU Nantes). This biocollection was approved by the local ethic committee of Nantes hospital (GNDES) and registered under the CNIL number “1278197”.
Generation of tumor-infiltrating DP, SP CD4+ and SP CD8+ T-cell lines and culture
TILs were previously obtained from tumor-invaded lymph nodes of eight melanoma patients (M125, M256, M261, M265, M288, M291, M298, M305, M314 and M329). TILs were expanded by stimulation with PHA-L (Sigma-Aldrich, L4144) and 150 U/mL human rIL-2 (Proleukin, Novartis) in the presence of allogeneic irradiated feeder cells (PBMCs and B-EBV B cells) in RPMI-1640 medium supplemented with 8% human serum (local production), 2 mM L-glutamine, 100 U/mL penicillin and 0.1 mg/mL streptomycin, as previously described.66 Pure DP, SP CD4+ and SP CD8+ T-cell polyclonal populations were generated by cell sorting using a BD FACSAria III cell sorter (BD Biosciences). TILs were stained with APC-conjugated anti-CD4+ MAB (Clone SK3, BD Biosciences, 340672) and BV421-conjugated anti-CD8+ mAb (Clone RPA-T8, BD Biosciences, 562428) for 30 min at 4°C in PBS 0.1% BSA and then washed two times in PBS 0.1% BSA (Sigma-Aldrich, A9576) before cell sorting. To ensure that DP T-cell phenotype does not result from doublets of SP T cells, doublets were excluded using FSC-A/FSC-H and SSC-A/SSC-H dot plots. The purity of each sorted T-cell population was checked right after cell-sorting and routinely by flow cytometry. After 24 h, sorted cell populations were amplified in culture as described above. In order to isolate SP CD8+ CD40L− T cells, SP CD8+ T cells were stimulated for 6 h by anti-CD3 stimulation (1 µg/mL, local production) in the presence of anti-CD40L-mAb (Clone 24-31, BioLegend, BLE310802), and FACS-sorted.
Microarray analysis
Total RNA was extracted from 5 × 106 sorted DP, SP CD4+ and SP CD8+ TIL populations from eight melanoma patients. After cell lysis with Trizol reagent (Life Technologies, 15596026), total RNA was extracted using the RNeasy Micro kit (Qiagen, 74004) and quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.) according to manufacturer's recommendations. Integrity of the extracted RNAs was assessed with a Bioanalyzer 2100 using the RNA6000 Nano kit (Agilent Technologies, Inc.). An RNA integrity number (RIN) greater or equal to 7.00 was achieved for all samples. No sign of DNA contamination was detected. The starting amount of total RNA used for the reactions was 400 ng per sample. The Illumina Total Prep RNA Amplification kit (Ambion, Life Technologies, AMIL1791) was used to generate biotinylated, amplified cRNA according to the manufacturer's recommendations. Hybridization on Illumina HumanHT-12 v3 Expression BeadChips, staining and detection of cRNAs with the I-Scan system were performed according to manufacturer's protocol (Illumina, Inc.). The HumanHT-12 v3 Expression BeadChip assesses a total of 48,803 marker probes, of which 27,455 are NM coding transcripts; and 7,870 are XM coding transcripts (RefSeq Content, Build 36.2, Release 22). It also contains 12,837 experimentally confirmed mRNA sequences that align to EST clusters (UniGene, Build 199). This entire process was performed in duplicate. GenomeStudio 2011 v1 and its Expression Analysis Module (version 1.9.0) were used for signal extraction and quantile normalization (Illumina, Inc.). Omics Explorer 3.2 software (Qlucore AB) was used on log-transformed data for hierarchical clustering analysis, and paired t-test comparisons with adjusted p-values (or q-values).
Analysis of transcript expression
Total RNA from different TIL sub-populations, prepared as described above, was reverse transcribed using the Superscript II reverse transcriptase (Life Technologies, 18064014). The expression of CD40L mRNA was analyzed by quantitative RT-PCR. Amplification was done by using iQ SYBR Green Supermix (Bio-Rad, 1708880). Relative gene expression was calculated using the EΔCt method with ΔCt values calculated from the sample with the largest Ct (fewest gene copies) and then normalized against the GAPDH (determined as the most stably expressed housekeeping gene using the geNorm method). Primer sequences are available upon request.
CD40L membrane expression
Intra-melanoma DP, SP CD4+ and SP CD8+ TILs were stimulated for 6 h with coated anti-CD3 mAb (1 µg/mL, OKT3) before being stained with anti-CD40L mAb or isotype control mAb (Clone MOPC-21, BioLegend, 400101) for 20 min at 4°C in PBS 0.1% BSA. CD40L membrane expression was analyzed on BD FACSCanto II (BD Biosciences). Relative fluorescence intensity (RFI) was calculated as sample mean fluorescence intensity divided by isotype control mean fluorescence intensity.
B-cell helper assay
CD19+ B cells were isolated from healthy donors PBMCs by negative immunomagnetic selection (STEMCELL Technologies, 19054) and cultured in RPMI supplemented with 10% SVF. After 24 h, naive (CD27−) and memory B (CD27+) cell fractions were then separated with anti-human-CD27 microbeads (Miltenyi Biotec, 130-051-601). For proliferation assay, B cells were labeled with 1 µM CFSE (Invitrogen, C34554) for 10 min at 37°C and incubated in RPMI 10% FBS at a 1:1 ratio with intra-melanoma SP CD4+, DP and SP CD8+ CD40L− T-cell subpopulations pre-irradiated at 35 Gy. The co-cultures were performed in microwells, pre-coated or not with anti-CD3 mAb (1 µg/mL, OKT3) to induce T-cell activation. At day 4, cells were recovered and stained with anti-CD19-APC mAb and Zombie NIR™ viability dye (BioLegend, 423105) before analysis on a BD FACS Canto II cytometer (BD Biosciences).
For the blockade of CD40L/CD40 interactions, 10 µg/mL of recombinant human CD40 Fc chimera protein (R&D Systems, 1493-CD-050) was added at the beginning of T-cell–B-cell co-culture. The recombinant human IgG1 Fc chimera protein (R&D Systems, 110-HG-100) was used as a negative control. For differentiation assay, unlabeled memory B cells were cultured as described above for 7 d and stained with anti-CD19-APC (Clone SJ25C1, 363029), anti-CD20-BV421 (Clone 2H7, 302329), anti-CD38-PE-Cy7 (Cone HIT2, BioLegend, 303516), anti-CD27-FITC (Clone M-T271, 555440) and anti-CD138-PE (BD Biosciences, Clone MI15, 347215) mAbs. At day 7, supernatants were collected and the level of IgG was quantified with the human IgG ELISA kit (eBioscience, 88-50550-22) according to the manufacturer's instructions.
DC maturation assay
Monocytes were obtained by elutriation (DTC Facility, Federative Structure Research François Bonamy, Nantes) and differentiated into immature monocyte-derived DCs (Mo-DCs) in RPMI containing 2% human albumin, GM-CSF (1,000 U/mL, CellGenix, 1412-050) and IL-4 (200 U/mL, CellGenix, 1003-050) for 5 d. Then, immature Mo-DCs (iDCs) were cultured at a 1:2 ratio in a 24-well plate for 36 h with intra-melanoma SP CD4+ or DP T-cell subpopulations pre-activated for 6 h with anti-CD3 mAb (1 µg/mL, OKT3). DC maturation was analyzed by flow cytometry after staining with anti-CD80-PE (Clone 2D10, 305207), anti-CD83-FITC (Clone HB15e, 305305), anti-CD86-PE anti-HLA-DR-PE (Clone LN3, 327007) mAbs and their respective isotype controls (BioLegend). To evaluate the cell contact dependency of DC maturation, iDCs were seeded at a 1:2 ratio into the bottom chamber of a 24-well Transwell (0.4 µm pores) plate (Costar, 3413), whereas pre-activated intra-melanoma DP T cells were seeded into the upper chamber. After 36 h, DC maturation profile was evaluated by flow cytometry as described above.
Priming of Melan-A specific CD8+ T-cell responses
For CD8+ T-cell priming, HLA-A2+ DCs were pulsed with the modified Melan-A16-40 A27L peptide for 24 h before maturation with activated T-cell subsets. This modified long peptide was chosen for its ability to generate the highly immunogenic class-I-restricted Melan-A26-35 ELAGIGILTV epitope (carrying the substitution of Ala for Leu at position 2 from the NH2 terminus hereafter A27L) that forms stable complexes with HLA-A*0201, conferring it a more immunogenic potential than the natural Melan-A peptide. The superior efficiency of cross-priming with this long peptide loaded on DC has already been documented in comparison with the non-modified peptide.67 DCs were then separated from the remaining allogeneic T cells with anti-CD3-positive immunomagnetic selection (eBioscience, 8802-6830-74) and cultured at a 1:10 ratio with autologous CD3+ T cells enriched by anti-CD3-negative selection (STEMCELL Technologies, 19051) in RPMI 8% human serum and 50 U/mL IL-2. At day 15, Melan-A-specific CD8+ T cells were detected using tetramer (Recombinant Protein Facility, Federative Structure Research François Bonamy, Nantes) labeling. Microcultures were considered positive when the percentage of Melan-A-specific CD8+ T cells was ≥ 0.2%.
Melan-A-specific CD8+ T-cell sorting and functional evaluation
Sorting and amplification of Melan-A-specific CD8+ T cells were performed as previously described.68 Melan-A-specific CD8+ T cells were stimulated against HLA-A2+ Melan-A+ M113 and HLA-A2+ Melan-A− M25 melanoma cell lines at a 1:2 ratio for 6 h in the presence of 10 µg/mL Brefeldin A (Sigma, B7651). After fixation with PBS 4% paraformaldehyde (VWR, 100504-858) for 10 min, cells were stained with anti-TNF-α-APC (Clone cA2, 130-098-882), anti-IFN-γ-APC (Clone 45-15, 130-097-944) (Miltenyi Biotec) or anti-IL-2-PE (Clone 5344.111, BD Biosciences, 340450) mAbs for 30 min in PBS 0.1% BSA and 0.1% saponin (Sigma-Aldrich).
Cytolytic activity of Melan-A-specific CD8+ T cells was tested against the 51Cr-labeled M113 melanoma cell line. Briefly, 1 × 106 M113 cells were labeled with 100 µCi of Na2CrO4 (2,77MBq) (NEZ030, PerkinElmer) for 1 h at 37°C. After five washes in RPMI 1640 10% FCS, 51Cr-labeled M113 cells were plated at 1 × 103 cells/well. 5 × 104–0.7 × 103 Melan-A-specific CD8+ T cells were added (50:1–0.78:1 effector/target ratios). After 4 h at 37°C, 25 µL of supernatants was mixed with 100 µL of scintillation liquid (Optiphase Supermix, Wallack) for measurement of radioactive content on a beta plate counter (Microbeta Jet 1450, PerkinElmer). Percentage of target cell lysis was calculated according to the following formula: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Maximum and spontaneous releases were respectively determined by adding 0.1% Triton X-100 or RPMI 1640 10% FCS on 51Cr-labeled M113 cells.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Recombinant Protein Facility for HLA-A2/peptide monomers' production, the Clinical Development and Transfer DTC Facility for monocyte production and the Cytometry Facility « CytoCell » for expert technical assistance all from the Federative Structure Research François Bonamy, Nantes.
Funding
This work was supported by grants awarded by the “Cancéropôle Grand Ouest” and the “Région Pays de la Loire.” This work was realized in the context of the LabEX IGO program supported by the National Research Agency via the investment of the future program ANR-11-LABX-0016-01. Tiphaine Parrot was supported by an allocation from the «Ligue Nationale contre le Cancer».
References
- 1.Blue ML, Daley JF, Levine H, Branton KR Jr., Schlossman SF. Regulation of CD4 and CD8 surface expression on human thymocyte subpopulations by triggering through CD2 and the CD3-T cell receptor. J Immunol 1989; 142:374-80; PMID:2783435 8461016 [PubMed] [Google Scholar]
- 2.Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem Biophys Res Commun 1993; 191:601-9; PMID:8461016; http://dx.doi.org/ 10.1006/bbrc.1993.1260 [DOI] [PubMed] [Google Scholar]
- 3.Blue ML, Daley JF, Levine H, Schlossman SF. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol 1985; 134:2281-6; PMID:2982943 15044252 [PubMed] [Google Scholar]
- 4.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 2004; 104:478-86; PMID:15044252; http://dx.doi.org/ 10.1182/blood-2003-12-4395 [DOI] [PubMed] [Google Scholar]
- 5.Parel Y, Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun Rev 2004; 3:215-20; PMID:15110234; http://dx.doi.org/ 10.1016/j.autrev.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Zloza A, Al-Harthi L. Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. J Leukoc Biol 2006; 79:4-6; PMID:16380600; http://dx.doi.org/ 10.1189/jlb.0805455 [DOI] [PubMed] [Google Scholar]
- 7.Bonomo A, Kehn PJ, Shevach EM. Premature escape of double-positive thymocytes to the periphery of young mice. Possible role in autoimmunity. J Immunol 1994; 152:1509-14; PMID:81203653262830 [PubMed] [Google Scholar]
- 8.Jimenez E, Sacedon R, Vicente A, Hernandez-Lopez C, Zapata AG, Varas A. Rat peripheral CD4+CD8+ T lymphocytes are partially immunocompetent thymus-derived cells that undergo post-thymic maturation to become functionally mature CD4+ T lymphocytes. J Immunol 2002; 168:5005-13; PMID:11994452; http://dx.doi.org/3262830 10.4049/jimmunol.168.10.5005 [DOI] [PubMed] [Google Scholar]
- 9.Paliard X, Malefijt RW, de Vries JE, Spits H. Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature 1988; 335:642-4; PMID:3262830; http://dx.doi.org/ 10.1038/335642a0 [DOI] [PubMed] [Google Scholar]
- 10.Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L. Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology 2001; 103:270-80; PMID:11454056; http://dx.doi.org/ 10.1046/j.1365-2567.2001.01243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraldo NA, Bolanos NI, Cuellar A, Guzman F, Uribe AM, Bedoya A, Olaya N, Cucunubá ZM, Roa N, Rosas F et al.. Increased CD4+/CD8+ double-positive T cells in chronic Chagasic patients. PLoS Negl Trop Dis 2011; 5:e1294; PMID:21886854; http://dx.doi.org/ 10.1371/journal.pntd.0001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimbeni M, Pol S, Saunier B. Distinct CD4+ CD8+ double-positive T cells in the blood and liver of patients during chronic hepatitis B and C. PLoS One 2011; 6:e20145; PMID:21647449; http://dx.doi.org/ 10.1371/journal.pone.0020145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss L, Roux A, Garcia S, Demouchy C, Haeffner-Cavaillon N, Kazatchkine MD, Gougeon ML. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis 1998; 178:1158-62; PMID:9806050; http://dx.doi.org/ 10.1086/515674 [DOI] [PubMed] [Google Scholar]
- 14.Zloza A, Schenkel JM, Tenorio AR, Martinson JA, Jeziorczak PM, Al-Harthi L. Potent HIV-specific responses are enriched in a unique subset of CD8+ T cells that coexpresses CD4 on its surface. Blood 2009; 114:3841-53; PMID:19700667; http://dx.doi.org/ 10.1182/blood-2009-02-202481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parel Y, Aurrand-Lions M, Scheja A, Dayer JM, Roosnek E, Chizzolini C. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum 2007; 56:3459-67; PMID:17907151; http://dx.doi.org/ 10.1002/art.22927 [DOI] [PubMed] [Google Scholar]
- 16.Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS One 2014; 9:e93293; PMID:24667579; http://dx.doi.org/ 10.1371/journal.pone.0093293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waschbisch A, Sammet L, Schroder S, Lee DH, Barrantes-Freer A, Stadelmann C, Linker RA. Analysis of CD4+ CD8+ double-positive T cells in blood, cerebrospinal fluid and multiple sclerosis lesions. Clin Exp Immunol 2014; 177:404-11; PMID:24730443; http://dx.doi.org/ 10.1111/cei.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carton J, Byrne B, Madrigal-Estebas L, O'Donoghue DP, O'Farrelly C. CD4+CD8+ human small intestinal T cells are decreased in coeliac patients, with CD8 expression downregulated on intra-epithelial T cells in the active disease. Eur J Gastroenterol Hepatol 2004; 16:961-8; PMID:15371918; http://dx.doi.org/ 10.1097/00042737-200410000-00003 [DOI] [PubMed] [Google Scholar]
- 19.Sarrabayrouse G, Bossard C, Chauvin JM, Jarry A, Meurette G, Quevrain E, Bridonneau C, Preisser L, Asehnoune K, Labarrière N et al.. CD4CD8alphaalpha lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol 2014; 12:e1001833; PMID:24714093; http://dx.doi.org/ 10.1371/journal.pbio.1001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senju M, Wu KC, Mahida YR, Jewell DP. Coexpression of CD4 and CD8 on peripheral blood T cells and lamina propria T cells in inflammatory bowel disease by two colour immunofluorescence and flow cytometric analysis. Gut 1991; 32:918-22; PMID:1885074; http://dx.doi.org/ 10.1136/gut.32.8.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagot M, Echchakir H, Mami-Chouaib F, Delfau-Larue MH, Charue D, Bernheim A, Chouaib S, Boumsell L, Bensussan A. Isolation of tumor-specific cytotoxic CD4+ and CD4+CD8dim+ T-cell clones infiltrating a cutaneous T-cell lymphoma. Blood 1998; 91:4331-41; PMID:9596682 [PubMed] [Google Scholar]
- 22.Desfrancois J, Derre L, Corvaisier M, Le Mevel B, Catros V, Jotereau F, Gervois N. Increased frequency of nonconventional double positive CD4CD8 alphabeta T cells in human breast pleural effusions. Int J Cancer 2009; 125:374-80; PMID:19358272; http://dx.doi.org/ 10.1002/ijc.24366 [DOI] [PubMed] [Google Scholar]
- 23.Desfrancois J, Moreau-Aubry A, Vignard V, Godet Y, Khammari A, Dreno B, Jotereau F, Gervois N. Double positive CD4CD8 alphabeta T cells: a new tumor-reactive population in human melanomas. PLoS One 2010; 5:e8437; PMID:20052413; http://dx.doi.org/ 10.1371/journal.pone.0008437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahemtullah A, Reichard KK, Preffer FI, Harris NL, Hasserjian RP. A double-positive CD4+CD8+ T-cell population is commonly found in nodular lymphocyte predominant Hodgkin lymphoma. Am J Clin Pathol 2006; 126:805-14; PMID:17050078; http://dx.doi.org/ 10.1309/Y8KD32QGRYFN1XQX [DOI] [PubMed] [Google Scholar]
- 25.Sarrabayrouse G, Corvaisier M, Ouisse LH, Bossard C, Le Mevel B, Potiron L, Meurette G, Gervois N, Jotereau F. Tumor-reactive CD4+ CD8alphabeta+ CD103+ alphabetaT cells: a prevalent tumor-reactive T-cell subset in metastatic colorectal cancers. Int J Cancer 2011; 128:2923-32; PMID:20824715; http://dx.doi.org/ 10.1002/ijc.25640 [DOI] [PubMed] [Google Scholar]
- 26.Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J Leukoc Biol 2015; 97:31-8; PMID:25360000; http://dx.doi.org/ 10.1189/jlb.1RU0814-382 [DOI] [PubMed] [Google Scholar]
- 27.Parrot T, Allard M, Oger R, Benlalam H, Raingeard de la Bletiere D, Coutolleau A, Preisser L, Desfrançois J, Khammari A, Dréno B et al.. IL-9 promotes the survival and function of human melanoma-infiltrating CD4(+) CD8(+) double-positive T cells. Eur J Immunol 2016; 46:1770-82; PMID:27094152; http://dx.doi.org/ 10.1002/eji.201546061 [DOI] [PubMed] [Google Scholar]
- 28.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol 2000; 67:2-17; PMID:10647992 [DOI] [PubMed] [Google Scholar]
- 29.Frentsch M, Stark R, Matzmohr N, Meier S, Durlanik S, Schulz AR, Stervbo U, Jürchott K, Gebhardt F, Heine G, et al.. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood 2013; 122:405-12; PMID:23719298; http://dx.doi.org/ 10.1182/blood-2013-02-483586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermann P, Van-Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol 1995; 25:2972-7; PMID:7589100; http://dx.doi.org/ 10.1002/eji.1830251039 [DOI] [PubMed] [Google Scholar]
- 31.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol 1992; 22:2573-8; PMID:1382991; http://dx.doi.org/ 10.1002/eji.1830221016 [DOI] [PubMed] [Google Scholar]
- 32.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998; 188:1679-89; PMID:9802980; http://dx.doi.org/ 10.1084/jem.188.9.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol 2007; 7:479-85; PMID:17479128; http://dx.doi.org/ 10.1038/nri2091 [DOI] [PubMed] [Google Scholar]
- 34.Cheroutre H, Husain MM. CD4 CTL: living up to the challenge. Semin Immunol 2013; 25:273-81; PMID:24246226; http://dx.doi.org/ 10.1016/j.smim.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 2002; 297:2060-3; PMID:12242444; http://dx.doi.org/ 10.1126/science.1072615 [DOI] [PubMed] [Google Scholar]
- 36.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 2001; 58:4-43; PMID:11229815; http://dx.doi.org/ 10.1007/PL00000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol 2006; 36:810-6; PMID:16541472; http://dx.doi.org/ 10.1002/eji.200535744 [DOI] [PubMed] [Google Scholar]
- 38.Le Gallou S, Caron G, Delaloy C, Rossille D, Tarte K, Fest T. IL-2 requirement for human plasma cell generation: coupling differentiation and proliferation by enhancing MAPK-ERK signaling. J Immunol 2012; 189:161-73; PMID:22634617; http://dx.doi.org/6438535 10.4049/jimmunol.1200301 [DOI] [PubMed] [Google Scholar]
- 39.Mingari MC, Gerosa F, Carra G, Accolla RS, Moretta A, Zubler RH, Waldmann TA, Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature 1984; 312:641-3; PMID:6438535; http://dx.doi.org/ 10.1038/312641a0 [DOI] [PubMed] [Google Scholar]
- 40.Howard M, Farrar J, Hilfiker M, Johnson B, Takatsu K, Hamaoka T, Paul WE. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med 1982; 155:914-23; PMID:6977612; http://dx.doi.org/ 10.1084/jem.155.3.914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kindler V, Zubler RH. Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J Immunol 1997; 159:2085-90; PMID:92782937537388 [PubMed] [Google Scholar]
- 42.Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science 1995; 268:720-2; PMID:7537388; http://dx.doi.org/ 10.1126/science.7537388 [DOI] [PubMed] [Google Scholar]
- 43.Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol 1998; 28:508-15; PMID:9521060; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199802)28:02%3c508::AID-IMMU508%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 44.Cocks BG, de Waal Malefyt R, Galizzi JP, de Vries JE, Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol 1993; 5:657-63; PMID:7688562; http://dx.doi.org/ 10.1093/intimm/5.6.657 [DOI] [PubMed] [Google Scholar]
- 45.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol 2006; 177:5236-47; PMID:17015709; http://dx.doi.org/22566959 10.4049/jimmunol.177.8.5236 [DOI] [PubMed] [Google Scholar]
- 46.Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, Ettinger R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 2007; 179:5886-96; PMID:17947662; http://dx.doi.org/22566959 10.4049/jimmunol.179.9.5886 [DOI] [PubMed] [Google Scholar]
- 47.Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front Immunol 2012; 3:78; PMID:22566959; http://dx.doi.org/ 10.3389/fimmu.2012.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen M, Sun Q, Wang J, Pan W, Ren X. Positive and negative functions of B lymphocytes in tumors. Oncotarget 2016; PMID:27331871; http://dx.doi.org/23454746 10.18632/oncotarget.10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A et al.. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Investig 2013; 123:1457-74; PMID:23454746; http://dx.doi.org/ 10.1172/JCI65579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karagiannis P, Villanova F, Josephs DH, Correa I, Van Hemelrijck M, Hobbs C, Saul L, Egbuniwe IU, Tosi I, Ilieva KM et al.. Elevated IgG4 in patient circulation is associated with the risk of disease progression in melanoma. Oncoimmunology 2015; 4:e1032492; PMID:26451312; http://dx.doi.org/ 10.1080/2162402X.2015.1032492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, de Vries JE. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol 1991; 147:8-13; PMID:1711085 8097323 [PubMed] [Google Scholar]
- 52.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, de Waal Malefyt R, de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA 1993; 90:3730-4; PMID:8097323; http://dx.doi.org/ 10.1073/pnas.90.8.3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer 2016; 4:40; PMID:27437104; http://dx.doi.org/ 10.1186/s40425-016-0145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemoine S, Morva A, Youinou P, Jamin C. Human T cells induce their own regulation through activation of B cells. J Autoimmun 2011; 36:228-38; PMID:21316922; http://dx.doi.org/ 10.1016/j.jaut.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 55.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529-42; PMID:25367570; http://dx.doi.org/ 10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miro F, Nobile C, Blanchard N, Lind M, Filipe-Santos O, Fieschi C, Chapgier A, Vogt G, de Beaucoudrey L, Kumararatne DS et al.. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J Immunol 2006; 177:3625-34; PMID:16951322; http://dx.doi.org/22688982 10.4049/jimmunol.177.6.3625 [DOI] [PubMed] [Google Scholar]
- 57.Simon T, Tanguy-Royer S, Royer PJ, Boisgerault N, Frikeche J, Fonteneau JF, Grégoire M. Human dendritic cells sequentially matured with CD4(+) T cells as a secondary signal favor CTL and long-term T memory cell responses. Biol Res 2012; 45:33-43; PMID:22688982; http://dx.doi.org/ 10.4067/S0716-97602012000100005 [DOI] [PubMed] [Google Scholar]
- 58.Wong KL, Lew FC, MacAry PA, Kemeny DM. CD40L-expressing CD8 T cells prime CD8alpha(+) DC for IL-12p70 production. Eur J Immunol 2008; 38:2251-62; PMID:18600823; http://dx.doi.org/ 10.1002/eji.200838199 [DOI] [PubMed] [Google Scholar]
- 59.Hernandez MG, Shen L, Rock KL. CD40-CD40 ligand interaction between dendritic cells and CD8+ T cells is needed to stimulate maximal T cell responses in the absence of CD4+ T cell help. J Immunol 2007; 178:2844-52; PMID:17312128; http://dx.doi.org/24498563 10.4049/jimmunol.178.5.2844 [DOI] [PubMed] [Google Scholar]
- 60.Llopiz D, Huarte E, Ruiz M, Bezunartea J, Belsue V, Zabaleta A, Lasarte JJ, Prieto J, Borrás-Cuesta F, Sarobe P. Helper cell-independent antitumor activity of potent CD8+ T cell epitope peptide vaccines is dependent upon CD40L. Oncoimmunology 2013; 2:e27009; PMID:24498563; http://dx.doi.org/ 10.4161/onci.27009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stark R, Hartung A, Zehn D, Frentsch M, Thiel A. IL-12-mediated STAT4 signaling and TCR signal strength cooperate in the induction of CD40L in human and mouse CD8+ T cells. Eur J Immunol 2013; 43:1511-7; PMID:23765345; http://dx.doi.org/ 10.1002/eji.201243218 [DOI] [PubMed] [Google Scholar]
- 62.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol 2010; 185:4977-82; PMID:20962266; http://dx.doi.org/18802153 10.4049/jimmunol.1001323 [DOI] [PubMed] [Google Scholar]
- 63.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L et al.. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410-7; PMID:18802153; http://dx.doi.org/ 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 64.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009; 4:e6412; PMID:19641607; http://dx.doi.org/ 10.1371/journal.pone.0006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nedergaard BS, Ladekarl M, Nyengaard JR, Nielsen K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol 2008; 108:106-11; PMID:17945335; http://dx.doi.org/ 10.1016/j.ygyno.2007.08.089 [DOI] [PubMed] [Google Scholar]
- 66.Gervois N, Labarriere N, Le Guiner S, Pandolfino MC, Fonteneau JF, Guilloux Y, Diez E, Dreno B, Jotereau F. High avidity melanoma-reactive cytotoxic T lymphocytes are efficiently induced from peripheral blood lymphocytes on stimulation by peptide-pulsed melanoma cells. Clin Cancer Res 2000; 6:1459-67; PMID:10778978 [PubMed] [Google Scholar]
- 67.Chauvin JM, Larrieu P, Sarrabayrouse G, Prevost-Blondel A, Lengagne R, Desfrancois J, Labarrière N, Jotereau F. HLA anchor optimization of the melan-A-HLA-A2 epitope within a long peptide is required for efficient cross-priming of human tumor-reactive T cells. J Immunol 2012; 188:2102-10; PMID:22291187; http://dx.doi.org/17646986 10.4049/jimmunol.1101807 [DOI] [PubMed] [Google Scholar]
- 68.Labarriere N, Gervois N, Bonnin A, Bouquie R, Jotereau F, Lang F. PBMC are as good a source of tumor-reactive T lymphocytes as TIL after selection by Melan-A/A2 multimer immunomagnetic sorting. Cancer Immunol Immunother 2008; 57:185-95; PMID:17646986; http://dx.doi.org/ 10.1007/s00262-007-0361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.