ABSTRACT
The correct regulation of tissue barriers is of utmost importance for health. Barrier dysfunction accompanies inflammatory disorders and, if not controlled properly, can contribute to the development of chronic diseases. Tissue barriers are formed by monolayers of epithelial cells that separate organs from their environment, and endothelial cells that cover the vasculature, thus separating the blood stream from underlying tissues. Cells within the monolayers are connected by intercellular junctions that are linked by adaptor molecules to the cytoskeleton, and the regulation of these interactions is critical for the maintenance of tissue barriers. Many endogenous and exogenous molecules are known to regulate barrier functions in both ways. Proinflammatory cytokines weaken the barrier, whereas anti-inflammatory mediators stabilize barriers. Adrenomedullin (ADM) and intermedin (IMD) are endogenous peptide hormones of the same family that are produced and secreted by many cell types during physiologic and pathologic conditions. They activate certain G-protein-coupled receptor complexes to regulate many cellular processes such as cytokine production, actin dynamics and junction stability. In this review, we summarize current knowledge about the barrier-stabilizing effects of ADM and IMD in health and disease.
KEYWORDS: actin cytoskeleton, adherens junction, cAMP, intermedin, PKA, Rap1, RhoA, ROCK, tight junction
Introduction
Tissue functionality is guaranteed by the proper regulation of its barriers, i.e. the endothelium and the epithelium.1 The endothelium lines the vasculature and ensures tissue supply with nutrients and oxygen. On the other hand, the epithelium forms the barrier between tissues and the outer environment thus protecting organs from invading harmful agents. Both barriers also play a critical role in the innate immune response to injury and infection. During inflammatory processes, the endothelium actively supports the recruitment of immune cells across the blood vessel wall into affected tissue areas.2-4 The epithelium forms a first line defense barrier for invading pathogens, however, if compromised, it also supports immune cells in their fight against microorganisms.5 Both, endothelium and epithelium form a cellular monolayer that is connected and sealed by so called junctions. Both cell types form tight junctions (TJ) that regulate the permeability of the monolayer and subjacent adherens junctions (AJ) that mediate intercellular adhesive interactions.6,7 Epithelial cells also form desmosomes that are located more basally and that further strengthen adhesive interactions. All these junctions are composed of several types of transmembrane proteins and cytosolic adaptor molecules that connect the adhesion molecules to the cytoskeleton. Desmosomes are connected to cytokeratins, whereas TJ and AJ are connected to actin. The connection of TJ and AJ to the actin cytoskeleton is critical for barrier functionality. Actin remodeling during inflammation regulates cell contact stability and permeability, and permits the transmigration of immune cells.8,9 If not controlled properly, this inflammatory response can also cause tissue damage. The inflammatory response of tissue barriers is initiated by pro-inflammatory cytokines produced by resident immune cells and the endothelium and epithelium themselves. Subsequently, other regulatory molecules are produced that help to control the inflammatory response by affecting actin dynamics and junction integrity. These molecules include peptide hormones such as adrenomedullin (ADM) and intermedin (IMD, also called ADM2).10-12 In this review, we summarize what is known about their regulation and explain how they affect endothelial and epithelial barriers. In particular, we will focus on the dynamics of the actin cytoskeleton and intercellular junctions downstream of ADM and IMD signaling.
Adrenomedullins: Gene and protein structure and regulation of expression
ADM is a peptide which was first isolated from pheochromocytoma (neuroendocrinal tumor of adrenal gland medullae) and demonstrated to be able to increase cAMP production in rat platelets. The authors also detected a high amount of ADM in the adrenal medulla, hence its name.13 ADM is composed of 52 amino acids in humans. It contains a disulfide bridge between their cysteine residues 16 and 21, thus forming a loop structure, and its C-terminal Tyr-residue is amidated.13 Both, the loop structure and amidation are characteristics conserved between species.
ADM belongs to the calcitonin gene peptide superfamily, including also calcitonin (CT), calcitonin gene-related peptide (CGRP), and amylin. ADM has a 24% amino acid homology with CGRP and both molecules have hypotensive effects.14
The functional human ADM is obtained from a precursor consisting of 185 amino acids termed preproadrenomedullin (preADM) including a 21 amino acid secretory signal peptide.15 PreADM is proteolytically processed yielding the 164 amino-acid peptide proadrenomedullin (proADM).15,16 ProADM is further proteolytically cleaved and amidated to generate the functional ADM peptide hormone and the proadrenomedullin N-terminal 20 peptide (PAMP)17 which both have hypotensive effects in rats.13,15,18
Mouse and rat ADM consist of 50 amino acids and their precursors contain 184 amino acids in mouse and 185 in rat.19,20 The porcine ADM precursor contains 188 amino acids and its mature ADM has 52 amino acids.21
Because in pufferfish cDNA 5 ADM paralogues were identified (ADM1-5),22 the presence of more ADM-like peptides was suspected in mammals. Genome database searching indeed revealed the presence of a sequence similar to fish ADM2 in human, mouse and rat. Thus, this peptide is referred to as ADM2, but also as intermedin (IMD), due to simultaneous discovery by another research group that identified its expression in the intermediate lobe of the pituitary gland.23,24 IMD is processed from a 148 amino acid peptide and contains a 24 amino acid secretory signal peptide localized in its N-terminus.23 This year the first report of IMD plasma concentrations in healthy humans was published.25 IMD was determined at 6.3±0.6 pg ml−1, a concentration much lower than that of ADM identified in the same study at 25.8 ± 1.8 pg ml−1.
Gene structure
Cloning, molecular analysis, and fluorescence in situ hybridization (FISH) revealed that the human ADM gene is located in the short arm of chromosome 11, region p15.4 (Fig. 1).26,27 The gene is formed by 4 exons and 3 introns. The first exon is not transcribed, the second and third exon encode for PAMP, while the whole coding sequence for ADM is located in the fourth exon.26 The 5′-end-ADM gene promoter region contains TATA, CAAT and GC boxes (Fig. 1). Its 5′-end also harbors binding sites for transcription factors such as activator protein-2 (AP-2), nuclear factor for interleukin-6 expression (NF-IL6), hypoxia-inducible factor-1 (HIF-1), cAMP response element (CRE), shear stress responsive element (SSRE), and the oncogenic transcription factor c-myc.26,28-33 A stem-loop structure in exon 1 of the human ADM gene has been reported that participates in the regulation of ADM transcription.34
Figure 1.
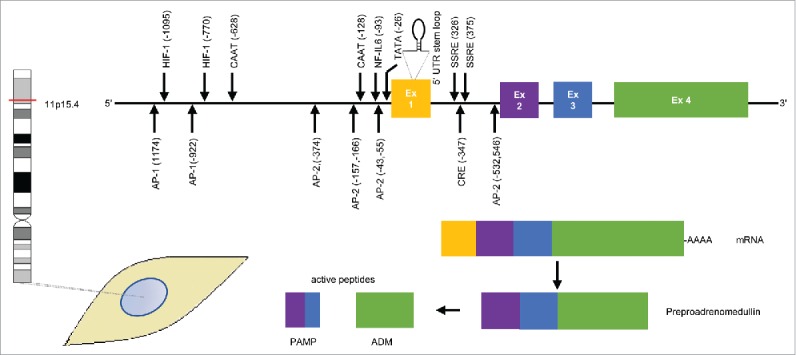
Gene and protein structures of adrenomedullin (ADM). The ADM gene located in chromosome 11 locus p15.4 contains 4 exons of which only 3 are translated into preproadrenomedullin which is then cleaved into the active peptides proadrenomedullin N-terminal 20 peptide (PAMP) and ADM. The second and third exon encode for PAMP, while the fourth exon encodes for ADM. There are several binding sites for transcription factors such as: TATA box; CAAT box; activator protein (AP)-1 and AP-2; HIF-1, hypoxia inducible factor-1; NF-IL-6, nuclear factor for interleukin-6 expression; CRE, cAMP responsive element; and SSRE, shear stress responsive elements (modified from Ref 31).
In mice, the ADM gene is located in chromosome 7. It has an amino acid similarity of 60% with the human ADM gene. The mouse ADM sequence also contains binding sites for AP-1, AP-2, specific-protein 1 (Sp1), GATA-2, nuclear factor kappa-light-chain-enhancer of activated B cells (NK-κB), NF-IL6, a shear-stress responsive element (SSRE), glucocorticoid response elements (GREs), and thyroid or T3 response elements (TREs).20
The mouse ADM2 or IMD gene is located in chromosome 22 in humans and chromosome 15 in mouse.23 The human IMD precursor is formed by 148-amino-acids. This precursor is cleaved into a 53 amino-acids peptide which is subsequently processed into a mature peptide of 47 amino-acids or another shorter variant of 40 amino-acids.24,35
Expression patterns of ADM and IMD
Hitherto, many studies have been published demonstrating human ADM mRNA expression in several tissues including pheochromocytoma, adrenal medulla, heart, lung and kidney.17 Expression of the ADM precursor was also found in all above mentioned tissues in rats and additionally in spleen, duodenum and submandibular glands.19 ADM expression was also detected in endothelial cells (ECs) isolated from rat thoracic aorta with mRNA levels even higher than in rat adrenal medulla.36 Later, expression of ADM in brain and neural tumors was demonstrated.37 ADM expression was confirmed in human umbilical vein endothelial cells (HUVEC) and in human glomerular epithelial cell lines.38,39
ADM expression has also been detected in haematopoietic cell lines including the human monocytic cell line THP-1 and the murine macrophage-like cell line RAW 264.7,40,41 and in primary human leukocytes.42
In the case of IMD, high mRNA expression levels have been found in brain and kidney, and low expression was detected in the pituitary gland and heart of human postmortem tissues.43 IMD is also strongly expressed in the mouse gastrointestinal tract, with highest expression in the stomach, in the submaxillary gland and kidney, whereas low expression was detected in the pituitary gland, lung, pancreas, spleen and thymus.44 Interestingly, IMD expression was detected in mouse ovaries but not in testes suggesting a specific role in the female reproductive system. 45
Transcriptional regulation
Vascular smooth muscle cells (VSMC) treated with TNF-α showed an increase in ADM expression as early as 1 h post-treatment and peaking after 48 h.46 Treatment of VSMC with IL-1α, IL-1β, TNF-β and lipopolysaccharide (LPS) also caused increases in both, ADM mRNA expression and peptide secretion.47 By contrast, human glomerular epithelial cells treated with TNF-α showed decreased ADM expression.39
Since the ADM gene harbors GREs and TREs, the effects of hormones on ADM expression have been analyzed. EC and VSCM cells stimulated with dexamethasone (DEX) strongly induced ADM mRNA expression, while T3 had only a slight effect.48 HUVEC treated with DEX also showed higher ADM expression.38 Hydrocortisone and cycloheximide also increased ADM expression in VSMC. As expected, the transcriptional inhibitor actinomycin D led to a decrease in ADM mRNA.49
In RAW 264.7 cells, incubation with LPS, retinoic acid (RA), 12-O-tetradecanoyl phorbol-13-acetate (TPA) and IFN-γ led to an increase in ADM transcription, whereas glucocorticoids decreased ADM expression.40 By contrast, forskolin, a substance known to increase levels of cAMP, and 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP), an activator of protein kinase A (PKA), had no effect on ADM expression.40
During many pathologic conditions such as hypertension, heart failure, burns, pancreatitis, systemic inflammatory response syndrome (SIRS), sepsis, and endocrine diseases such as hyperthyroidism, ADM mRNA expression was increased and correlated in most cases with higher ADM plasma levels suggesting that the principal regulation of ADM production occurs during transcription.50-55
Much less is known about IMD expression regulation. For example, human aortic endothelial cells (HAEC) and HUVEC increased expression of both IMD and ADM mRNA under different stress conditions such as serum starvation and oxidative stress induced by H2O2, with ADM mRNA levels being always higher compared to IMD.56 However, in the NCIH460 large lung epithelial carcinoma cell line the opposite was seen.56 Of note, serum deprivation of these cells for 12 or 24h and oxidative stress led to a stronger increase of IMD mRNA levels compared to ADM.56 IMD expression is regulated by an estrogen response element (ERE), and a hypoxia response element (HRE).24,57 Recently, it was reported that IMD is upregulated by an integrated stress response element (ISR), as demonstrated in the cervical cancer cell line HeLa and the colon carcinoma cell line HCT116 treated with wyxothiazol, an inhibitor of the mitochondrial cytochrome bc1 complex.58 This IMD mRNA overexpression was mediated by the activating transcription factor 4 (ATF4), a signal integrator which is activated downstream of different stress stimuli.
More studies are required to clearly define the differential transcriptional profile of ADM and IMD under pathophysiologic conditions, as well as the regulatory mechanisms involved.
Adrenomedullin receptors
ADM-mediated signal transduction occurs through receptor complexes consisting of the G-protein coupled receptor calcitonin-receptor-like receptor (CRLR) and one of the receptor activity-modifying proteins (RAMP), a family comprised of the 3 members RAMP1, 2 and 3. RAMPs contribute to CRLR translocation toward the plasma membrane. CRLR/RAMP2 and CRLR/RAMP3 are known as AM1 and AM2 receptors, respectively.59,60 ADM can also bind to CRLR/RAMP1, the receptor for CGRP, but with less affinity than CGRP.61
IMD is capable of binding to all AM1, AM2 and CGRP receptor, but with lower affinity than ADM as revealed by cAMP production levels in the cell line COS-7 expressing different combinations of CRLR and RAMPs.23
CRLR and RAMP2 mRNAs are expressed in rat heart, lung, kidney, brain, thymus, stomach, skeletal muscle, spinal cord, liver, spleen, and testis. Similar results were observed for RAMP1, with the exception of heart and kidney, where only very little expression was detected. RAMP3 was also expressed but at lower levels than RAMP1 and RAMP2 in the same tissues.62,63 However, in several human tissues RAMP3 mRNA is well expressed.64 mRNA expression of CRLR and RAMP2 has also been detected in human aortic smooth muscle cells (HASMC), human aortic endothelial cells (HAEC) and HUVEC, but neither RAMP1 nor RAMP3 expression was found in these cell types.65
mRNA expression of CRLR, RAMP1 and RAMP2 was also detected in the human colon epithelial cell line Col-29 and the neuroepithelial cell line SK-N-MC. However RAMP3 mRNA was not detectable in those cells.66 CRLR is expressed in endometrial and myometrial blood vessels but expression was very low in endometrial epithelium.67 Of the RAMPs, only RAMP1 was expressed in endometrial blood vessels, whereas all RAMP mRNAs were present in endometrial epithelium.
Hypoxia stimulates CRLR mRNA expression in human microvascular endothelial cells. However, expression of RAMP mRNA was unaffected.68 In the human colon carcinoma cell line Caco-2, hypoxia also induced CRLR mRNA expression, but not RAMP2.69
Additionally, lamina propria mononuclear cells and intra-epithelial lymphocytes isolated from Wistar rats expressed CRLR and all 3 RAMP transcripts.70
Because RAMP proteins determine the ligand and signaling specificity of the AMD and IMD receptor complexes, their distinct expression patterns may determine function specificity for AMD and IMD in different tissues.
Endothelial barrier functions regulated by adrenomedullin
EC from different species (human, rat, porcine and bovine) are known to actively produce ADM and secrete it into the blood stream where it contributes to regulate vascular tension.36 When ADM was first isolated by Kitamura and colleagues,13 they analyzed a potential role for this peptide in the regulation of vascular functions by monitoring blood pressure in Wistar rats injected i.v. with ADM. ADM treatment had a strong hypotensive effect that lasted up to 60 mins and was comparable to effects obtained with CGRP, one of the most potent vasorelaxants known. The authors suggested that ADM may exert its vasodilator effects via cAMP since other vasorelaxant molecules such as CGRP, peptide histidine isoleucine (PHI) and vasoactive intestinal polypeptide (VIP) are thought to bind receptors on platelets that increase cAMP levels.13 Later, the same group observed that inhibition of the CGRP receptor blocked ADM activity in the rat mesenteric vasculature suggesting that this is the main receptor for ADM in the mesentery.71 ADM indeed increases intracellular cAMP levels in different ECs (HUVEC, bovine aortic endothelial cells (BAEC), rat aorta endothelial cells (RAEC)),72,73,74 and this is the most prominent mechanism of ADM action, given that stimulation of rat cardiac myocytes and nonmyocytes with either CGRP or ADM resulted in similar cAMP increases, and inhibition using the CGRP antagonist CGRP(8–37) inhibited this increase completely. By contrast, no increases in cGMP levels were observed after stimulation with either ADM or CGRP.75
ADM-KO embryos die in utero at midgestation, due to massive edema and heart defects.76 The thoracic cavity of ADM-KO embryos was enlarged when compared to control littermates and hydrops fetalis could be observed all over the body and in neural structures. Hearts of ADM-KO were only 2 thirds the size of controls with increased left ventricular trabecular development resulting in smaller chamber sizes. The aorta and carotid from ADM-KO also showed abnormalities including thinner walls and irregular form.76 All these results show that ADM is important for cardiovascular development. Given the fact that ADM is produced by EC and its deletion resulted in hydrops fetalis in mice, it is reasonable to think that ADM is important for the regulation of endothelial barrier integrity (Table 1). Indeed, ADM treatment ameliorated H2O2- and thrombin-induced permeability in HUVEC.74 This effect was a result of diminished myosin light chain (MLC) phosphorylation, actomyosin contractility and intercellular gap (cell-cell contact discontinuities) formation. As expected, this was accompanied by increased cAMP levels with no changes in the basal content of cGMP, basal intracellular Ca2+-concentrations, or H2O2-, thrombin- or E. coli-induced increases in Ca2+ concentrations in HUVEC.74 cAMP activation downstream of ADM can also lead to the activation of protein kinase A (PKA) and the Rap1 guanylate exchange factor (GEF) exchange protein directly activated by cAMP (EPAC) consequently inhibiting RhoA activation and actomyosin contractility (Fig. 2).77,78
Table 1.
Molecular mechanisms by which ADM and IMD contribute to endothelial barrier homeostasis.
| Model | Protein | Mechanism | Ref |
|---|---|---|---|
| ADRENOMEDULLIN | |||
| RAMP2 KO mice | RAMP2 | Activation of Rac1, inhibition of RhoA | 79 |
| HUVEC | cAMP | Reduction of MLC2 phosphorylation, stress fibers and gap formation. Increased VE-cadherin-mediated adhesion | 74 |
| HMEC, MLEC, HUVEC, cortactin KO mice | Cortactin | Regulation of ADM secretion, inhibition of MLC2 phosphorylation and stress fibers and reversal of increased vascular permeability | 83 |
| INTERMEDIN | |||
| Wistar Rat | p38MAPK/NF-κB | Reduced p38MAPK phosphorylation and ICAM-1 expression | 93 |
| HMVEC-L | CRLR/RAMP2/PKA-VASP | Activation of PKA and phosphorylation of VASP | 94,96 |
| RCEC, HUVEC | RhoA/ROCK-Rac1 CRLR/RAMP2/cAMP/EPAC/PKA |
Inhibition of Rac1 and RhoA/ROCK pathway. Inhibition of RhoA/ROCK, increased activation of Rac1 and VE-cadherin-mediated adhesion | 96,97 |
| HPMVEC C57BL/6 mice with VILI |
Not described (RAMP3?) |
Increased endothelial TER. Reduced VILI-induced hyperpermeability | 98 |
Figure 2.
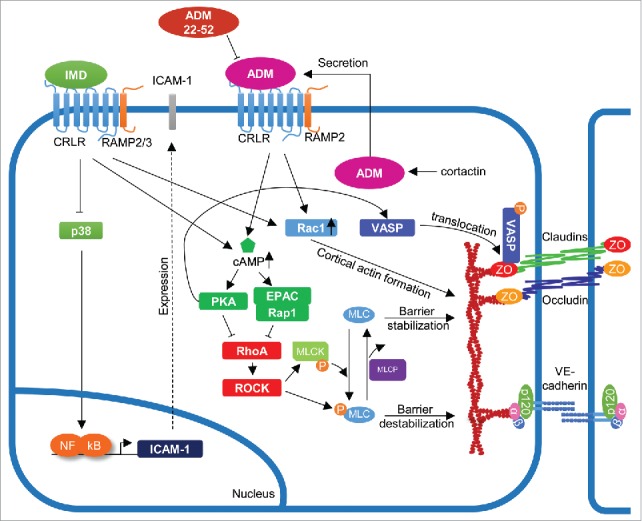
Signaling pathways involved in endothelial barrier regulation by adrenomedullin (AMD) and intermedin (IMD). ADM and IMD trigger similar signaling cascades, leading to increased production of cAMP and activation of PKA and EPAC/Rap1. Generally, these pathways lead to endothelial barrier stabilization by inhibiting activation of RhoA/ROCK and promoting Rac1 function, thus maintaining inter-endothelial cell contacts.
Both ADM and CGRP act through 7-transmembrane-domain-containing G-protein-coupled receptors (GPCRs) (Fig. 2).64 Xenopus oocytes transfected with human calcitonin-receptor-like receptor (CRLR) and different receptor-activity-modifying proteins (RAMP) revealed that CGRP was able to stimulate CRLR coupled to RAMP1 but not to RAMP2 or RAMP3. On the other hand, ADM was able to stimulate CRLR when coupled to RAMP2 at concentrations as low as 0.3 nM. Importantly, RAMP2 was not able to respond without CRLR, and HEK294T cells that co-expressed CRLR and RAMP2 were able to induce cAMP production upon ADM stimulation.64 Recently, the activity of RAMP2 was found to be important for EC viability, integrity and homeostasis.11 In this study, RAMP2-KO mice died at midgestation, while EC-specific RAMP2-KO survived until later development but most of these surviving embryos died after birth (only 5% survived until adulthood). These mice showed systemic edema, interstitial edema in the intestinal villi and the lung and severe hemorrhage in the liver. Additionally, the animals presented vascular abnormalities such as malformation of aortic EC. The few animals surviving into adulthood showed thinner aortic walls accompanied by lower systemic blood pressure and deformed EC detaching from the basement membrane. EC-specific drug-inducible RAMP2-KO in adult mice resulted in systemic edema formation due to increased vascular permeability.79 This phenotype was a consequence of reduced Rac1 activity and increased RhoA activation leading to a decrease in stabilizing cortical actin and increased actin stress fiber formation. These data demonstrate the importance of the CRLR/RAMP2 axis for ADM-mediated endothelial barrier development and homeostasis. By contrast, EC-specific ADM-KO survive relatively unaffected, most likely due to the fact that other cell types also secrete ADM that can compensate for the lack of EC-derived ADM. Association of CRLR with RAMP3 also gives an ADM-responsive receptor that is important for lymphatic vessel functions.80
In vivo, ADM is also implicated in lung homeostasis and repair. For example, Itoh and colleagues found that during LPS-induced acute lung injury (ALI) in rats, ADM administration reduced neutrophil numbers in bronchoalveolar lavage fluid (BALF) and improved the histologic lung injury score and vascular permeability.81 In addition, ADM was critical for normal alveolar development in a model of bronchopulmonary dysplasia (BPD) induced by hyperoxia in newborn rats, since intranasal ADM treatment was capable of reducing hyperoxia-induced right ventricular hypertrophy and artery thickness leading to reduced lung hypertension.82 ADM treatment in this model also blocked BPD-mediated arrest in alveolar growth.
We have recently described that depletion of the actin-binding protein (ABP) cortactin in EC and cortactin deficiency in mice caused reduced ADM expression and secretion resulting in increased MLC phosphorylation, a higher content of contractile actin stress fibers and increased vascular permeability.83 Importantly, ADM administration was sufficient to completely rescue the permeability increase in vitro and in vivo. Cortactin deficiency also caused reduced basal Rap1 activation.83,84 Thus, reduced ADM secretion in the absence of cortactin leads to reduced cAMP production in EC causing less activation of EPAC/Rap1 and increased actomyosin contractility. Rap1 has been reported to inhibit RhoA activity via Rasip1/ArhGAP29 in endothelial and epithelial cells,85,86 thus providing a possible explanation of how ADM mediates its protective effects on the endothelial barrier via Rap1 activation and RhoA inhibition downstream of cAMP production (Fig. 2).
ADM contributes to the stabilization of lymphatic endothelial cells since ADM treatment of human dermal lymphatic microvascular EC (HMVEC-dLys) prevented endothelial hyperpermeability caused by vascular endothelial growth factor A (VEGFA) due to enhanced ZO-1 and VE-Cadherin expression at cell contacts; although no changes in the total protein or mRNA levels were observed.87 In brain microvascular endothelial cells (BMEC) obtained from rats, ADM treatment for 6 h increased protein levels of claudin-5 and changed its distribution from a zipper-like to a linear pattern at cell-cell contacts in a dose dependent manner.88 These changes were accompanied by reduced transendothelial electrical resistance and increased permeability to sodium fluorescein. However, no changes in Evans Blue permeability were observed after ADM treatment. Another study found no changes in the protein expression levels of junctional markers such as occludin, claudin-1 or ZO-1 after ADM treatment, while still having a protective effect.89 These data suggest that ADM likely promotes endothelial barrier stabilization by conserving the location of junctional molecules at intercellular contacts rather than modulating their expression.
It is important to remember that different pro-inflammatory stimuli such as LPS90 or hypoxia91 increase ADM production demonstrating that ADM is also important for vascular homeostasis under pathological conditions by counteracting the barrier destabilizing effects of pro-inflammatory mediators.73
Endothelial barrier functions regulated by intermedin
IMD has also been shown to be important for vascular barrier regulation by binding to receptor complexes formed by CRLR and RAMPs, and is thus also capable of raising intracellular cAMP levels and to protect against hypertension (Fig. 2; Table 1).92 Overexpression of human IMD in rats after treatment with deoxycorticosterone acetate salt to induce hypertension and renal damage, reduced systolic blood pressure when compared to control animals. Additionally, IMD also attenuated renal histological damage and inflammation as demonstrated by reduced expression of intercellular adhesion molecule 1 (ICAM-1) and monocyte/macrophage accumulation in kidney immunohistochemical sections. The protective effect mediated by IMD in this model was due to reduced p38MAPK activation and reduced NF-κB-dependent ICAM-1 expression.93
Interestingly, hypoxia-induced lung damage in mice led to an increase in IMD mRNA expression levels in lungs.57 Similar results were observed with primary pulmonary microvascular endothelial cells (PMEC) that were subjected to hypoxia in vitro. This increase in IMD levels was mediated by hypoxia-inducible factor 1 (HIF-1).57 Human microvascular endothelial cells of the lung (HMVEC-L) treated with IMD showed reduced permeability for trypan-blue-labeled albumin under basal conditions and after thrombin treatment that was accompanied by increased phosphorylation of vasodilator-stimulated phosphoprotein (VASP) at serine 157.94 VASP is a well-known PKA substrate involved in endothelial barrier regulation and its phosphorylation at serine 157 induces the translocation of VASP to cell contacts where it binds to ZO-1 and stabilizes the endothelial barrier. Thus, IMD may act in a similar way to ADM by activating PKA via cAMP (Fig. 2).78 Of note, IMD levels were higher than that of ADM in PMEC.57
ADM predominantly signals through CRLR/RAMP2 and CRLR/RAMP3,62 whereas IMD primarily signals via CRLR/RAMP1 or CRLR/RAMP3, and to a lesser extent also via CRLR/RAMP2.44 Given that hypoxia induces an increase in RAMP1 and RAMP3 in rat lungs but not of RAMP2,95 it seems likely that IMD has a more critical role in vascular barrier homeostasis in the lung than ADM.
IMD may have different effects on vascular function depending on the vascular bed. While rat coronary microvascular endothelial cells (RCEC) showed increased permeability for macromolecules, HUVEC showed reduced permeability when treated with IMD under basal conditions.96 Additionally, RCEC treated with IMD showed less VE-cadherin at cell borders and intercellular gap formation, whereas control cells had a more linear distribution of VE-cadherin at cell contacts. Under basal conditions RCEC had many actin stress fibers and only a thin line of cortical actin at the cell periphery. After IMD treatment, the actin cytoskeleton appeared as so called knots of filaments leading to the loss of cell limits and shape alterations. By contrast, in HUVEC, the same treatment concentrated VE-cadherin at cell borders thus preventing gap formation and the actin cytoskeleton shifted from actin stress fibers to cortical actin thus stabilizing the cell contacts. These data suggest that effects of IMD on the actin cytoskeleton dictate the outcome for barrier integrity in different EC types. Interestingly, in both cell types, IMD led to reduced RhoA/ROCK pathway activity, which explains less presence of stress fibers in HUVEC. However, in RCEC, IMD also reduced the activity of Rac1, while in HUVEC, IMD rather promoted Rac1 activity. Inhibition of both RhoA/Rac1 by IMD in RCEC may explain why these cells lost their morphology, while HUVEC still retained Rac1 activity which reinforced the content of cortical actin.96 It is unknown how IMD can exert such differential effects on GTPase activation and this will be critical to unravel in the future for a better understanding of IMD signaling. Later, the same group found that IMD could protect HUVEC from thrombin-induced permeability. This effect was mediated by the binding of IMD to the CRLR/RAMP2 receptor complex since HUVEC cells only expressed CRLR and RAMP2 as measured by real-time PCR. In addition, treatment of these cells with the ADM receptor antagonist AM22-52 prevented IMD-mediated permeability reduction. IMD induced the production of cAMP to activate EPAC and PKA signaling pathways leading to activation of myosin light chain phosphatase and Rac1 and inhibition of the RhoA/ROCK axis, stress fiber formation and an increase of VE-cadherin at cell contacts (Fig. 2).97 In human pulmonary microvascular endothelial cells (HPMVEC), IMD improved endothelial barrier stability as measured by transendothelial electrical resistance (TER).98 In the same study, IMD also attenuated vascular hyperpermeability in a model of ventilator-induced lung injury (VILI) in C57BL/6 mice when compared to non-treated animals. However, the treatment was not capable of reducing pulmonary inflammation induced by VILI since the pulmonary and plasma levels of several pro-inflammatory cytokines including IL-1β, IL-6, KC, MCP-1 or IL-10 did not change.
In summary, both ADM and IMD are key regulators of vascular functions in different tissues (Fig. 2). Thus, these peptide hormones could be used as therapeutic agents in inflammatory diseases where improved vascular homeostasis is required for a better outcome, but more studies are needed in order to further understand the molecular pathways regulated by these molecules. This is important in light of the controversial results that have been obtained with ADM and IMD as therapeutic agents during sepsis. In one study, blocking of ADM resulted in an improved catecholamine response attenuating the systemic inflammatory response in mice.99 Moreover, ADM administration via the carotid artery proved to be beneficial in female C57BL/6 mice with VILI as mice showed less lung injury and permeability to human serum albumin.100 However, another report showed a positive correlation between ADM levels in plasma and disease severity in patients suffering from SIRS suggesting that ADM could be used as marker for sepsis progression.55 In line with this, combined blocking of ADM and eNOS in rats with LPS-induced endotoxemia improved survival by 50% compared to septic animals without inhibitors.101 ADM could rather be detrimental during late phases of sepsis because of its hypotensive effects, thus compromising oxygen/blood delivery to different vascular beds. On the other hand, IMD treatment in rats with cecal-ligation and puncture (CLP)-induced sepsis improved survival when compared to septic non-treated animals, an effect mediated by improved cardiac function leading to better tissue perfusion/oxygenation.102 Thus, the potent vasodilatory capabilities of ADM and IMD may under certain conditions outweigh the beneficial effects on vascular barrier function, so that their use in certain diseases such as sepsis has to be strictly supervised as it may also worsen the conditions of patients.
Epithelial barrier functions regulated by adrenomedullin
Similar to the endothelial barrier, the epithelial barrier is regulated by dynamic interactions between the epithelial cell-cell junctions, the actin cytoskeleton and several signaling pathways (Fig. 3). Dysregulation of these well-orchestrated interactions can lead to acute inflammation or, in severe cases, to chronic disorders such as inflammatory bowel diseases (IBD), chronic obstructive pulmonary disease (COPD) and asthma.
Figure 3.
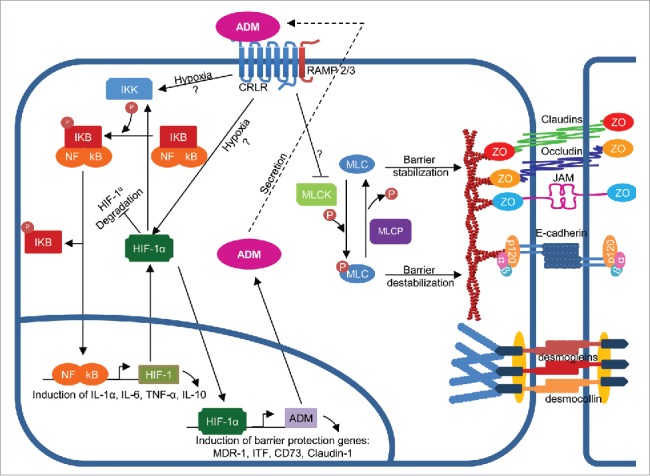
Signaling pathways involved in epithelial barrier regulation by adrenomedullin (AMD). ADM signaling contributes to epithelial barrier stabilization. During hypoxia, ADM activates NFĸB that in turn activates transcription of cytokines and HIF-1α. ADM inhibits HIF-1α degradation allowing it to translocate to the nucleus thus promoting transcription of barrier protecting genes and more ADM resulting in a positive feedback loop. ADM also inhibits MLCK activity to reduce p-MLC, thus preventing internalization of TJ and AJ proteins. Question marks: unknown mechanisms.
ADM is basally expressed by many types of human, mouse and rat epithelial cells including bronchial and uterine epithelium,103 oral and skin keratinocytes,104 and the human gastric adenocarcinoma cell line AGS.105,106 ADM gene expression is up-regulated in the presence of pro-inflammatory cytokines such as TNFα, IFN-γ, IL-1β and IL-6.106,107 While the diverse effects of ADM in endothelial barrier functions are well studied, less is known about its involvement in epithelial barrier regulation. The protective effects of ADM have been tested in various animal models of experimental colitis (Table 2). Amelioration of clinical severity, prevention of histological damage and downregulation of TNF-α, IFN-γ, IL-6, IL-1β and IL-12 were observed in animals with TNBS-induced colitis after ADM treatment.108-110 During DSS-induced colitis, ADM treatment resulted in a significant decrease of inflammatory features such as intestinal bleeding, weight loss and shortening of the colon. Histological damage was reduced and proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IFN-γ, IL-4, IL-12 and KC were downregulated.69,111,112 In this model, ADM also triggered wound healing and tissue regeneration.111 In a clinical pilot study with 7 refractory ulcerative colitis (UC) patients, intravenous ADM application for 14 d lowered the DAI and improved mucosal healing highlighting the potential of ADM as treatment option for IBD patients.113 This is in line with findings that ADM promotes gastric epithelial wound healing.114,115 Such anti-inflammatory, protective effects of ADM were also observed in animals after induction of colitis with acetic acid.116 Barrier stabilizing effects of ADM have also been reported in vitro (Table 3). For example, treatment of the intestinal epithelial cell line CMT93 with IFN-γ to induce inflammation increased permeability and induced downregulation of occludin, E-cadherin and β-catenin, and these effects were prevented when cells were co-treated with ADM.112 A decrease of permeability for macromolecules and ions was also observed in Caco-2 cells with ADM when cells were challenged with staphylococcal α-toxin or H2O2.117 The exact mechanism of how ADM contributes to epithelial barrier integrity still remains elusive. Numerous studies have demonstrated that ADM contributes to the stabilization of endothelial barrier function by increasing intracellular cAMP levels and, in consequence, activation of the EPAC and PKA signaling pathways.74,118,119 ADM-induced elevation of cAMP has also been reported in epithelial cells, but in these studies a direct effect of ADM-induced barrier function has not been investigated. 120,121 Nevertheless, many other studies have shown that increased cAMP concentrations can improve epithelial barriers,122-124 suggesting that ADM-induced cAMP would contribute to epithelial barrier protection. By contrast, a recent study suggests that ADM may stabilize the epithelial barrier via cAMP-independent mechanisms.117 Caco-2 cells with barrier dysfunction induced by H2O2 or Staphylococcus aureus α-toxin showed improved barrier function when co-treated with ADM. Surprisingly, this occurred without changes in intracellular cAMP levels. In the same model, specific inhibitors for phosphodiesterases 3 and 4 were tested to observe whether they were preventing cAMP accumulation, but cAMP levels were not elevated and epithelial barrier function was still improved.117 These data suggest that the barrier-stabilizing effects of ADM are not cAMP-dependent in epithelial cells. Alternatively, several kinases participate in the regulation of epithelial barrier function. For example, PKC regulates TJ integrity,125-127 PI3K enhances epithelial cell restitution and upregulates the expression of TJ proteins,128,129 and ERK and p38 also upregulate TJ protein expression.130-132 ADM-treated Caco-2 cells in which barrier dysfunction had been induced by H2O2 were co-incubated with inhibitors against PKC, PI3K, ERK or p38. However, the barrier-stabilizing effect of ADM was not prevented by the inhibition of these kinases.117 Even though Caco-2 cells show several morphological and biochemical characteristics of intestinal enterocytes and they are widely being used as in vitro model of the intestinal epithelial barrier, it is important to keep in mind that different factors such as passage number, substrate and medium composition/pH can affect cell morphology and functionality.133 Thus, more studies are needed using different epithelial cell lines or mouse models and applying various barrier disruptive agents to clearly define the molecular mechanisms of ADM action in different types of epithelia. Certainly other kinases are also involved in barrier regulation, for example ROCK and MLCK phosphorylate MLC and induce actomyosin contractility. However, these pathways cause differential effects. MLC phosphorylation by ROCK induces a strong contraction causing junction disassembly and epithelial barrier dysfunction. By contrast, MLC phosphorylation by MLCK results in a moderate increase in permeability without disassembly of cell-cell junctions.9 ADM has been shown to downregulate MLCK-dependent phosphorylation of MLC during TNB-Sinduced colitis,134 suggesting that this pathway could be responsible for mediating the protective effects of ADM on intestinal epithelial barrier functions (Fig. 3). This is also in line with our observations that inhibition of ROCK-dependent MLC phosphorylation ameliorates intestinal epithelial barrier dysfunction in the absence of cortactin (unpublished own data) indicating that MLC phosphorylation is indeed a major event to induce epithelial barrier dysfunction.
Table 2.
Effects of ADM on cytokine production in experimental colitis models in vivo.
| Model | Species | Route | Time of admn. | Molecules | Ref |
|---|---|---|---|---|---|
| TNBS-colitis | Balb/c mice SJL mice Wistar rats |
I.P. I.P. |
Treatment Pre-treatment |
↓ TNF-α ↓ IL-6 ↓ MIP-2 ↓ IL-1β ↑ IL-10 ↓ IL-12 ↓ IFN-γ |
108-110 |
| DSS-colitis | BALB/c mice C57BL/6 mice C57BL/6 mice |
I.P. I.R. I.P. |
Pre-treatment Treatment Treatment |
↓ TNF-α ↓ IL-1β ↓ IL-6 ↓ IFN-γ ↑ TGF-β ↓ P-STAT1 ↓ P-STAT3 ↓ KC ↓ IL-4 ↓ IL-12 |
69,111,112 |
| Acetic acid-colitis | Wistar rats | I.R. | Treatment | ↓ IL-6 | 116 |
Table 3.
Effects of ADM on epithelial barrier function in vitro.
During inflammation, hypoxia occurs which induces activation of transcription factors such as NF-kB and hypoxia-inducible factor-1 (HIF-1). NF-kB is involved in the expression of many proinflammatory cytokines such as TNF-α and IL-1β. Elevated levels of these cytokines can induce epithelial barrier dysfunction.135 Thus, a role of ADM in NK-kB-mediated barrier regulation was expected. Indeed, the keratinocyte cell line HaCaT incubated with ADM showed increased IĸB degradation, NF-ĸB translocation to the nucleus and elevated levels of IL-6 (Fig. 3).136 Thus, the cellular context and the inflammatory environment are obviously important factors that determine the outcome of ADM signaling. Interestingly, exogenous ADM activates HIF in Caco-2 cells.69 HIFs are central regulators of metabolism during hypoxia. There are 3 different HIF isoforms in mammals: HIF-1α, HIF-2α, and HIF-3α. HIF-1α activation confers barrier protection during inflammation by inducing the expression of barrier protective genes such as multidrug resistance gene-1 (MDR1), intestinal trefoil factor (ITF), CD73 and claudin-1.137,138 Recently, ADM expression has been shown to be also regulated by HIF,139,140 suggesting a potential feedback loop through which the ADM-mediated barrier stabilizing effect could be potentiated (Fig. 3).
Moreover, ADM has also been characterized as a bactericidal peptide. It was demonstrated by disc diffusion and broth microdilution assays that at high concentrations (60 pmol 1−1) ADM inhibited the growth of microorganisms derived from the skin (Propionibacterium acnes, Staphylococcus aureus and Micrococcus luteus), the mouth (Porphyromonas gingivalis, Actinomyces naeslundii, Streptococcus mutans, Eikenella corrodens and Actinobacillus actinomycetemcomitans), the respiratory tract (Streptococcus pneumoniae, Haemophilus in≤uenzae and Streptococcus pyogenes) and the gastrointestinal tract (Bacteroides fragilis, Escherichia coli and Helicobacter pylori).141 Of note, ADM is a more potent inhibitor of E. coli growth than β-defensin-2 and α-defensin-1.142 The secretion of ADM is augmented in the presence of both Gram-positive and Gram-negative bacteria.105 When human H357 oral keratinocytes were exposed to microorganisms commonly found in the oral cavity such as Porphyromonas gingivalis, Streptococcus mutans and Eikenella corrodens, ADM mRNA and protein levels were upregulated. However, this was not observed with the yeast Candida albicans. These data suggest that ADM might participate in the maintenance of host-microbiota homeostasis that greatly affects epithelial barrier integrity. By doing so, ADM could contribute to the prevention of bacterial overgrowth and translocation in IBD patients.
As previously mentioned, IMD is widely expressed in various tissues and its function as an endothelial barrier stabilizer has been explored. However, the participation of IMD in epithelial barrier function has not been investigated yet.
Common pathways regulated by ADM in endothelium and epithelium
As mentioned before, ADM is very well known for its anti-inflammatory and barrier protective effects in both endothelial and epithelial cells.69,83,87,88,117,134 Most of our knowledge comes from studies in endothelial cells, whereas less is known about epithelial cells. However, in both cell types ADM increases intracellular cAMP levels,72-74,120,121 suggesting that ADM exerts its barrier effects via common molecular effectors. cAMP activates the EPAC/Rap1 pathway to enhance cortical actin formation via Rac1 and thus barrier stability in endothelial cells.143 Rap1 has been shown to enhance E-cadherin-mediated adhesion via Rac1 in the epithelial cell line HEK293,144 implicating that ADM, via cAMP, stabilizes the epithelial barrier also through activation of EPAC/Rap1. The cAMP-EPAC/Rap1 pathway inhibits the RhoA-ROCK axis to prevent vascular permeability.83,85 These data suggest that ADM activates a canonical cAMP-dependent pathway to regulate endothelial and epithelial barriers.
Another consequence of ADM treatment, observed in both endothelial and epithelial cells, is the downregulation of pro-inflammatory cytokines by preventing NF-kB activation.110-112,145 During hypoxia, IMD and AMD levels are increased by HIF-1α activation in both endothelial and epithelial cells,57,69 suggesting that both peptides might be exerting their barrier function protective effects in response to the activation of this transcription factor. Despite several similarities of ADM functions in endothelial and epithelial cells, ADM effects in epithelial cells require better characterization to conclude that ADM has redundant functions in both cell types. It is likely that IMD also stabilizes the epithelial barrier as IMD binds the same receptor complexes, albeit with different affinities. However, this will have to be carefully studied in the future.
Conclusions
Both ADM and IMD have been show to enhance endothelial barrier function by inducing cAMP production and thus stabilizing cortical actin and junction architecture. However, protection of the intestinal epithelial barrier by ADM may also occur independent of cAMP. As observed in basic in vivo and clinical studies, improved epithelial wound healing is involved but the underlying mechanisms are still incompletely understood and require further investigation. Whether IMD also protects the epithelial barrier during inflammation remains to be analyzed. Given the promising in vivo data on tissue barrier protection, it is tempting to propose the use of ADM and IMD as therapeutic agents for chronic inflammatory diseases. However, the differential effects that ADM causes during different stages of sepsis clearly show the Janus-like properties of these hormones. Carefully executed animal in vivo studies will be needed to determine the effects of ADM and IMD during various stages of a given disease, the correct doses and route of application to avoid adverse effects in subsequent clinical studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Work in the laboratory of MS is supported by grants from the Mexican Council for Science and Technology (Conacyt, 207268 and 233395)
References
- [1].Schnoor M, Parkos CA. Disassembly of endothelial and epithelial junctions during leukocyte transmigration. Front Biosci 2008; 13:6638-52; PMID:18508684; http://dx.doi.org/ 10.2741/3178 [DOI] [PubMed] [Google Scholar]
- [2].Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol 2014; 184:886-96; PMID:24655376; http://dx.doi.org/ 10.1016/j.ajpath.2013.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schnoor M. Endothelial actin-binding proteins and actin dynamics in leukocyte transendothelial migration. J Immunol 2015; 194:3535-41; PMID:25848070; http://dx.doi.org/ 10.4049/jimmunol.1403250 [DOI] [PubMed] [Google Scholar]
- [4].Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol 2015; 15:692-704; PMID:26471775; http://dx.doi.org/ 10.1038/nri3908 [DOI] [PubMed] [Google Scholar]
- [5].Parkos CA. Neutrophil-Epithelial Interactions: A Double-Edged Sword. Am J Pathol 2016; 186:1404-16; PMID:27083514; http://dx.doi.org/ 10.1016/j.ajpath.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ivanov AI, Naydenov NG. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol 2013; 303:27-99; PMID:23445808; http://dx.doi.org/ 10.1016/B978-0-12-407697-6.00002-7 [DOI] [PubMed] [Google Scholar]
- [7].Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 2014; 36:157-65; PMID:25171873; http://dx.doi.org/ 10.1016/j.semcdb.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Garcia-Ponce A, Citalan-Madrid AF, Velazquez-Avila M, Vargas-Robles H, Schnoor M. The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb Haemost 2015; 113:20-36; PMID:25183310; http://dx.doi.org/ 10.1160/TH14-04-0298 [DOI] [PubMed] [Google Scholar]
- [9].Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010; 177:512-24; PMID:20581053; http://dx.doi.org/ 10.2353/ajpath.2010.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kato J, Kitamura K. Bench-to-bedside pharmacology of adrenomedullin. Eur J Pharmacol 2015; 764:140-8; PMID:26144371; http://dx.doi.org/ 10.1016/j.ejphar.2015.06.061 [DOI] [PubMed] [Google Scholar]
- [11].Koyama T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Shindo T. Adrenomedullin-RAMP2 System in Vascular Endothelial Cells. J Atheroscler Thromb 2015; 22:647-53; PMID:26040754; http://dx.doi.org/ 10.5551/jat.29967 [DOI] [PubMed] [Google Scholar]
- [12].Martinez-Herrero S, Martinez A. Adrenomedullin regulates intestinal physiology and pathophysiology. Domest Anim Endocrinol 2016; 56 Suppl:S66-83; PMID:27345325; http://dx.doi.org/ 10.1016/j.domaniend.2016.02.004 [DOI] [PubMed] [Google Scholar]
- [13].Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993; 192:553-60; PMID:8387282; http://dx.doi.org/ 10.1006/bbrc.1993.1451 [DOI] [PubMed] [Google Scholar]
- [14].Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol 1997; 11:167-239; PMID:9209829; http://dx.doi.org/ 10.1615/CritRevNeurobiol.v11.i2-3.40 [DOI] [PubMed] [Google Scholar]
- [15].Ishiyama Y, Kitamura K, Ichiki Y, Nakamura S, Kida O, Kangawa K, Eto T. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol 1993; 241:271-3; PMID:8243562; http://dx.doi.org/ 10.1016/0014-2999(93)90214-3 [DOI] [PubMed] [Google Scholar]
- [16].Saito H, Minamiya Y, Kitamura M, Saito S, Enomoto K, Terada K, Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol 1998; 161:1533-40; PMID:9686621 [PubMed] [Google Scholar]
- [17].Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun 1993; 194:720-5; PMID:7688224; http://dx.doi.org/ 10.1006/bbrc.1993.1881 [DOI] [PubMed] [Google Scholar]
- [18].Kitamura K, Kangawa K, Ishiyama Y, Washimine H, Ichiki Y, Kawamoto M, Minamino N, Matsuo H, Eto T. Identification and hypotensive activity of proadrenomedullin N-terminal 20 peptide (PAMP). FEBS Lett 1994; 351:35-7; PMID:8076689; http://dx.doi.org/ 10.1016/0014-5793(94)00810-8 [DOI] [PubMed] [Google Scholar]
- [19].Sakata J, Shimokubo T, Kitamura K, Nakamura S, Kangawa K, Matsuo H, Eto T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun 1993; 195:921-7; PMID:7690563; http://dx.doi.org/ 10.1006/bbrc.1993.2132 [DOI] [PubMed] [Google Scholar]
- [20].Okazaki T, Ogawa Y, Tamura N, Mori K, Isse N, Aoki T, Rochelle JM, Taketo MM, Seldin MF, Nakao K. Genomic organization, expression, and chromosomal mapping of the mouse adrenomedullin gene. Genomics 1996; 37:395-9; PMID:8938454; http://dx.doi.org/ 10.1006/geno.1996.0576 [DOI] [PubMed] [Google Scholar]
- [21].Kitamura K, Kangawa K, Kojima M, Ichiki Y, Matsuo H, Eto T. Complete amino acid sequence of porcine adrenomedullin and cloning of cDNA encoding its precursor. FEBS Lett 1994; 338:306-10; PMID:8043068; http://dx.doi.org/ 10.1016/0014-5793(94)80289-0 [DOI] [PubMed] [Google Scholar]
- [22].Ogoshi M, Inoue K, Takei Y. Identification of a novel adrenomedullin gene family in teleost fish. Biochem Biophys Res Commun 2003; 311:1072-7; PMID:14623291; http://dx.doi.org/ 10.1016/j.bbrc.2003.10.111 [DOI] [PubMed] [Google Scholar]
- [23].Takei Y, Hyodo S, Katafuchi T, Minamino N. Novel fish-derived adrenomedullin in mammals: structure and possible function. Peptides 2004; 25:1643-56; PMID:15476931; http://dx.doi.org/ 10.1016/j.peptides.2004.06.026 [DOI] [PubMed] [Google Scholar]
- [24].Lin Chang C, Roh J, Park JI, Klein C, Cushman N, Haberberger RV, Hsu SY. Intermedin functions as a pituitary paracrine factor regulating prolactin release. Mol Endocrinol 2005; 19:2824-38; PMID:16002435; http://dx.doi.org/ 10.1210/me.2004-0191 [DOI] [PubMed] [Google Scholar]
- [25].Bell D, Gordon BJ, Lavery A, Megaw K, Kinney MO, Harbinson MT. Plasma levels of intermedin (adrenomedullin-2) in healthy human volunteers and patients with heart failure. Peptides 2016; 76:19-29; PMID:26767798; http://dx.doi.org/ 10.1016/j.peptides.2015.12.003 [DOI] [PubMed] [Google Scholar]
- [26].Ishimitsu T, Kojima M, Kangawa K, Hino J, Matsuoka H, Kitamura K, Eto T, Matsuo H. Genomic structure of human adrenomedullin gene. Biochem Biophys Res Commun 1994; 203:631-9; PMID:8074714; http://dx.doi.org/ 10.1006/bbrc.1994.2229 [DOI] [PubMed] [Google Scholar]
- [27].Ishimitsu T, Hosoya K, Tsukada K, Minami J, Ono H, Ohrui M, Hino J, Kangawa K, Matsuoka H. Chromosomal sublocalization and microsatellite DNA polymorphism of human adrenomedullin gene. Peptides 2001; 22:1739-44; PMID:11754959; http://dx.doi.org/ 10.1016/S0196-9781(01)00531-9 [DOI] [PubMed] [Google Scholar]
- [28].Cormier-Regard S, Nguyen SV, Claycomb WC. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 1998; 273:17787-92; PMID:9651380; http://dx.doi.org/ 10.1074/jbc.273.28.17787 [DOI] [PubMed] [Google Scholar]
- [29].Ishimitsu T, Miyata A, Matsuoka H, Kangawa K. Transcriptional regulation of human adrenomedullin gene in vascular endothelial cells. Biochem Biophys Res Commun 1998; 243:463-70; PMID:9480831; http://dx.doi.org/ 10.1006/bbrc.1998.8110 [DOI] [PubMed] [Google Scholar]
- [30].Shinoki N, Kawasaki T, Minamino N, Okahara K, Ogawa A, Ariyoshi H, Sakon M, Kambayashi J, Kangawa K, Monden M. Shear stress down-regulates gene transcription and production of adrenomedullin in human aortic endothelial cells. J Cell Biochem 1998; 71:109-15; PMID:9736459; http://dx.doi.org/ 10.1002/(SICI)1097-4644(19981001)71:1%3c109::AID-JCB11%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- [31].Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 2000; 21:138-67; PMID:10782362 [DOI] [PubMed] [Google Scholar]
- [32].Ishimitsu T, Tsukada K, Minami J, Ono H, Matsuoka H. Adrenomedullin gene In: Nishikimi T, ed. Adrenomedullin in Cardiovascular Disease. Boston, MA: Springer US, 2005; 41-60 [Google Scholar]
- [33].Ozawa N, Shichiri M, Fukai N, Yoshimoto T, Hirata Y. Regulation of adrenomedullin gene transcription and degradation by the c-myc gene. Endocrinology 2004; 145:4244-50; PMID:15192039; http://dx.doi.org/ 10.1210/en.2003-0402 [DOI] [PubMed] [Google Scholar]
- [34].Brenet F, Dussault N, Delfino C, Boudouresque F, Chinot O, Martin PM, Ouafik LH. Identification of secondary structure in the 5′-untranslated region of the human adrenomedullin mRNA with implications for the regulation of mRNA translation. Oncogene 2006; 25:6510-9; PMID:16715138; http://dx.doi.org/ 10.1038/sj.onc.1209672 [DOI] [PubMed] [Google Scholar]
- [35].Yang JH, Jia YX, Pan CS, Zhao J, Ouyang M, Yang J, Chang JK, Tang CS, Qi YF. Effects of intermedin(1-53) on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun 2005; 327:713-9; PMID:15649405; http://dx.doi.org/ 10.1016/j.bbrc.2004.12.071 [DOI] [PubMed] [Google Scholar]
- [36].Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun 1994; 201:1160-6; PMID:8024557; http://dx.doi.org/ 10.1006/bbrc.1994.1827 [DOI] [PubMed] [Google Scholar]
- [37].Satoh F, Takahashi K, Murakami O, Totsune K, Sone M, Ohneda M, Abe K, Miura Y, Hayashi Y, Sasano H, et al.. Adrenomedullin in human brain, adrenal glands and tumor tissues of pheochromocytoma, ganglioneuroblastoma and neuroblastoma. J Clin Endocrinol Metab 1995; 80:1750-2; PMID:7745031 [DOI] [PubMed] [Google Scholar]
- [38].Ishihara T, Kato J, Kitamura K, Katoh F, Fujimoto S, Kangawa K, Eto T. Production of adrenomedullin in human vascular endothelial cells. Life Sci 1997; 60:1763-9; PMID:9150416; http://dx.doi.org/ 10.1016/S0024-3205(97)00136-7 [DOI] [PubMed] [Google Scholar]
- [39].Lai KN, Leung JC, Yeung VT, Lewis LK, Nicholls MG. Gene transcription and synthesis of adrenomedullin by cultured human renal cells. Biochem Biophys Res Commun 1998; 244:567-72; PMID:9514865; http://dx.doi.org/ 10.1006/bbrc.1998.8167 [DOI] [PubMed] [Google Scholar]
- [40].Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, Dohi K, Matsuo H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem 1998; 273:16730-8; PMID:9642228; http://dx.doi.org/ 10.1074/jbc.273.27.16730 [DOI] [PubMed] [Google Scholar]
- [41].Nakayama M, Takahashi K, Kitamuro T, Murakami O, Shirato K, Shibahara S. Transcriptional control of adrenomedullin induction by phorbol ester in human monocytic leukemia cells. Eur J Biochem 2000; 267:3559-66; PMID:10848972; http://dx.doi.org/ 10.1046/j.1432-1327.2000.01384.x [DOI] [PubMed] [Google Scholar]
- [42].Trollmann R, Schoof E, Beinder E, Wenzel D, Rascher W, Dotsch J. Adrenomedullin gene expression in human placental tIssue and leukocytes: a potential marker of severe tIssue hypoxia in neonates with birth asphyxia. Eur J Endocrinol 2002; 147:711-6; PMID:12444904; http://dx.doi.org/ 10.1530/eje.0.1470711 [DOI] [PubMed] [Google Scholar]
- [43].Takahashi K, Kikuchi K, Maruyama Y, Urabe T, Nakajima K, Sasano H, Imai Y, Murakami O, Totsune K. Immunocytochemical localization of adrenomedullin 2/intermedin-like immunoreactivity in human hypothalamus, heart and kidney. Peptides 2006; 27:1383-9; PMID:16359754; http://dx.doi.org/ 10.1016/j.peptides.2005.11.004 [DOI] [PubMed] [Google Scholar]
- [44].Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 2004; 279:7264-74; PMID:14615490; http://dx.doi.org/ 10.1074/jbc.M305332200 [DOI] [PubMed] [Google Scholar]
- [45].Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 2004; 556:53-8; PMID:14706825; http://dx.doi.org/ 10.1016/S0014-5793(03)01368-1 [DOI] [PubMed] [Google Scholar]
- [46].Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun 1994; 203:719-26; PMID:8074727; http://dx.doi.org/ 10.1006/bbrc.1994.2241 [DOI] [PubMed] [Google Scholar]
- [47].Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Interleukin-1, tumor necrosis factor and lipopolysaccharide additively stimulate production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun 1995; 207:25-32; PMID:7857273; http://dx.doi.org/ 10.1006/bbrc.1995.1148 [DOI] [PubMed] [Google Scholar]
- [48].Imai T, Hirata Y, Iwashina M, Marumo F. Hormonal regulation of rat adrenomedullin gene in vasculature. Endocrinology 1995; 136:1544-8; PMID:7895664 [DOI] [PubMed] [Google Scholar]
- [49].Minamino N, Shoji H, Sugo S, Kangawa K, Matsuo H. Adrenocortical steroids, thyroid hormones and retinoic acid augment the production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun 1995; 211:686-93; PMID:7794283; http://dx.doi.org/ 10.1006/bbrc.1995.1866 [DOI] [PubMed] [Google Scholar]
- [50].Wilson C, Nikitenko LL, Sargent IL, Rees MC. Adrenomedullin: multiple functions in human pregnancy. Angiogenesis 2004; 7:203-12; PMID:15609075; http://dx.doi.org/ 10.1007/s10456-004-4183-5 [DOI] [PubMed] [Google Scholar]
- [51].Kohno M, Hanehira T, Kano H, Horio T, Yokokawa K, Ikeda M, Minami M, Yasunari K, Yoshikawa J. Plasma adrenomedullin concentrations in essential hypertension. Hypertension 1996; 27:102-7; PMID:8591870; http://dx.doi.org/ 10.1161/01.HYP.27.1.102 [DOI] [PubMed] [Google Scholar]
- [52].Nishikimi T, Horio T, Sasaki T, Yoshihara F, Takishita S, Miyata A, Matsuo H, Kangawa K. Cardiac production and secretion of adrenomedullin are increased in heart failure. Hypertension 1997; 30:1369-75; PMID:9403555; http://dx.doi.org/ 10.1161/01.HYP.30.6.1369 [DOI] [PubMed] [Google Scholar]
- [53].Hirata Y, Mitaka C, Sato K, Nagura T, Tsunoda Y, Amaha K, Marumo F. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. J Clin Endocrinol Metab 1996; 81:1449-53; PMID:8636349 [DOI] [PubMed] [Google Scholar]
- [54].Taniyama M, Kitamura K, Ban Y, Eto T, Katagiri T. Elevated plasma adrenomedullin level in hyperthyroidism. Eur J Clin Invest 1996; 26:454-6; PMID:8817157; http://dx.doi.org/ 10.1046/j.1365-2362.1996.155307.x [DOI] [PubMed] [Google Scholar]
- [55].Ueda S, Nishio K, Minamino N, Kubo A, Akai Y, Kangawa K, Matsuo H, Fujimura Y, Yoshioka A, Masui K, et al.. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med 1999; 160:132-6; PMID:10390390; http://dx.doi.org/ 10.1164/ajrccm.160.1.9810006 [DOI] [PubMed] [Google Scholar]
- [56].Pearson LJ, Yandle TG, Nicholls MG, Evans JJ. Intermedin (adrenomedullin-2): a potential protective role in human aortic endothelial cells. Cell Physiol Biochem 2009; 23:97-108; PMID:19255504; http://dx.doi.org/ 10.1159/000204098 [DOI] [PubMed] [Google Scholar]
- [57].Pfeil U, Aslam M, Paddenberg R, Quanz K, Chang CL, Park JI, Gries B, Rafiq A, Faulhammer P, Goldenberg A, et al.. Intermedin/adrenomedullin-2 is a hypoxia-induced endothelial peptide that stabilizes pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol 2009; 297:L837-45; PMID:19684198; http://dx.doi.org/ 10.1152/ajplung.90608.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kovaleva IE, Garaeva AA, Chumakov PM, Evstafieva AG. Intermedin/Adrenomedullin 2 is a stress-inducible gene controlled by Activating Transcription Factor 4. Gene 2016; PMID:27328454 [DOI] [PubMed] [Google Scholar]
- [59].Hay DL, Conner AC, Howitt SG, Smith DM, Poyner DR. The pharmacology of adrenomedullin receptors and their relationship to CGRP receptors. J Mol Neurosci 2004; 22:105-13; PMID:14742915; http://dx.doi.org/ 10.1385/JMN:22:1-2:105 [DOI] [PubMed] [Google Scholar]
- [60].Kuwasako K, Kitamura K, Nagata S, Hikosaka T, Takei Y, Kato J. Shared and separate functions of the RAMP-based adrenomedullin receptors. Peptides 2011; 32:1540-50; PMID:21645567; http://dx.doi.org/ 10.1016/j.peptides.2011.05.022 [DOI] [PubMed] [Google Scholar]
- [61].Hay DL, Walker CS, Poyner DR. Adrenomedullin and calcitonin gene-related peptide receptors in endocrine-related cancers: opportunities and challenges. Endocr Relat Cancer 2011; 18:C1-14; PMID:21051558; http://dx.doi.org/ 10.1677/ERC-10-0244 [DOI] [PubMed] [Google Scholar]
- [62].Chakravarty P, Suthar TP, Coppock HA, Nicholl CG, Bloom SR, Legon S, Smith DM. CGRP and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) in rat tissues. Br J Pharmacol 2000; 130:189-95; PMID:10781016; http://dx.doi.org/ 10.1038/sj.bjp.0702975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nagae T, Mukoyama M, Sugawara A, Mori K, Yahata K, Kasahara M, Suganami T, Makino H, Fujinaga Y, Yoshioka T, et al.. Rat receptor-activity-modifying proteins (RAMPs) for adrenomedullin/CGRP receptor: cloning and upregulation in obstructive nephropathy. Biochem Biophys Res Commun 2000; 270:89-93; PMID:10733909; http://dx.doi.org/ 10.1006/bbrc.2000.2390 [DOI] [PubMed] [Google Scholar]
- [64].McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998; 393:333-9; PMID:9620797; http://dx.doi.org/ 10.1038/30666 [DOI] [PubMed] [Google Scholar]
- [65].Kamitani S, Asakawa M, Shimekake Y, Kuwasako K, Nakahara K, Sakata T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett 1999; 448:111-4; PMID:10217420; http://dx.doi.org/ 10.1016/S0014-5793(99)00358-0 [DOI] [PubMed] [Google Scholar]
- [66].Choksi T, Hay DL, Legon S, Poyner DR, Hagner S, Bloom SR, Smith DM. Comparison of the expression of calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) with CGRP and adrenomedullin binding in cell lines. Br J Pharmacol 2002; 136:784-92; PMID:12086988; http://dx.doi.org/ 10.1038/sj.bjp.0704761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nikitenko LL, Brown NS, Smith DM, MacKenzie IZ, Bicknell R, Rees MC. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol Hum Reprod 2001; 7:655-64; PMID:11420389; http://dx.doi.org/ 10.1093/molehr/7.7.655 [DOI] [PubMed] [Google Scholar]
- [68].Nikitenko LL, Smith DM, Bicknell R, Rees MC. Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. FASEB J 2003; 17:1499-501; PMID:12824306 [DOI] [PubMed] [Google Scholar]
- [69].MacManus CF, Campbell EL, Keely S, Burgess A, Kominsky DJ, Colgan SP. Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J 2011; 25:1856-64; PMID:21350119; http://dx.doi.org/ 10.1096/fj.10-170316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hagner S, Knauer J, Haberberger R, Goke B, Voigt K, McGregor GP. Calcitonin receptor-like receptor is expressed on gastrointestinal immune cells. Digestion 2002; 66:197-203; PMID:12592095; http://dx.doi.org/ 10.1159/000068365 [DOI] [PubMed] [Google Scholar]
- [71].Nuki C, Kawasaki H, Kitamura K, Takenaga M, Kangawa K, Eto T, Wada A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun 1993; 196:245-51; PMID:8216298; http://dx.doi.org/ 10.1006/bbrc.1993.2241 [DOI] [PubMed] [Google Scholar]
- [72].Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem 1995; 270:4412-7; PMID:7876206; http://dx.doi.org/ 10.1074/jbc.270.9.4412 [DOI] [PubMed] [Google Scholar]
- [73].Isumi Y, Shoji H, Sugo S, Tochimoto T, Yoshioka M, Kangawa K, Matsuo H, Minamino N. Regulation of adrenomedullin production in rat endothelial cells. Endocrinology 1998; 139:838-46; PMID:9492011 [DOI] [PubMed] [Google Scholar]
- [74].Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krull M, Seybold J, Seeger W, Rascher W, Schutte H, et al.. Adrenomedullin reduces endothelial hyperpermeability. Circ Res 2002; 91:618-25; PMID: 12364390; http://dx.doi.org/ 10.1161/01.RES.0000036603.61868.F9 [DOI] [PubMed] [Google Scholar]
- [75].Nishikimi T, Horio T, Yoshihara F, Nagaya N, Matsuo H, Kangawa K. Effect of adrenomedullin on cAMP and cGMP levels in rat cardiac myocytes and nonmyocytes. Eur J Pharmacol 1998; 353:337-44; PMID:9726664; http://dx.doi.org/ 10.1016/S0014-2999(98)00400-2 [DOI] [PubMed] [Google Scholar]
- [76].Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci U S A 2001; 98:615-9; PMID:11149956; http://dx.doi.org/ 10.1073/pnas.98.2.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 2005; 105:1950-5; PMID:15374886; http://dx.doi.org/ 10.1182/blood-2004-05-1987 [DOI] [PubMed] [Google Scholar]
- [78].Miyashita K, Itoh H, Sawada N, Fukunaga Y, Sone M, Yamahara K, Yurugi-Kobayashi T, Park K, Nakao K. Adrenomedullin provokes endothelial Akt activation and promotes vascular regeneration both in vitro and in vivo. FEBS Lett 2003; 544:86-92; PMID:12782295; http://dx.doi.org/ 10.1016/S0014-5793(03)00484-8 [DOI] [PubMed] [Google Scholar]
- [79].Koyama T, Ochoa-Callejero L, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Iinuma N, Arai T, Yoshizawa T, Iesato Y, Lei Y, et al.. Vascular endothelial adrenomedullin-RAMP2 system is essential for vascular integrity and organ homeostasis. Circulation 2013; 127:842-53; PMID:23355623; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.112.000756 [DOI] [PubMed] [Google Scholar]
- [80].Yamauchi A, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Igarashi K, Toriyama Y, Tanaka M, Liu T, Xian X, et al.. Functional differentiation of RAMP2 and RAMP3 in their regulation of the vascular system. J Mol Cell Cardiol 2014; 77:73-85; PMID:25264174; http://dx.doi.org/ 10.1016/j.yjmcc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- [81].Itoh T, Obata H, Murakami S, Hamada K, Kangawa K, Kimura H, Nagaya N. Adrenomedullin ameliorates lipopolysaccharide-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2007; 293:L446-52; PMID:17557801; http://dx.doi.org/ 10.1152/ajplung.00412.2005 [DOI] [PubMed] [Google Scholar]
- [82].Vadivel A, Abozaid S, van Haaften T, Sawicka M, Eaton F, Chen M, Thebaud B. Adrenomedullin promotes lung angiogenesis, alveolar development, and repair. Am J Respir Cell Mol Biol 2010; 43:152-60; PMID:19738161; http://dx.doi.org/ 10.1165/rcmb.2009-0004OC [DOI] [PubMed] [Google Scholar]
- [83].Garcia Ponce A, Citalan Madrid AF, Vargas Robles H, Chanez Paredes S, Nava P, Betanzos A, Zarbock A, Rottner K, Vestweber D, Schnoor M. Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility. Sci Rep 2016; 6:29003; PMID:27357373; http://dx.doi.org/ 10.1038/srep29003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med 2011; 208:1721-35; PMID:21788407; http://dx.doi.org/ 10.1084/jem.20101920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci U S A 2013; 110:11427-32; PMID:23798437; http://dx.doi.org/ 10.1073/pnas.1306595110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Post A, Pannekoek WJ, Ponsioen B, Vliem MJ, Bos JL. Rap1 Spatially Controls ArhGAP29 To Inhibit Rho Signaling during Endothelial Barrier Regulation. Mol Cell Biol 2015; 35:2495-502; PMID:25963656; http://dx.doi.org/ 10.1128/MCB.01453-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dunworth WP, Fritz-Six KL, Caron KM. Adrenomedullin stabilizes the lymphatic endothelial barrier in vitro and in vivo. Peptides 2008; 29:2243-9; PMID:18929609; http://dx.doi.org/ 10.1016/j.peptides.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, Niwa M. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell Mol Neurobiol 2006; 26:109-18; PMID:16763778; http://dx.doi.org/ 10.1007/s10571-006-9028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kis B, Snipes JA, Deli MA, Abraham CS, Yamashita H, Ueta Y, Busija DW. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir Suppl 2003; 86:565-8; PMID:14753507 [DOI] [PubMed] [Google Scholar]
- [90].Zaks-Zilberman M, Salkowski CA, Elsasser T, Cuttitta F, Vogel SN. Induction of adrenomedullin mRNA and protein by lipopolysaccharide and paclitaxel (Taxol) in murine macrophages. Infect Immun 1998; 66:4669-75; PMID:9746563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nagata D, Hirata Y, Suzuki E, Kakoki M, Hayakawa H, Goto A, Ishimitsu T, Minamino N, Ono Y, Kangawa K, et al.. Hypoxia-induced adrenomedullin production in the kidney. Kidney Int 1999; 55:1259-67; PMID:10200989; http://dx.doi.org/ 10.1046/j.1523-1755.1999.00361.x [DOI] [PubMed] [Google Scholar]
- [92].Owji AA, Chabot JG, Dumont Y, Quirion R. Adrenomedullin-2/intermedin induces cAMP accumulation in dissociated rat spinal cord cells: evidence for the existence of a distinct class of sites of action. J Mol Neurosci 2008; 35:355-61; PMID:18418734; http://dx.doi.org/ 10.1007/s12031-008-9062-x [DOI] [PubMed] [Google Scholar]
- [93].Hagiwara M, Bledsoe G, Yang ZR, Smith RS Jr., Chao L, Chao J. Intermedin ameliorates vascular and renal injury by inhibition of oxidative stress. Am J Physiol Renal Physiol 2008; 295:F1735-43; PMID:18829738; http://dx.doi.org/ 10.1152/ajprenal.90427.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 2002; 16:583-5; PMID:11919161 [DOI] [PubMed] [Google Scholar]
- [95].Qing X, Svaren J, Keith IM. mRNA expression of novel CGRP1 receptors and their activity-modifying proteins in hypoxic rat lung. Am J Physiol Lung Cell Mol Physiol 2001; 280:L547-54; PMID:11159039 [DOI] [PubMed] [Google Scholar]
- [96].Aslam M, Gunduz D, Schuler D, Li L, Sharifpanah F, Sedding D, Piper HM, Noll T. Intermedin induces loss of coronary microvascular endothelial barrier via derangement of actin cytoskeleton: role of RhoA and Rac1. Cardiovasc Res 2011; 92:276-86; PMID:21816966; http://dx.doi.org/ 10.1093/cvr/cvr213 [DOI] [PubMed] [Google Scholar]
- [97].Aslam M, Pfeil U, Gunduz D, Rafiq A, Kummer W, Piper HM, Noll T. Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin-induced barrier failure in endothelial cell monolayers. Br J Pharmacol 2012; 165:208-22; PMID:21671901; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Muller-Redetzky HC, Kummer W, Pfeil U, Hellwig K, Will D, Paddenberg R, Tabeling C, Hippenstiel S, Suttorp N, Witzenrath M. Intermedin stabilized endothelial barrier function and attenuated ventilator-induced lung injury in mice. PLoS One 2012; 7:e35832; PMID:22563471; http://dx.doi.org/ 10.1371/journal.pone.0035832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wagner K, Wachter U, Vogt JA, Scheuerle A, McCook O, Weber S, Groger M, Stahl B, Georgieff M, Moller P, et al.. Adrenomedullin binding improves catecholamine responsiveness and kidney function in resuscitated murine septic shock. Intensive Care Med Exp 2013; 1:21; PMID:26266790; http://dx.doi.org/ 10.1186/2197-425X-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Muller-Redetzky HC, Will D, Hellwig K, Kummer W, Tschernig T, Pfeil U, Paddenberg R, Menger MD, Kershaw O, Gruber AD, et al.. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Crit Care 2014; 18:R73; PMID:24731244; http://dx.doi.org/ 10.1186/cc13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hyvelin JM, Shan Q, Bourreau JP. Adrenomedullin: a cardiac depressant factor in septic shock. J Card Surg 2002; 17:328-35; PMID:12546081; http://dx.doi.org/ 10.1111/j.1540-8191.2001.tb01152.x [DOI] [PubMed] [Google Scholar]
- [102].Li T, Yu Z, Huiling W, Yue W, Jie Z, Xiaoyong P, Jiatao Z, Xinming X, Liu L. Beneficial effect of intermedin 1-53 in septic shock rats: contributions of RHO kinase and BKCA pathway-mediated improvement in cardiac function. Shock 2016; http://dx.doi.org/ 10.1097/SHK.0000000000000639 [DOI] [PubMed] [Google Scholar]
- [103].Cameron VA, Fleming AM. Novel sites of adrenomedullin gene expression in mouse and rat tissues. Endocrinology 1998; 139:2253-64; PMID:9564831 [DOI] [PubMed] [Google Scholar]
- [104].Kapas S, Tenchini ML, Farthing PM. Regulation of adrenomedullin secretion in cultured human skin and oral keratinocytes. J Invest Dermatol 2001; 117:353-9; PMID:11511315; http://dx.doi.org/ 10.1046/j.0022-202x.2001.01426.x [DOI] [PubMed] [Google Scholar]
- [105].Kapas S, Bansal A, Bhargava V, Maher R, Malli D, Hagi-Pavli E, Allaker RP. Adrenomedullin expression in pathogen-challenged oral epithelial cells. Peptides 2001; 22:1485-9; PMID:11514033; http://dx.doi.org/ 10.1016/S0196-9781(01)00470-3 [DOI] [PubMed] [Google Scholar]
- [106].Allaker RP, Kapas S. Adrenomedullin expression by gastric epithelial cells in response to infection. Clin Vaccine Immunol 2003; 10:546-51; PMID:12853384; http://dx.doi.org/ 10.1128/CDLI.10.4.546-551.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Udono T, Takahashi K, Nakayama M, Murakami O, Durlu YK, Tamai M, Shibahara S. Adrenomedullin in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2000; 41:1962-70; PMID:10845623 [PubMed] [Google Scholar]
- [108].Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn's disease. Gut 2006; 55:824-32; PMID:16401687; http://dx.doi.org/ 10.1136/gut.2005.084525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kono T, Kaneko A, Hira Y, Suzuki T, Chisato N, Ohtake N, Miura N, Watanabe T. Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn's disease mouse model. J Crohns Colitis 2010; 4:161-70; PMID:21122500; http://dx.doi.org/ 10.1016/j.crohns.2009.09.006 [DOI] [PubMed] [Google Scholar]
- [110].Talero E, Sanchez-Fidalgo S, de la Lastra CA, Illanes M, Calvo JR, Motilva V. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides 2008; 29:2001-12; PMID:18708104; http://dx.doi.org/ 10.1016/j.peptides.2008.07.013 [DOI] [PubMed] [Google Scholar]
- [111].Hayashi Y, Narumi K, Tsuji S, Tsubokawa T, Nakaya MA, Wakayama T, Zuka M, Ohshima T, Yamagishi M, Okada T. Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: insights from in vitro and in vivo experimental studies. Int J Colorectal Dis 2011; 26:1453-62; PMID:21674139; http://dx.doi.org/ 10.1007/s00384-011-1254-0 [DOI] [PubMed] [Google Scholar]
- [112].Ashizuka S, Inagaki-Ohara K, Kuwasako K, Kato J, Inatsu H, Kitamura K. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol 2009; 53:573-81; PMID:19780971; http://dx.doi.org/ 10.1111/j.1348-0421.2009.00159.x [DOI] [PubMed] [Google Scholar]
- [113].Ashizuka S, Inatsu H, Kita T, Kitamura K. Adrenomedullin therapy in patients with refractory ulcerative colitis: a case series. Dig Dis Sci 2016; 61:872-80; PMID:26470867; http://dx.doi.org/ 10.1007/s10620-015-3917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fukuda K, Tsukada H, Oya M, Onomura M, Kodama M, Nakamura H, Hosokawa M, Seino Y. Adrenomedullin promotes epithelial restitution of rat and human gastric mucosa in vitro. Peptides 1999; 20:127-32; PMID:10098633; http://dx.doi.org/ 10.1016/S0196-9781(98)00146-6 [DOI] [PubMed] [Google Scholar]
- [115].Hashimoto H, Akimoto M, Maeda A, Shigemoto M, Yamashita K, Yokoyama I. Changes in vasoactive substances during gastric ulcer healing. J Cardiovasc Pharmacol 2000; 36:S278-81; PMID:11078398; http://dx.doi.org/ 10.1097/00005344-200036051-00082 [DOI] [PubMed] [Google Scholar]
- [116].Ashizuka S, Ishikawa N, Kato J, Yamaga J, Inatsu H, Eto T, Kitamura K. Effect of adrenomedullin administration on acetic acid-induced colitis in rats. Peptides 2005; 26:2610-5; PMID:15978699; http://dx.doi.org/ 10.1016/j.peptides.2005.05.007 [DOI] [PubMed] [Google Scholar]
- [117].Temmesfeld-Wollbruck B, Brell B, zu Dohna C, Dorenberg M, Hocke AC, Martens H, Klar J, Suttorp N, Hippenstiel S. Adrenomedullin reduces intestinal epithelial permeability in vivo and in vitro. Am J Physiol Gastrointest Liver Physiol 2009; 297:G43-51; PMID:19423749; http://dx.doi.org/ 10.1152/ajpgi.90532.2008 [DOI] [PubMed] [Google Scholar]
- [118].Liverani E, Paul C. Adrenomedullin and Glucocorticoids interaction at the glial/endothelial interface: two sides of the same regulatory coin? Recept Clin Investigat 2014; 1:e25; http://dx.doi.org/ 10.14800/rci25 [DOI] [Google Scholar]
- [119].Nikitenko LL, MacKenzie IZ, Rees MC, Bicknell R. Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod 2000; 6:811-9; PMID:10956553; http://dx.doi.org/ 10.1093/molehr/6.9.811 [DOI] [PubMed] [Google Scholar]
- [120].Kawaguchi T, Kanazawa H, Hirata K, Kurihara N, Yoshikawa J. Adrenomedullin stimulates cyclic AMP production in the airway epithelial cells of guinea-pigs and in the human epithelial cell line. Allergol Int 1999; 48:53-9; http://dx.doi.org/ 10.1046/j.1440-1592.1999.00117.x [DOI] [Google Scholar]
- [121].Disa J, Dang K, Tan KB, Aiyar N. Interaction of adrenomedullin with calcitonin receptor in cultured human breast cancer cells, T 47D. Peptides 1998; 19:247-51; PMID:9493856; http://dx.doi.org/ 10.1016/S0196-9781(97)00376-8 [DOI] [PubMed] [Google Scholar]
- [122].Duffey ME, Hainau B, Ho S, Bentzel CJ. Regulation of epithelial tight junction permeability by cyclic AMP. Nature 1981; 294:451-3; PMID:6273740; http://dx.doi.org/ 10.1038/294451a0 [DOI] [PubMed] [Google Scholar]
- [123].Friedman GB, Taylor CT, Parkos CA, Colgan SP. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: role of intracellular cAMP. J Cell Physiol 1998; 176:76-84; PMID:9618147; http://dx.doi.org/ 10.1002/(SICI)1097-4652(199807)176:1%3c76::AID-JCP9%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- [124].Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol 2002; 282:C1235-45; PMID:11997237; http://dx.doi.org/ 10.1152/ajpcell.00288.2001 [DOI] [PubMed] [Google Scholar]
- [125].Koizumi J, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, Harimaya A, Murata M, Osanai M, Chiba H, et al.. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol 2008; 74:432-42; PMID:18477669; http://dx.doi.org/ 10.1124/mol.107.043711 [DOI] [PubMed] [Google Scholar]
- [126].Lu R, Dalgalan D, Mandell EK, Parker SS, Ghosh S, Wilson JM. PKCiota interacts with Rab14 and modulates epithelial barrier function through regulation of claudin-2 levels. Mol Biol Cell 2015; 26:1523-31; PMID:25694446; http://dx.doi.org/ 10.1091/mbc.E14-12-1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci U S A 2009; 106:61-6; PMID:19114660; http://dx.doi.org/ 10.1073/pnas.0802741106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Babbin BA, Jesaitis AJ, Ivanov AI, Kelly D, Laukoetter M, Nava P, Parkos CA, Nusrat A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol 2007; 179:8112-21; PMID:18056353; http://dx.doi.org/ 10.4049/jimmunol.179.12.8112 [DOI] [PubMed] [Google Scholar]
- [129].Lin N, Xu LF, Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem Biophys Res Commun 2013; 440:143-9; PMID:24051092; http://dx.doi.org/ 10.1016/j.bbrc.2013.09.049 [DOI] [PubMed] [Google Scholar]
- [130].Kiely B, Feldman G, Ryan MP. Modulation of renal epithelial barrier function by mitogen-activated protein kinases (MAPKs): mechanism of cyclosporine A-induced increase in transepithelial resistance. Kidney Int 2003; 63:908-16; PMID:12631071; http://dx.doi.org/ 10.1046/j.1523-1755.2003.00804.x [DOI] [PubMed] [Google Scholar]
- [131].Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol 2005; 167:1587-97; PMID:16314472; http://dx.doi.org/ 10.1016/S0002-9440(10)61243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med 2012; 29:202-8; PMID:22089663 [DOI] [PubMed] [Google Scholar]
- [133].Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol 2005; 21:1-26; PMID:15868485; http://dx.doi.org/ 10.1007/s10565-005-0085-6 [DOI] [PubMed] [Google Scholar]
- [134].Yi Z, Fan H, Liu X, Tang Q, Zuo D, Yang J. Adrenomedullin improves intestinal epithelial barrier function by downregulating myosin light chain phosphorylation in ulcerative colitis rats. Mol Med Rep 2015; 12:3615-20; PMID:26043783 [DOI] [PubMed] [Google Scholar]
- [135].Utech M, Mennigen R, Bruewer M. Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol 2010; 2010:484987; PMID:20011071; http://dx.doi.org/ 10.1155/2010/484987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Pleguezuelos O, Hagi-Pavli E, Crowther G, Kapas S. Adrenomedullin signals through NF-kappaB in epithelial cells. FEBS Lett 2004; 577:249-54; PMID:15527794; http://dx.doi.org/ 10.1016/j.febslet.2004.10.019 [DOI] [PubMed] [Google Scholar]
- [137].Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Investigat 2004; 114:1098-106; PMID:15489957; http://dx.doi.org/ 10.1172/JCI200421086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, et al.. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell 2015; 26:2252-62; PMID:25904334; http://dx.doi.org/ 10.1091/mbc.E14-07-1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Garayoa M, Martinez A, Lee S, Pio R, An WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, et al.. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol 2000; 14:848-62; PMID:10847587; http://dx.doi.org/ 10.1210/mend.14.6.0473 [DOI] [PubMed] [Google Scholar]
- [140].Agorreta J, Zulueta JJ, Montuenga LM, Garayoa M. Adrenomedullin expression in a rat model of acute lung injury induced by hypoxia and LPS. Am J Physiol Lung Cell Mol Physiol 2005; 288:L536-45; PMID:15579624; http://dx.doi.org/ 10.1152/ajplung.00314.2004 [DOI] [PubMed] [Google Scholar]
- [141].Allaker RP, Zihni C, Kapas S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol 1999; 23:289-93; PMID:10225288; http://dx.doi.org/ 10.1111/j.1574-695X.1999.tb01250.x [DOI] [PubMed] [Google Scholar]
- [142].Marutsuka K, Nawa Y, Asada Y, Hara S, Kitamura K, Eto T, Sumiyoshi A. Adrenomedullin and proadrenomudullin N-terminal 20 peptide (PAMP) are present in human colonic epithelia and exert an antimicrobial effect. Exp Physiol 2001; 86:543-5; PMID:11571480; http://dx.doi.org/ 10.1113/eph8602250 [DOI] [PubMed] [Google Scholar]
- [143].Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol 2008; 294:H1188-96; PMID:18178724; http://dx.doi.org/ 10.1152/ajpheart.00937.2007 [DOI] [PubMed] [Google Scholar]
- [144].Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol 2004; 24:6690-700; PMID:15254236; http://dx.doi.org/ 10.1128/MCB.24.15.6690-6700.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Talero E, Di Paola R, Mazzon E, Esposito E, Motilva V, Cuzzocrea S. Anti-inflammatory effects of adrenomedullin on acute lung injury induced by Carrageenan in mice. Mediators Inflamm 2012; 2012:717851; PMID:22685374 [DOI] [PMC free article] [PubMed] [Google Scholar]


