Abstract
It is well established that central nervous system norepinephrine (NE) and corticotropin releasing factor (CRF) systems are important mediators of behavioral responses to stressors. More recent studies have defined a role for delta opioid receptors (DOPR) in maintaining emotional valence including anxiety. The amygdala plays an important role in processing emotional stimuli, and has been implicated in the development of anxiety disorders. Activation of DOPR or inhibition of CRF in the amygdala reduces baseline and stress-induced anxiety-like responses. It is not known whether CRF- and DOPR-containing amygdalar neurons interact or whether they are regulated by NE afferents. Therefore, the present study sought to better define interactions between the CRF, DOPR and NE systems in the basolateral (BLA) and central nucleus of the amygdala (CeA) of the male rat using anatomical and functional approaches. Irrespective of the amygdalar subregion, dual immunofluorescence microscopy showed that DOPR was present in CRF-containing neurons. Immunoelectron microscopy confirmed that DOPR was localized to both dendritic processes and axon terminals in the BLA and CeA. Semi-quantitative dual immunoelectron microscopy analysis of gold-silver labeling for DOPR and immunoperoxidase labeling for CRF revealed that 55% of the CRF neurons analyzed contained DOPR in the BLA while 67% of the CRF neurons analyzed contained DOPR in the CeA. Furthermore, approximately 41% of DOPR-labeled axon terminals targeted BLA neurons that expressed CRF while 29% of DOPR-labeled axon terminals targeted CeA neurons that expressed CRF. Triple label immunofluorescence microscopy revealed that DOPR and CRF were co-localized in common cellular profiles that were in close proximity to NE-containing fibers in both subregions. These anatomical results indicate significant interactions between DOPR and CRF in this critical limbic region and reveal that NE is poised to regulate these peptidergic systems in the amygdala. Functional studies were performed to determine if activation of DOPR could inhibit the anxiety produced by elevation of NE in the amygdala using the pharmacological stressor yohimbine. Administration of the DOPR agonist, SNC80, significantly attenuated elevated anxiogenic behaviors produced by yohimbine as measured in the rat on the elevated zero maze. Taken together, results from this study demonstrate the convergence of three important systems, NE, CRF, and DOPR, in the amygdala and provide insight into their functional role in modulating stress and anxiety responses.
Keywords: amygdala, delta opioid receptor, corticotropin-releasing factor, norepinephrine
Introduction
The amygdala is composed of multiple subregions that play a critical role in the processing of emotional stimuli (LeDoux, 2000, McGaugh et al., 2002). Considered to be the gatekeeper of the amygdala, the lateral nucleus (LA) receives inputs from multiple sensory systems (Sah et al., 2003). From the LA, information is processed through the basolateral amygdala (BLA), which is involved in the consolidation of highly salient emotional stimuli (Sah and Lopez De Armentia, 2003) and receives dense noradrenergic projections (Asan, 1998). The central nucleus of the amygdala (CeA) acts as the amygdala’s output station mediating behavioral and autonomic responses to emotionally arousing stimuli through its highly connected afferents to endocrine and autonomic centers in the hypothalamus and brainstem (Veening et al., 1984, Petrovich et al., 2001). Amygdalar dysfunction is seen in stress-induced anxiety as well as anxiety associated with substance abuse and withdrawal (Retson et al., 2014, Spanagel et al., 2014, Radley et al., 2015, Silberman and Winder, 2015).
Physiological and emotional responses to stress are tightly linked to the hypothalamic pituitary adrenal axis, and the neuropeptide corticotropin releasing factor (CRF) is integral to this response. CRF in the amygdala mediates the behavioral and emotional consequences of stress including anxiety (Heinrichs et al., 1994, Francis et al., 1999, Tovote et al., 2015) and CRF mRNA levels increased in the CeA following stress (Mamalaki et al., 1992). In fact, CRF or CRF receptor agonist administered directly into the amygdala produces an aversive state including anxiety-like behavior (Gray and Bingaman, 1996, Sajdyk et al., 2006). It has been shown that CRF in the amygdala is critical for the reconsolidation of contextual fear memories (Pitts et al., 2009), and application of a variety of acute stressors result in elevations of CRF expression in the central nucleus of the amygdala of the rat (Iwasaki-Sekino et al., 2009, Ciccocioppo et al., 2014). Norepinephrine (NE) is another neuromodulator in the amygdala that is also involved in behavioral and emotional responses to stressors (Koob, 1999). Stressors have been shown to alter NE activity. For example, stressors increase NE release in the amygdala (Tanaka et al., 1991a, Tanaka et al., 1991b, Galvez et al., 1996, Quirarte et al., 1998) and induce high density of NE transporter binding sites in the amygdala (Tejani-Butt et al., 1994). Clinical studies using functional magnetic resonance imaging and a strategy of pharmacologically potentiating NE neurotransmission in healthy individuals have demonstrated that elevated NE neurotransmission enhances basolateral amygdalar nuclear complex (BLA) responses to fear signals (Onur et al., 2009). Furthermore, NE system in the BLA is implicated in memory modulation by stress (McGaugh et al., 2002, Ferry and McGaugh, 2008, McGaugh and Roozendaal, 2009).
In addition to CRF, the delta opioid system has been implicated in the control of emotion-regulated behaviors including anxiety. The endogenous opioid peptides, met- and leu-enkephalin, reduce anxiety through activation of delta opioid receptors (DOPR) (Nieto et al., 2005). Selective DOPR agonists decrease anxiety-like behaviors in rodent models and have a modulatory effect on a variety of stress-induced responses (Nieto et al., 2005, Saitoh et al., 2005, Perrine et al., 2006, Perrine et al., 2008, Randall-Thompson et al., 2010). Conversely, genetic deletion of the DOPR gene (Filliol et al., 2000) or administration of selective DOPR antagonists (Saitoh et al., 2005, Perrine et al., 2006) increases anxiety-like responses, suggesting a tonic dampening of anxiety mediated by the delta opioid system. The amygdala appears to be an important site of action for the anxiolytic effects of DOPR agonists. Direct infusion of the selective DOPR agonist, DPDPE, into the amygdala reduces basal and stress-induced anxiety-like behaviors in a rat model (Randall-Thompson et al., 2010).
The present study focused on the amygdalar complex as the site of the anxiolytic effects DOPR agonists. Our previous results demonstrating that activation of DOPR specifically in the CeA can attenuate stress-induced anxiety (Randall-Thompson et al., 2010), suggest that DOPR may interact with CRF neurons in the amygdala. Although the role of amygdalar CRF in anxiety has been studied extensively (Heinrichs et al., 1992, Liang et al., 1992, Swiergiel et al., 1993, Merlo Pich et al., 1995, Rodriguez de Fonseca et al., 1997, Merali et al., 1998, Bruchas et al., 2009, Knoll et al., 2011, Lam and Gianoulakis, 2011, Andero et al., 2013, McCall et al., 2015), there is a gap in our knowledge regarding whether DOPR directly modulates CRF activity. Considering the importance of this stress-integrative circuitry, we sought to determine whether amygdalar neurons that express CRF were positioned to be modulated by opioids via DOPR. We also investigated the anatomical interactions between noradrenergic afferents and amygdalar DOPR- and CRF-containing neurons due to the established role of NE in anxiety and stress responses. Finally, this study tested the ability of a DOPR agonist to reduce anxiety-like behaviors produced by a noradrenergic pharmacological stressor.
Materials and Methods
All procedures used in the present study were approved by the Institutional Animal Care and Use Committees of Drexel University and Temple University and conformed to National Institute of Health’s Guide for the Care and Use of Laboratory Animals. Rats were housed two – three per cage on a 12-h light schedule in a temperature controlled colony room (20 ºC). They were allowed access to standard rat chow and water ad libitum and were acclimated to the housing facility for several days prior to handling. All efforts were made to utilize the minimum number of animals necessary to produce reliable scientific data.
Experimental animals and brain preparation for anatomical analysis
Eleven adult male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) weighing 250–300g were used to examine the cellular associations of DOPR and CRF with norepinephrine transporter (NET) in the BLA and CeA. Out of 11 male rats, five rats were used for light microscopy and immunofluorescence experiments and six rats were used for electron microscopy. Of the six rats used for electron microscopy and ultrastructural analysis, three rats received injections of colchicine, a microtubule inhibitor (50 μg; Sigma-Adrich, St. Louis, MO), into their lateral ventricles to elevate peptide levels in soma and dendrites. Three rats were anesthetized with cocktail of ketamine hydrochloride (100 mg/kg: Phoenix Pharmaceutical Inc., St. Joseph, MO) and xylazine hydrochloride (2 mg/kg; Phoenix Pharmaceutical Inc.), and placed in stereotaxic apparatus. Using a Picospritzer (General Valve Corporation, Fairfield, NJ), colchicine was injected at 24–26 psi, 10 ms duration and 0.2 Hz. Coordinates for the lateral ventricle infusions were taken from the rat brain atlas of Paxinos and Watson (1986) as follows: 0.26 mm posterior from the bregma, 1.2 mm medial/lateral and 3.6 mm ventral from the top of the skull. About eighteen hours after colchicine administration, rats were deeply anesthetized with sodium pentobarbital (Beuthanasia) at 60 mg/kg via an intraperitoneal route and transcardially perfused through the ascending aorta with (1) 10 mL heparin, (2) 50 ml of 3.75% acrolein (Electron Microscopy Sciences, Fort Washington, PA), and (3) 2% formaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed, cut in half and immersed in 2% formaldehyde overnight at 4°C.
Antibody characterization
DOPR antibody (Alomone Laboratories, Jerusalem, Israel) was raised in rabbit and directed against an extracellular epitope of the mouse DOPR. Previous studies using this DOPR antibody have demonstrated that DOPR immunoreactivity was abolished following preadsorption with antigenic peptide (Salman et al., 2013, Salman et al., 2014). In the present study to ensure antibody specificity, we performed control experiments and replicated these results with both immunohistochemistry and Western blotting technique.
The CRF antibody (Peninsula Laboratories, San Carlos, CA) in this study was used to identify CRF-containing amygdalar neurons. This CRF serum was raised in guinea pig and was generated against human/mouse/rat CRF peptide (SEEPPISLDLTFHLLREVLEMARAEQLAQQAHSNRKLMEII) (Peninsula Laboratories). The specificity of CRF immunolabeling was tested by lack of immunoreactivity in areas not known to expresss CRF. In addition, blocking with the antigenic CRF peptide via preabsorption abolished CRF immunoreactivity as previously conducted (Bachtell et al., 2003, Lim et al., 2007, Sawada et al., 2008).
NE transporter (NET) was used to identify NE terminals. The monoclonal antibody directed against NET (MAb Technologies, Stone Mountain, GA) was generated using a peptide (amino acids 5–17) of a mouse and rat NET coupled to a keyhole limpet hemocyanin by the addition of a C-terminal cysteine. We have previously tested the specificity of NET antibody by preabsorption of this antibody with the antigenic peptide (MAb Technologies). This resulted in the absence of immunoreactivity in rat tissues that were expected to show NET immunolabeling including nucleus accumbens and amygdala (Carvalho et al., 2010, Kravets et al., 2015).
Dual immunofluorescence
Coronal tissue sections from five naïve rats were cut through the amygdala at 40 μm using a Vibratome (Technical Product International, St Louis, MO) and collected in 0.1 M PB. Free-floating tissue sections were treated with 1% sodium borohydride in 0.1 M PB for 30 minutes. They were then washed in 0.1 M PB followed by 0.1 M Tris buffered saline (TBS, pH 7.6). The tissue sections were blocked in 0.5% bovine serum albumin (BSA) in 0.1 M TBS for 30 minutes and washed in 0.1 M TBS twice for 5 minutes. Tissue sections were incubated overnight at room temperature with a rabbit antibody directed against DOPR (1:1000; Alomone) and mouse anti-NET (1:1000; MAb Technologies) or guinea pig anti-CRF (1:2000, Peninsula Laboratories) in 0.1% BSA with 0.25% Triton X in 0.1 M TBS. The tissue sections were washed in 0.1 M TBS for 10 min, 3 times. Then for 2h at room temperature the tissue sections were incubated in secondary antibodies, fluorescein isothiocyanate-conjugated donkey anti-rabbit (1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for visualization of DOPR and rhodamine isothiocyanate-conjugated donkey anti-guinea pig (1:200; Jackson Immunoresearch) to visualize CRF. The tissue sections were washed 3 times for 10 min each in 0.1 M TBS followed by a wash for 10 min in 0.1 M PB and then 0.05 M PB. The tissue sections were mounted on gelatin coated slides and dehydrated in ascending series of alcohols and xylene. Some mounted tissue sections were also incubated with 4′,6-diamidino-2-phenylindole (DAPI; EMD Millipore, Billerica, MA) at 1:10,000 for 5 minutes and washed 3 times with 0.05 M PB prior to dehydration in alcohols and xylene. The tissue sections were coverslipped using DPX mounting medium (Sigma-Aldrich, St. Louis, MO).
Triple immunofluorescence
The procedures used for dual immunofluorescence described earlier were also followed for triple immunofluorescence except that the tissue sections were incubated overnight at room temperature with a mouse antibody directed against NET (1:1000; MAb Technologies), rabbit anti-DOPR (1:1000; Alomone), and guinea pig anti-CRF (1:2000, Peninsula Laboratories). Moreover, the tissue sections were incubated in secondary antibodies, namely: rhodamine isothiocyanate-conjugated donkey anti-guinea pig (1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) to visualize CRF; fluorescein isothiocyanate-conjugated donkey anti-rabbit (1:200; Jackson Immunoresearch) for visualization of DOPR, and cyanine dye Cy5-conjugated donkey anti-mouse (1:200, Jackson Immunoresearch) to visualize NET. The tissue sections were washed 3 times for 10 min each in 0.1 M TBS followed by a wash for 10 min in 0.1 M PB and then 0.05 M PB. The tissue sections were mounted on gelatin coated slides and dehydrated in ascending series of alcohols and xylene. The tissue sections were coverslipped using DPX mounting medium (Sigma-Aldrich, St. Louis, MO).
Dual immunolabeling: immunoperoxidase and immunogold-silver labeling
Using immunoelectron microscopy, CRF was labeled with immunoperoxidase while DOPR was detected using the immunogold-silver labeling. The brains were removed, cut in half and immersed in 2% formaldehyde overnight at 4°C. CRF was visualized by immunohistochemical reaction. The tissue sections were processed following the protocol described for immunofluorescence, but without the use of Triton-X 100 in the antibody incubation solutions. The tissue sections were incubated overnight at room temperature in primary antibody solution containing rabbit anti-DOPR (1:1000) and guinea pig anti-CRF (1:2000) with 0.1% BSA in 0.1 M TBS. CRF antibody was visualized using immunoperoxidase detection by incubating the tissue sections in biotinylated goat anti-guinea pig IgG (1:400, Jackson ImmunoResearch Laboratories) for 30 min followed by a 30 min incubation in avidin-biotin complex (ABC Elite Kit, Vector Laboratories, Burlingame, CA). The tissue sections were washed in 0.1 M TBS three times for 10 min each. The tissue sections were then immersed in 22 mg of 3–3′ diaminobenzidine (DAB, Sigma-Aldrich) with 10 μL of 30% hydrogen peroxide in 100 mL of 0.1 M TBS for 10 min. After visualization of CRF, the tissue sections were processed for immunogold-silver labeling for DOPR as previously described (Reyes et al., 2008, Reyes et al., 2011, Kravets et al., 2015).
Gold-silver localization of DOPR was carried out by rinsing the tissue sections three times with 0.1 M TBS, followed by rinses with 0.1 M PB and 0.01 M phosphate buffered saline (PBS; pH 7.4). Sections were then incubated in a 0.2% gelatin-PBS and 0.8% BSA buffer for 10 min. This was followed by incubation in goat anti-mouse IgG conjugate in 1 nm gold particles (Amersham Bioscience Corp., Piscataway, NJ) at room temperature for 2 hours. Sections were then rinsed in buffer containing the same concentration of gelatin and BSA as above and subsequently rinsed with 0.01 M PBS. Sections were then incubated in 2% glutaraldehyde (Electron Microscopy Sciences) in 0.01 M PBS for 10 min followed by washes in 0.01 M PBS and 0.2 M sodium citrate buffer (pH 7.4). A silver enhancement kit (Amersham Bioscience Corp.) was used for silver intensification of the gold particles. The optimal times for silver enhancement were determined by empirical observation for each experiment and ranged 7–8 min. Following intensification, tissue sections were rinsed in 0.2 M citrate buffer and 0.1 M PB, and incubated in 2% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M PB for 1 h, washed in 0.1 M PB, dehydrated in an ascending series of ethanol followed by propylene oxide and flat embedded in Epon 812 (Electron Microscopy Sciences; (Leranth and Pickel, 1989).
Ultrathin sections of approximately 70 nm were cut with a diamond knife (Diatome-US, Fort Washington, PA, USA) using a Leica Ultracut ultramicrotome (Leica Microsystems, Wetzlar, Germany). Sections were collected on copper mesh grids and counterstained with 5% aqueous uranyl acetate for 20 minutes followed by Reynold’s lead citrate for 5–7minutes. Captured images of selected sections were compared with light microscopic images of the block-face before sectioning to verify that the area of interest was targeted/collected. Tissue sections were examined with a Morgagni 268 transmission electron microscope (Fei Company, Hillsboro, OR). Digital images were obtained using the AMT advantage HR/HR-B CCD camera system (Advance Microscopy Techniques Corp., Danvers, MA). The TEM has an accelerating voltage of 80kv and we used the magnification of 11,000 to 14,000 in a field size of 9.3 μm × 9.3 μm for 11000× and 7.3 μm × 7.3 μm for 14000×. Figures were adjusted in brightness and contrast using Adobe Photoshop CS4 software (Adobe Systems, Inc., San Jose, CA).
Controls and Data analysis
Tissue sections for electron microscopy were taken from rats with the best immunohistochemical labeling and preservation of ultrastructural morphology. The semi-quantitative approach used in the present study is well established and has been described previously (Van Bockstaele et al., 1996a, Van Bockstaele et al., 1996b, Reyes et al., 2006, Reyes et al., 2007). Three rats used for semi-quantitative analysis were injected with colchicine. Colchicine has been shown to induce stress-like responses and increased mRNA expression levels for certain neuropeptides (Swanson et al., 1983; Moga et al., 1990). Since the goal of the present study was to use antibodies to determine the neurochemical signature of neurons rather than to quantify their expression levels, the use of colchicine in the present studies should not impact the interpretation of our electron microscopy results.
While acrolein fixation optimizes the preservation of ultrastructural morphology, the caveat of limited and or differential penetration of immunoreagents in thick tissue sections exists (Leranth and Pickel, 1989, Jensen et al., 2009). Consequently, the limited penetration of CRF and DOPR may result in an underestimation of the relative frequencies of their distribution. We mitigated this limitation by collecting the tissue sections exclusively near the tissue-Epon interface where penetration is maximal and profile were sampled only when all the markers were present in the surrounding neuropil included in the analysis.
The cellular elements were identified based on the description of Peters and colleagues (Peters and Palay, 1996). Somata contained a nucleus, Golgi apparatus and smooth endoplasmic reticulum. Proximal dendrites contained endoplasmic reticulum, were postsynaptic to axon terminals and were larger than 0.7 μm in diameter. A terminal was considered to form a synapse if it showed a junctional complex, a restricted zone of parallel membranes with slight enlargement of the intercellular space, and/or associated with postsynaptic thickening. A synaptic specialization was only limited to the profiles that form clear morphological characteristics of either Type I or Type II (Gray, 1959). Asymmetric synapses were identified by thick postsynaptic densities (Gray’s type I; (Gray, 1959). In contrast, symmetric synapses had thin densities (Gray’s type II; (Gray, 1959) both pre- and post-synaptically. An undefined synapse was defined as an axon terminal plasma membrane juxtaposed to that of a dendrite or soma devoid of recognizable membrane specializations and no intervening glial processes.
Semi-quantitative analysis include only tissue sections that contained both DOPR and CRF immunoreactivities. In sections dually labeled for DOPR and CRF, the number of DOPR-labeled dendrites and axon terminals were grouped from randomly selected sections taken from four nonadjacent ultrathin sections from each animal (n = 5). At least five sections were examined per animal. From the surface of the individual epon block containing the tissue, at least four to eight ultrathin sections were collected. Fields of at least 11,000X magnification showing DOPR-labeled dendrites or axon terminals and CRF-labeled profiles were captured and classified. This approach resulted in total profiles of 577 and 475 DOPR-labeled dendrites from the BLA and CeA, respectively as well as total profiles of 198 and 147 DOPR-labeled axon terminals from the BLA and CeA, respectively. All potential neuronal targets of DOPR-labeled axon terminals throughout the analysis were examined by defining their total associations with other profiles regardless of whether a membrane specialization was seen within the plane of section. The postsynaptic targets considered included dendrites containing gold-silver labeling for CRF and dendrites lacking gold-silver labeling for CRF-labeled profiles forming clear synaptic specializations were classified as symmetric (Gray’s Type II) or asymmetric (Gray’s Type I). On the other hand, undefined contacts were characterized by a junctional complex that was not clearly distinguishable to allow a classification as either symmetric or asymmetric in the plane of section analyzed.
Identification of immunogold-silver labeling in profiles
Selective immunogold-silver labeled profiles were identified by the presence, in single thin sections, of at least two immunogold-silver particles within a cellular compartment. As we previously reported (Reyes, et al., 2006), single spurious immunogold-silver labeling can contribute to false positive labeling and can be detected on blood vessels, myelin or nuclei. Although minimal spurious labeling was identi ed in the present study, the criteria for de ning a process as immunolabeled was therefore set at 2 immunogold-silver particles in a cellular pro le. Whenever possible, the more lightly labeled somatic and dendritic labeling for DOPR was confirmed by detection in at least two adjacent sections.
Quantification of DOPR distribution
The analysis of DOPR distribution in the BLA and CeA was carried out by calculating the ratio of cytoplasmic to total immunogold-silver particles for each singly immunolabeled dendritic profile in individual rats. Cytoplasmic immunogold-silver particles are defined by their localization within the cytoplasmic compartment and are not contacting the plasma membrane. The total immunogold-silver particles include particles that are located within the cytoplasmic compartment and those that are located along or in direct contact with the plasma membrane.
Anxiety measurement
Thirty-two adult male Sprague Dawley rats were tested on the elevated zero maze for anxiety-like behavior (Braun et al., 2011). The zero maze apparatus (San Diego Instruments) is a beige circular runway 4 inches wide with a 48-inch diameter outer wall and 40-inch diameter inner wall. The platform is elevated 30 inches above the ground and divided into equal quadrants. Two opposing quadrants are “closed” with 8-inch high walls, separated by “open” quandrants with a half-inch high ledge. The lighting conditions were adjusted to produce light levels of ~75 lux in the open sections and ~30 lux in the closed sections of the maze. Rats were allowed to acclimate in the testing room to the dim (~75lux) lighting for 1 hour prior to testing. Testing took place between 10:00 AM and 2:30 PM. Each rat was placed in the same orientation in the same closed section of the maze and behavior was video recorded for 10 minutes. The maze was cleaned and dried between rats. Anxiety-like behavior was assessed by the amount of time each rat spent in the open quadrants of the maze and reported as a percent of the total time on the maze. A rat was considered to be in an open quadrant when at least the head and both front legs of the rat moved into an open section of the maze.
In order to assess stress-induced anxiety, yohimbine (2 mg/kg sc) or vehicle (sterile water 1 ml/kg sc) was administered 30 minutes prior to testing on the elevated zero maze. The selective DOPR agonist SNC80 (10 mg/kg s.c.) or vehicle (sterile water at pH 5 with HCl, 1 ml/kg sc) was administered 60 minutes prior to testing (30 minutes prior to yohimbine). Data were analyzed by a two-way ANOVA with pretreatment and treatment main factors.
Results
Specificity of antibodies and control experiments
Control experiments were carried out in order to rule out any cross-reactivity of antibodies. The following conditions were processed 1) primary antisera were omitted while secondary antibodies were added, 2) one of the primary antisera was omitted; 3) one of the secondary antibodies was omitted; and 4) all secondary antibodies were omitted. Thus, control tissue sections processed in the absence of either DOPR or CRF primary antibodies did not exhibit detectable immunoreactivity. As we previously reported (Ambrose-Lanci et al., 2008, Reyes et al., 2008, Carvalho et al., 2010, Reyes et al., 2011, Kravets et al., 2015), there was no detectable immunolabeling with the primary antibody omitted. We also evaluated the cross-reactivity of the primary antibodies with secondary antibodies by processing tissue sections with omission of one of each of the primary antisera. Furthermore, processing of additional controls included incubation of each primary antibody with an inappropriate secondary antibody. This resulted in the absence of immunolabeling for the incorrectly paired primary-secondary antibody combination. Thus, control sections that were incubated with rabbit DOPR followed by goat anti-mouse secondary antibody did not exhibit any detectable DOPR immunolabeling.
Region of interest selected for DOPR-CRF analysis
Different investigators have grouped the amygdalar nuclei in various ways (Price et al., 1987; McDonald, 1998; Sah et al., 2003). We use the nomenclature introduced by Price and colleagues (Price et al., 1987) with some modifications (McDonald, 1998; Sah et al., 2003). In the present study, the basolateral nucleus of the amygdala (BLA; Figure 1) is defined as the area encompassing the basolateral group as described by Sah and colleagues (Sah et al., 2003), and comprises the lateral and anterior subdivisions of the BLA (Zhang et al., 2013) at bregma level −1.80 through −3.0 (Paxinos and Watson, 1986). The CeA is located medially to the BLA and can be further subdivided based on nuclear density and shape (McDonald 1982; Cassell et al. 1986).
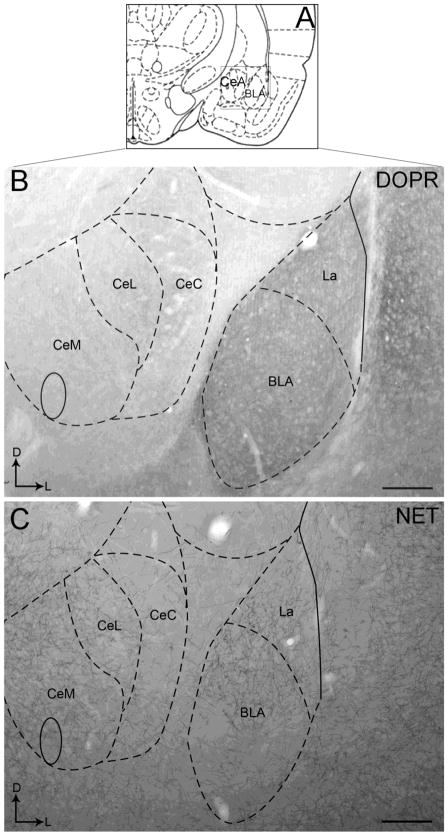
Figure 1.
Brightfield photomicrographs showing immunoperoxidase labeling of DOPR and NET immunoreactivities in the CeA and BLA. Dense DOPR immunoperoxidase labeling can be seen in the BLA and much less dense immunoperoxidase labeling is evident in the CeA as shown in panel B. Similar pattern of immunoperoxidase labeling is observed in NET immunoreactivity as shown in panel C. Panels B and C illustrate a representative section through the amygdalar region at bregma - 2.12. Arrows indicate dorsal (D) and medial (L) orientation of the tissue section. Schematic illustration adapted from the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1997; Panel A) showing the anterior-posterior level from which the photomicrographs in panel B and C are taken. The region denoted by the box in panel A is shown in panels B and C. BLA, basolateral nucleus of the amygdala; CeA, central nucleus of the amygdala; CeC, capsular part of the CeA; CeL, lateral part of the CeA; CeM, medial division of the CeA La, lateral nucleus of the amygdala. Scale bars: 200 μm.
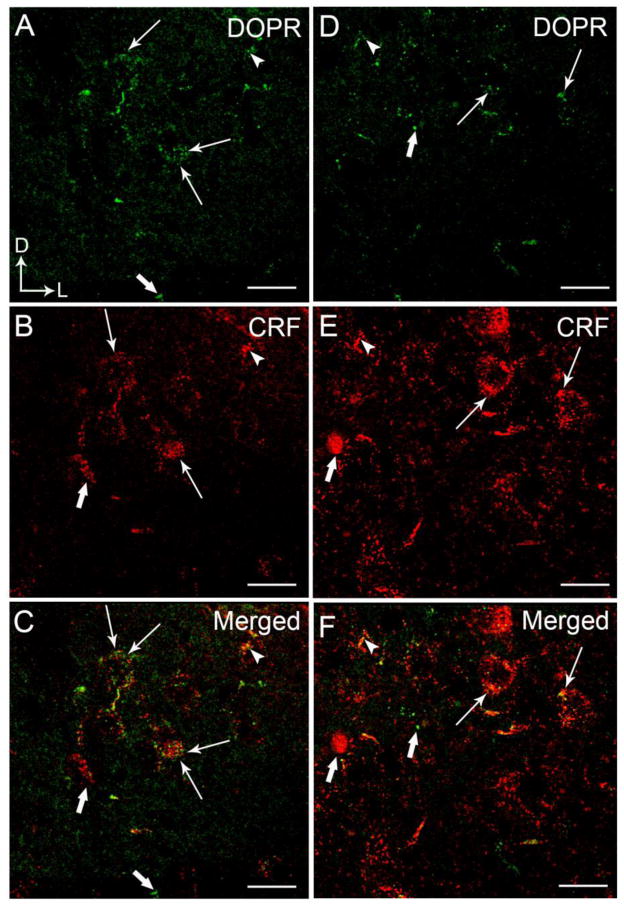
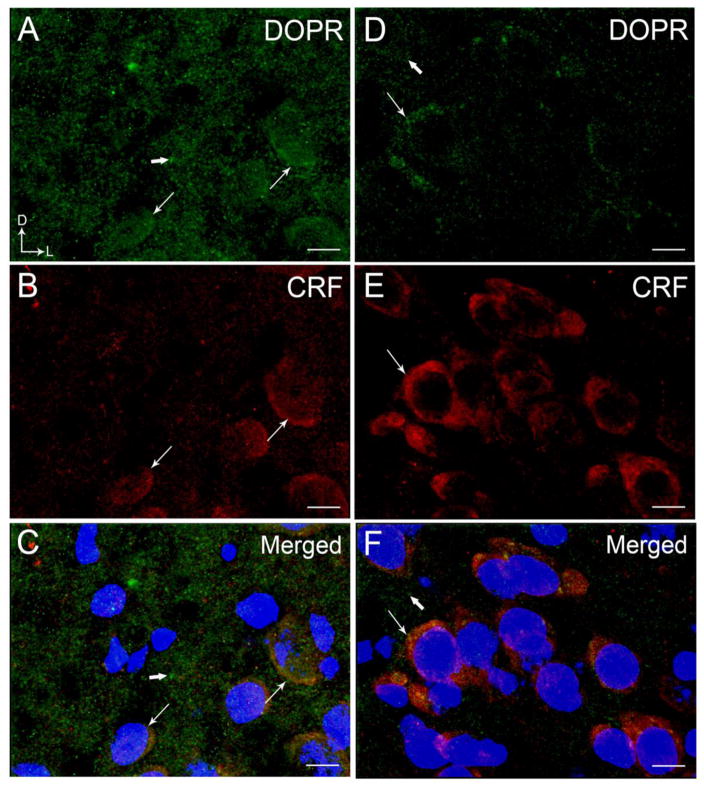
Using light microscopy, DOPR immunoreactivity appeared to be more robust and dense in the BLA compared to the CeA as shown in Figure 1. This is supported by the confocal immunofluorescence images showing a more abundant distribution of DOPR in the BLA (Figure 2A, 3A, 6A) than in the CeA (Figure 2D, 3D). The distribution of CRF in the BLA (Figure 2B) is consistent with our previous report showing less CRF immunoreactivity in this subregion when compared to the CeA (Figure 2E) (Kravets et al., 2014, Reyes et al., 2008, 2011). DOPR and CRF co-localization was present in both the BLA (Figure 2C) and the CeA (Figure 2F). Figure 3 illustrates nuclei staining in CRF-containing neurons that co-localized with DOPR in the BLA and the CeA.
Figure 2.
High magnification confocal immunofluorescence photomicrographs showing dual-labeling for DOPR and CRF in a coronal section through the BLA (A–C) and CeA (D–F). DOPR immunoreactivity was labeled with fluorescein isothiocyanate (green) and CRF was labeled with rhodamine isothicyanate (red). A–B. Thin arrows depict dual labeling for DOPR (A) and CRF (B) while thick arrows depict singly labeled DOPR and CRF, respectively, in the BLA. D–E. Thin arrows depict dual labeling for DOPR (D) and CRF (E) in the CeA while thick arrows depict singly labeled DOPR and CRF. Panels C and F show merged image. Corner arrows indicate dorsal (D) and lateral (L) orientation. Scale bar, 25 μm.
Figure 3.
High magnification confocal immunofluorescence photomicrographs showing labeling for DOPR and CRF in a coronal section through the BLA (A–C) and CeA (D–F). Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). DOPR immunoreactivity was labeled with fluorescein isothiocyanate (green), CRF was labeled with rhodamine isothicyanate (red) and DAPI in blue. A–C. Thin arrows depict labeling for DOPR (A) and CRF (B) with DAPI while thick arrows depict singly labeled DOPR and CRF, respectively, in the BLA. D–E. Thin arrows depict labeling for DOPR (D) and CRF (E) with DAPI in the CeA while thick arrows depict singly labeled DOPR and CRF. Panels C and F show merged image of DOPR, CRF and DAPI. Corner arrows indicate dorsal (D) and lateral (L) orientation. Scale bar, 25 μm.
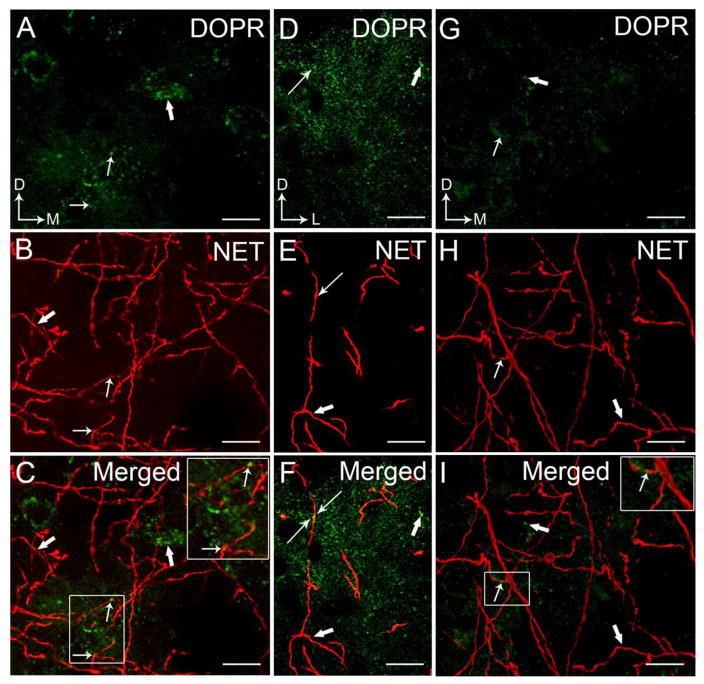
Figure 6.
High magnification confocal immunofluorescence photomicrographs showing dual-labeling for DOPR and NET in a coronal section through the BLA (A–F) and CeA (G–I). DOPR immunoreactivity was labeled with fluorescein isothiocyanate (green) and NET was labeled with rhodamine isothicyanate (red). Images of DOPR and CRF as collapsed Z-stack through the BLA (A–C) and CeA (G–I). Panels D–F are images of DOPR and CRF through the BLA, not as a collapsed Z-stack. Arrows depict site of dual labeling for DOPR (A,D) and NET (B,E) while thick arrow depicts singly labeled DOPR and NET, respectively, in the BLA. G–H. Arrows depict site of dual labeling for DOPR (G) and NET (H) while thick arrow depicts singly labeled DOPR and NET, respectively, in the CeA. Panels C, F, and I show merged images with dual labeling indicated by thin arrows. Arrows indicate dorsal (D) and lateral (L) orientation. Scale bar, 25 μm.
Localization of DOPR in the BLA and CeA
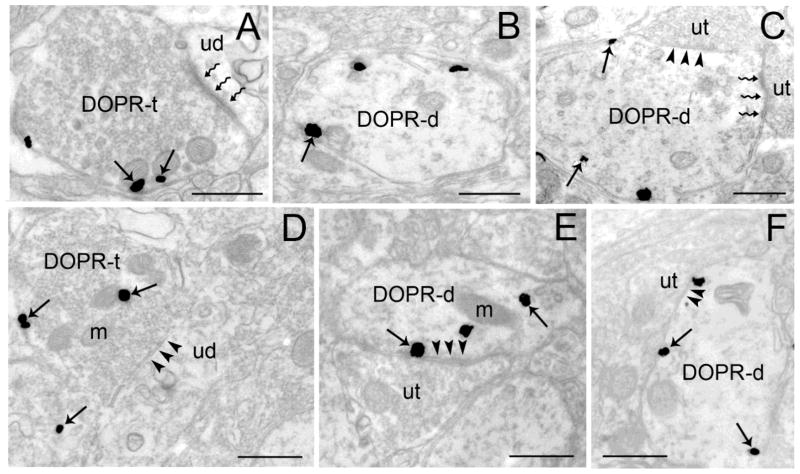
Using electron microscopy, DOPR was localized in dendrites and axon terminals in the BLA (Figure 4A–C) and CeA (Figure 4D–F). In the BLA, approximately 45% (237/528) and 55% (292/528) of DOPR was localized in axon terminals (Figure 4A, 5C) and dendrites (Figure 4B–C, 5A–B), respectively. In the CeA, approximately 31% (145/473) and 69% (328/473) of DOPR was localized in axon terminals (Figure 4D, 5F) and dendrites (Figure 4E–F, 5D–E), respectively.
Figure 4.
Ultrastructural evidence for DOPR immunoreactivity in axon terminals and dendrites in the BLA and CeA. Electron photomicrographs showing immunogold-silver labeling for DOPR in the BLA (A–C) and CeA (D–F). A. Immunogold-silver labeling is present in a BLA axon terminal (DOPR-t) which forms an asymmetric synapse (zigzag arrows) with an unlabeled dendrite (ud). Arrows point to DOPR. B–C. DOPR-labeled dendrites (DOPR-d) in the BLA. Panel C shows a DOPR-d that receives an asymmetric (zigzag arrows) and symmetric synapses (arrowheads) from unlabeled terminals (ut). D. DOPR-immunogold-silver labeling can be seen in CeA axon terminal (DOPR-t) that forms a symmetric synapse (arrowheads) with unlabeled dendrite (ud). E–F. DOPR-containing dendrites in the CeA. Both DOPR-d receive a symmetric synapse (arrowheads) from an unlabeled terminal (ut). Arrows depict DOPR-immunogold silver particles. m, mitochondria. Scale bars, 0.50μm.
Figure 5.
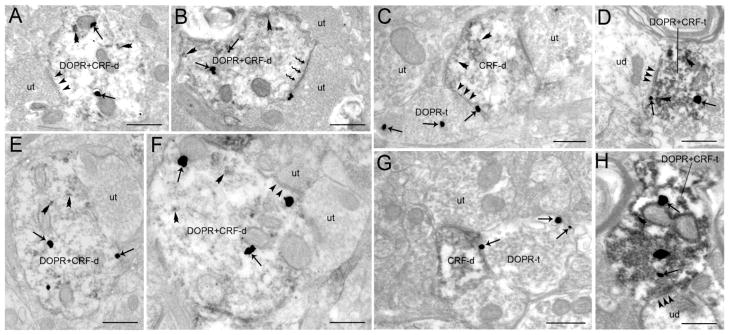
Electron photomicrographs showing dual labeling for DOPR and CRF in the BLA and CeA. A. A DOPR and CRF-labeled dendrite (DOPR+CRF-d) receives a symmetric synaptic contact (arrowheads) from an unlabeled terminal (ut) in the BLA. B. A DOPR+CRF-d receives an asymmetric synapse (zigzag arrow) from an unlabeled terminal (ut) in the BLA. C. A DOPR-labeled axon terminal (DOPR-t) in the BLA forms a symmetric synapse (arrowheads) with a CRF-labeled dendrite (CRF-d). D. A DOPR and CRF-labeled axon terminal (DOPR+CRF-t) contacting (arrowheads) an unlabeled dendrite (ud) in the BLA. E. An unlabeled axon terminal (ut) is contacting a DOPR+CRF-d in the CeA. F. A DOPR+CRF-d receives a symmetric synapse (arrowheads) from an unlabeled terminal (ut). G. A DOPR-labeled axon terminal (DOPR-t) in the CeA contacting a CRF-d. H. A DOPR and CRF-labeled axon terminal (DOPR+CRF-t) forming a symmetric synapse (arrowheads) with an unlabeled dendrite (ud) in the CeA. Arrows point to DOPR-immunogold silver particles while double arrowheads point to peroxidase immunolabeling indicative of CRF. ut, unlabeled terminal. Scale bar, 0.50μm.
DOPR-immunogold silver particles were distributed within the cytoplasmic compartment while some were associated with the plasma membrane (Fig. 4–5). Semi-quantitative analysis showed that the ratio of cytoplasmic to total DOPR immunogold-silver particles in the BLA dendrites was 0.54 ± 0.01 (n=115 dendritic profiles per rat analyzed). The ratio of cytoplasmic to total DOPR immunogold-silver particles in the CeA was 0.56 ± 0.04 (n=100 dendritic profiles analyzed). Likewise, semi-quantitative analysis showed that the ratio of cytoplasmic to total DOPR-immunogold silver particles in the BLA and CeA axon terminals was 0.48 ± 0.03 (n=108 axon terminals analyzed) and 0.53 ± 0.05 (n=108 axon terminals analyzed).
DOPR co-localization with CRF in the BLA
Using dual immunolabeling where CRF was labeled with immunoperoxidase and DOPR was labeled with immunogold-silver particles, approximately 55% (133/242; Table 1) of DOPR was co-localized with CRF in BLA dendrites (Figure 5A–B). Semi-quantitative analysis revealed that, of 133 DOPR+CRF-labeled dendrites analyzed, 49% (65/133) received symmetric synapses (arrowheads in Figure 5A) from unlabeled terminals, 23% (30/133) received asymmetric synapses (zigzag arrows in Figure 5B) and 29% (38/133) received synaptic specializations that could not be unequivocally determined. DOPR-labeled terminals targeting CRF-dendrites were also analyzed. Of 198 DOPR axon terminals analyzed in the BLA, 41% (81/198) targeted CRF-labeled dendrites (Figure 5C). Semi-quantitative analysis showed that of the 81 DOPR-labeled axon terminals contacting CRF-labeled dendrites 41% (40/81) formed symmetric synapses, 22% (18/81) formed asymmetric synapses and 28% (23/81) formed undefined synaptic specializations with the CRF-labeled dendrites. Asymmetric synapses had enlarged post-synaptic densities, while symmetric synapses exhibited densities that were comparable across pre- and post-synaptic membranes. In addition to co-localization of DOPR and CRF in dendrites in the BLA, we also observed some co-localization of DOPR and CRF in axon terminals albeit infrequently (Figure 5D).
Table 1.
Semi-quantitative analysis of DOPR and CRF interactions in the BLA and CeA
| BLA | CeA | |
|---|---|---|
|
| ||
| Frequency of co-localization of | 55% (133/242)
|
67% (212/315)
|
| CRF with DOPR in dendrites in different amygdalar subdivisions | Asymmetric 23% (30/133) | Asymmetric 20% (43/212) |
| Symmetric 49% (65/133) | Symmetric 60% (20/43) | |
| Undefined 29% (38/133) | Undefined 20% (42/212) | |
|
| ||
| Frequency of synapses formed by DOPR containing axon terminals with CRF containing dendrites in different amygdalar subdivisions | 41% (81/198)
|
29% (43/147)
|
| Asymmetric 22% (18/81) | Asymmetric 28% (12/43) | |
| Symmetric 41% (40/81) | Symmetric 49% (65/133) | |
| Undefined 28% (23/81) | Undefined 26% (11/43) | |
DOPR co-localization with CRF in the CeA
Using dual immunolabeling where CRF was labeled with peroxidase and DOPR was labeled with immunogold-silver particles, approximately 67% (212/315; Table 1) of DOPR was co-localized with CRF in CeA dendrites (Figure 5D–E). Semi-quantitative analysis revealed that, of 212 DOPR+CRF-labeled dendrites analyzed, 60% (127/212) received symmetric synapses (Figure 5E) from unlabeled terminals, 20% (43/212) received asymmetric synapses and 20% (42/212) received undefined synapses (Figure 5D). DOPR-labeled terminals targeting CRF-dendrites were also analyzed. Of the 147 DOPR axon terminals analyzed in the CeA, 29% (43/147) targeted CRF-labeled dendrites (Figure 5E). Semi-quantitative analysis showed that of the 43 DOPR-labeled axon terminals contacting CRF-labeled dendrites 46% (20/43) formed symmetric synapses, 28% (12/43) formed asymmetric synapses and 26% (11/43) formed undefined synaptic specializations with CRF-labeled dendrites. Infrequent co-localization of DOPR and CRF in axon terminals was observed (Figure 5H).
Anatomical substrates for NE innervation of CRF- and DOPR-containing amygdalar neurons
We examined the interaction between DOPR and NET. Consistent with our previous report (Kravets et al., 2014), NET is abundantly distributed in the BLA (Figure 6B–C, 6E–F 7C–D) with lesser density in the CeA (Figure 6H–I, 7G–H). DOPR-containing neurons in the BLA (Figure 6A–F, 7A, C–D) and CeA (Figure 6G–I, 7E, G–H) are in close proximity with the NET-immunolabeled fibers. Interestingly, some DOPR are co-localized with NET in the BLA (Figure 6C,F) and CeA (Figure 6I).
Figure 7.
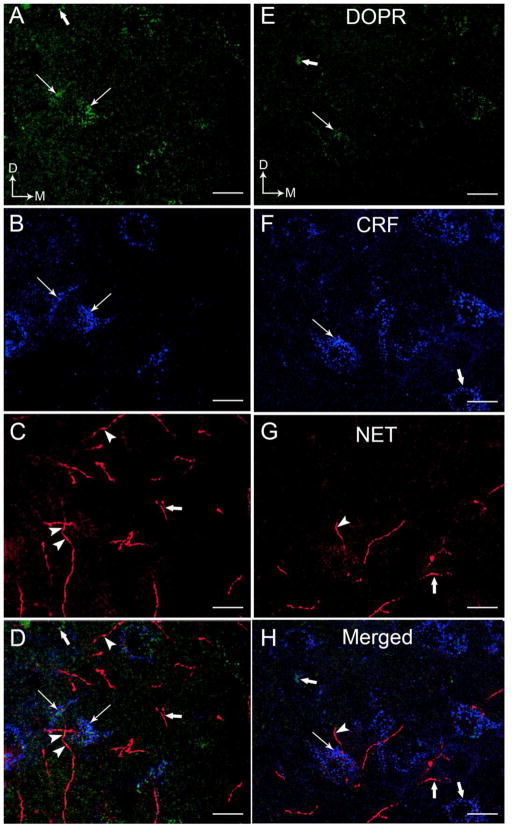
High magnification confocal immunofluorescence photomicrographs demonstrating DOPR (A,E), CRF (B,F) and NET (C,G) immunoreactivities in the coronal section through the BLA (A–D) and CeA (E–H). DOPR immunoreactivity was labeled with fluorescein isothiocyanate (green), CRF was labeled with rhodamine isothiocyanate and pseudocolored with blue, and NET was labeled with cyanine dye 5 (Cy5) and pseudocolored with red. We pseudocolored NET with red to allow easier identification of co-localized DOPR and NET which produces yellow color (arrowheads). The merged images illustrate DOPR and CRF-containing neurons that are in close proximity to NET-labeled fibers (D,H). Arrows illustrate neurons that co-express DOPR and CRF.. Thick arrows indicate single labeled DOPR, CRF or NET. Arrows indicate dorsal (D) and lateral (L) orientation. Scale bar, 25 μm.
To determine if NE axon terminals are positioned to directly regulate CRF- and DOPR-containing neurons in the BLA and CeA, immunoreactivities for NET, CRF, and DOPR were detected in the same section of tissue using immunofluorescence microscopy. Triple label immunofluorescence showed that DOPR (green labeling) are co-localized with CRF (pseudocolored with blue) labeled neurons and were in close proximity to NET-immunolabeled fibers (pseudocolored with red) in the BLA (Figure 7A–D) and CeA (Figure 7E–H), respectively. Consistent to our previous report (Kravets et al., 2015), NET was localized to processes that were highly varicose and punctate in appearance (Figure 7C–D, G–H). Moreover, NET immunoreactivity was more dense in the BLA (Figure 7C–D) compared to the CeA (Figure 7G–H). Likewise, CRF perikarya were more abundant in the CeA (Figure 7F, H) compared to the BLA (Figure 7B, D). While NET and DOPR co-localized in the BLA and CeA, co-localization of NET with DOPR and CRF was not observed (Figure 7D, 7H). In addition, we observed that the co-localized NET and DOPR in the BLA and CeA show a close proximity interaction with a CRF-labeled neuron (Figure 7D, 7H).
Functional interactions of DOPR, NE and CRF in stress-induced anxiety
In order to study the functional interactions of DOPR, NE, and CRF, the ability of the selective DOPR agonist, SNC80, to block anxiety produced by the pharmacological adrenergic stressor, yohimbine, a α2-adrenergic receptor antagonist, was tested. Increases in norepinephrine transmission result in heightened stress and anxiety responses in both humans and animal models (Moran Santa Maria et al., 2014; Cai L, et al., 2012; Kaplan JS et al., 2012; Braun AA et al., 2011). Anxiety-like behaviors of rats were measured using the elevated zero maze 30 minutes after an injection of yohimbine (2 mg/kg sc) or vehicle. The effect of the DOPR agonist on yohimbine-induced anxiety was tested; SNC80 (10 mg/kg sc) or its vehicle was administered 30 minutes prior to yohimbine (ie, 60 min prior to testing). The time spent in the open quadrants of the maze was expressed as a percentage of total time spent on the maze.
As shown in Figure 8, results indicate significant differences in open-arm times among treatment groups. Analysis of the data by two-way ANOVA revealed significant main effects of yohimbine stress (F1,27= 8.415, P=0.0073) and treatment with SNC80 (F1,27=47.03, P<0.0001). Bonferroni’s multiple comparison post hoc analysis revealed significantly lower time on the open arms in rats given the yohimbine stressor versus the vehicle controls (vehicle/yohimbine vs vehicle/vehicle, P<0.01) indicating that yohimbine produced heightened anxiety. Treatment with SNC80 significantly blocked the increase in anxiety-like behaviors produced by yohimbine, as indicated by significantly greater open arm time in SNC80 treated stressed rats versus yohimbine stress alone (SNC80/yohimbine vs vehicle/yohimbine, P<0.001). These results indicate that administration of SNC80 prevented the stress-induced anxiety produced by elevated NE transmission.
Figure 8.
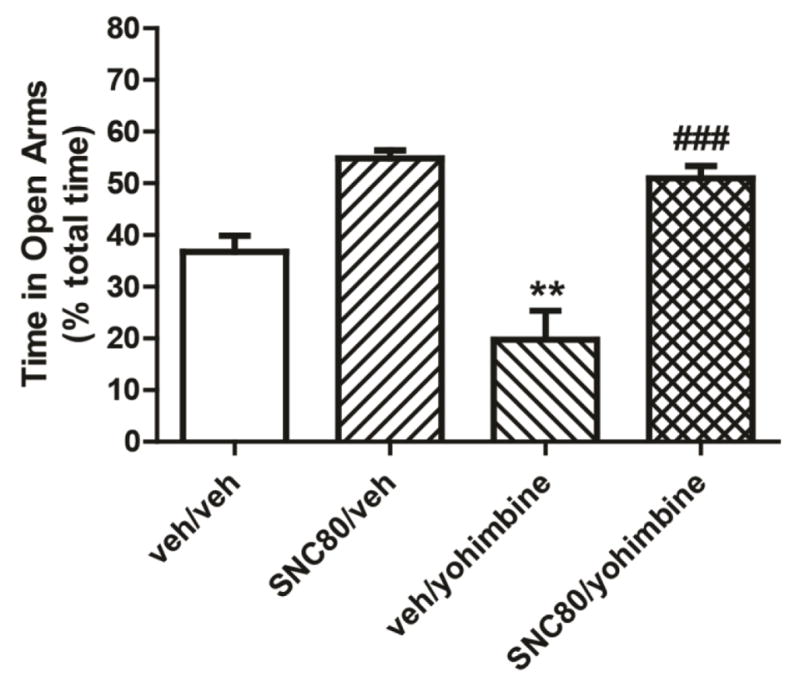
The DOPR agonist, SNC80, attenuated the anxiogenic effects of yohimbine. Rats were pretreated with the selective DOPR agonist, SNC80 10 mg/kg, or vehicle 30 minutes prior to yohimbine administration. Anxiety-like behaviors were measured using the elevated zero maze. Time spent on the open quadrants of the zero maze was lower after yohimbine administration as compared with vehicle controls, indicating an anxiogenic response. The anxiogenic effects of yohimbine were significantly blocked by treatment with SNC80. Data are expressed as means + SEM. ** P<0.01 veh/yohimbine vs veh/veh; ### P<0.001 SNC80/yohimbine vs veh/yohimbine. N=8/group.
Discussion
The present study provides anatomical evidence for a mechanism by which activation of DOPR could regulate amygdalar CRF activity and modulate stress responses including anxiety-like behaviors. An increased understanding of the fine synaptic organization of the amygdalar complex may lead to better treatments for anxiety disorders that are often precipitated by stress (de Kloet et al. 2005; Arborelius et al. 1999). In the present study, we advance knowledge of the intrinsic organization of limbic circuitry by providing evidence for co-localization of CRF and DOPR in individual neurons of the BLA and CeA. Additionally, our results demonstrate that DOPR and CRF-containing neurons in the BLA and CeA are in close proximity with NE afferents suggesting that NE modulation may impact amygdalar opioid-CRF circuitry. Taken together, these results provide anatomical evidence for the interaction of DOPR and CRF systems and suggest a role for NE in modulating DOPR and CRF-containing neurons in the amygdala that may be critical for the regulation of anxiety-like behaviors.
Amygdalar DOPR and anxiety
The opioid system continues to be an important target in the development of new therapies for stress and anxiety disorders because of its role in the modulation of emotional state and regulation of arousal and stress responses (Drolet et al., 2001, Primeaux et al., 2006). A prominent role of DOPR in emotional processing has been revealed in genetic mouse studies. Genetic deletion of the DOPR gene results in a phenotype of heightened anxiety (Filliol et al., 2000). This is specific to the DOPR as anxiogenic behavior is not observed in mu or kappa opioid receptor knockout mice (Konig et al., 1996, Filliol et al., 2000). In agreement with the DOPR knockout mouse model, systemic administration of the selective DOPR antagonist, naltrindole, increases anxiety-like behaviors (Perrine et al., 2006; Saitoh et al., 2005), as do conditions that produce dysfunction of DOPRs such as withdrawal from chronic cocaine administration (Perrine et al., 2008). The converse is also observed; enhanced DOPR function leads to reduced anxiety-like behaviors. This can be seen by administration of DOPR agonists (Saitoh et al., 2004; Perrine et al., 2006 and 2008; Nieto et al., 2005; Randall-Thompson et al., 2010) or manipulations that increase endogenous opioid peptides that activate DOPR receptors (Kang et al., 2005, Nieto et al., 2005). Together, these data indicate that DOPR plays an important role in the homeostatic regulation of anxiety states.
Amygdalar DOPR and CRF: anatomical considerations
Previous pharmacological and anatomical studies have demonstrated significant interactions between endogenous opioid peptide systems and CRF in the amygdalar complex (Drolet et al., 2001, Reyes et al., 2008, Van Bockstaele et al., 2010, Reyes et al., 2011, Andero et al., 2013, Kravets et al., 2015). Although CRF, DOPR and enkephalin-containing neurons are abundant in the amygdalar complex (Sharif and Hughes, 1989, Mansour et al., 1995, Criado and Morales, 2000, Randall-Thompson et al., 2010), there were no previous reports of localization of DOPR to amygdalar CRF neurons. Although it had been demonstrated that DOPR and CRF are co-localized in other neural circuits such as hippocampal interneurons and pyramidal cell dendrites (Williams et al., 2011, Williams and Milner, 2011), this is the first report demonstrating that DOPR are expressed in CRF-containing neurons in the amygdala. The present study is in agreement with previous reports showing that CRF-containing neurons are more abundant in the CeA compared to the BLA (Cassell et al., 1986, Sakanaka et al., 1986, Cassell and Gray, 1989, Van Bockstaele et al., 1998, Reyes et al., 2008, Iemolo et al., 2013). Interestingly, the present results reveal that DOPR exhibits a topographic distribution within the BLA and CeA where dense DOPR immunoreactivity was observed in the BLA compared to the CeA. The subcellular localization of DOPR within the BLA and CeA suggests that DOPR may function to inhibit the excitation of BLA and CeA neurons in the presence of a stressor (Herringa et al., 2004; Tye et al., 2011). The postsynaptic localization of DOPR is also supported by in situ hybridization and immunohistochemical data of enkephalin mRNA and immunoreactivity in the amygdalar complex including the BLA and CeA (Berube et al., 2013; Chang et al., 2014; Chieng et al., 2006; Zhang and McDonald, 2016).
The presence of DOPR and CRF postsynaptically in common dendrites suggests that the activation of DOPR can affect the postsynaptic excitability of CRF-containing neurons in both BLA and CeA. This in agreement with our previous reports indicating that endogenous opioids interact with CRF in an opposing fashion such that opioids may serve as a counter-regulatory mechanism to balance or limit excessive activation of the stress response (Van Bockstaele et al., 2010; Xu et al., 2004; Curtis et al., 2001). A distinct intracellular signaling pathway supports the functional opposition since opioids bind with Gi/Go coupled DOPR to inhibit the cAMP pathway while CRF binds to the Gs-coupled CRF receptor to stimulate the cAMP pathway (McKenzie and Milligan, 1990; Levitt et al., 2011; Grammatopoulos et al., 2001). While we demonstrated co-localization of CRF and DOPR in BLA dendrites, and evidence suggests that BLA sends unidirectional projections to the CeA (McDonald, 1998, LeDoux, 2000, Pitkänen, 2000, Tye et al., 2011), we were not able to determine the precise trajectory of BLA CRF efferents in the current study. Further investigation is needed to address this issue.
We also demonstrated that DOPR and CRF are co-localized in single axon terminals in the BLA and CeA, albeit infrequently. The anatomical interaction of DOPR and CRF suggests that DOPR may modulate CRF release in the BLA and CeA. Previous studies reveal that CeA and BLA receive afferents from several distinct brain regions (McDonald, 1998; Pittaken, 2000; Sah et al., 2003). Tract tracing studies demonstrate that potential sources of opioidergic input to the CeA arise from forebrain, thalamic, hypothalamic and hindbrain nuclei (Pitkanen et al., 2000). In particular, retrograde tracing studies have demonstrated that enkephalinergic afferents to the CeA originate from the bed nucleus of the stria terminalis (BNST) and from other amygdalar subnuclei (Poulin et al., 2006). With regard to afferents from amygdalar subnuclei, the major enkephalinergic afferents to the CeA arise from the anterolateral BNST, the cortical nucleus of the amygdala, the medial amygdaloid nucleus and basomedial nucleus (Poulin et al., 2006). Additionally, the ventromedial nucleus of the hypothalamus and the pontine parabrachial nucleus provide moderate enkephalinergic afferents to the CeA (Poulin et al., 2006). Intrinsic amygdalar enkephalinergic neurons, that are known to be distinct from amygdalar CRF neurons (Drolet et al., 2001; Reyes et al., 2008; Van Bockstaele et al., 2010; Reyes et al., 2011; Andero et al., 2013; Kravets et al., 2015), may play a role in the local circuit regulation of amygdalar neurons as there exists significant evidence describing such intrinsic connections in the CeA (Haubensak et al., 2010; Ciocci et al., 2010; Pomrenze et al., 2015). Potential opioidergic afferents to the BLA could also originate from local axonal arborizations of intrinsic enkephalinergic pyramidal neurons originating from the BLA (McDonald et al., 2005; Pitkanen et al., 2003). It is also likely that other sources of input could arise from the thalamus (Carlsen and Heimer, 1988; LeDoux et al., 1991) and cerebral cortex (Farb and Ledoux, 1999; Smith et al., 2000; Pinard et al., 2010). Since to our knowledge, this is the first report describing the ultrastructural analysis of DOPR in the BLA, future studies are necessary to define the source of opioidergic afferents in the BLA (Zhang and McDonald, 2016).
Noradrenergic modulation of amygdalar DOPR and CRF systems
Our semi-quantitative analysis indicates that DOPR and CRF-containing dendrites in the BLA and CeA primarily received symmetric synapses as compared to asymmetric synapses. Symmetric synapses are thought to be involved principally in inhibitory neurotransmission while asymmetric synapses are thought to be involved in excitatory neurotransmission (Peters et al., 1991, Peters and Palay, 1996). The preponderance of symmetric synapses onto DOPR and CRF neurons indicate that these dually labeled amygdalar neurons are potentially under inhibitory control (Gray, 1959). Future studies are needed to unequivocally establish this possibility as well as to investigate whether DOPR and CRF neurons are GABAergic themselves as there exists a large population of GABAergic neurons in the amygdala (Swanson and Petrovich, 1998, Ciocchi et al., 2010). Given that NET fibers targeted DOPR and CRF-containing neurons in our confocal results, it is possible that synaptic transmission occurs. In addition, the unlabeled axon terminals that target DOPR and CRF-containing dendrites may contain NET immunoreactivity. A triple EM can be utilized to address this possibility. There are reports of catecholaminergic neurotransmission occurring through conventional wired synaptic transmission and volume transmission, in which the neurotransmitter permeates through the extracellular space (Agnati et al., 1986). Wired synaptic transmission is associated with rapid responses to stress while volume transmission is associated with more long-term events (Perez de la Mora et al., 2008). Previous reports suggest that noradrenergic axon terminals exhibit a low frequency of synaptic contacts in the cortex and amygdala (Seguela et al., 1990, Asan, 1998). Our present results as well as studies from others have demonstrated that noradrenergic axon terminals more frequently form synaptic connections in both the BLA and CeA (Zhang et al., 2013, Kravets et al., 2015). It would be interesting to investigate whether CRF and NET are co-expressed at the ultrastructural level and whether they may contact DOPR-labeled dendrites or somata. Future investigations are needed to address this potential anatomical interaction using a triple immunolabeling electron microscopy approach (Reyes et al., 2011; Reyes et al., 2012; Kravets et al., 2013; Jaremko et al., 2013).
Consistent with our previous immunocytochemical studies (Rudoy and Van Bockstaele, 2005, Kravets et al., 2015) and those of Schroeter and colleagues (Schroeter et al., 2000), results from the present study show a moderate density of NET-ir fibers throughout the amygdalar complex with the BLA exhibiting a more robust distribution of NET-immunolabeling compared to the CeA. NET distribution and binding have demonstrated differential patterns of expression in the amygdalar complex. Quantitative autoradiography and in situ hybridization in rodents reveal a homogeneous binding pattern for the NET in the amygdalar complex, with higher binding in the BLA (Benmansour et al. 1992; Tejani-Butt 1992), whereas in non-human primates, NET has a heterogeneous distribution and moderate binding density in the CeA (Smith et al., 2006), and in humans, using spectrofluorimetric assay in postmortem brains, high concentrations of NE is found in the CeA (Farley and Hornykiewicz 1977).
NE neurotransmission in the in the BLA and CeA has been implicated in different segments of the stress response (Buffalari and Grace, 2007, Brown et al., 2011, Wenzel et al., 2014, Karkhanis et al., 2015, Rajbhandari et al., 2015). Stress-induced NE release occurs in both the BLA and CeA (Williams et al. 1998; Quirarte et al. 1998; Pacak et al. 1993; McIntyre et al. 2002). NE modulation of afferents to the BLA and CeA containing DOPR could possibly take place via α1- and β1-adrenergic receptors as NE could either excite or inhibit neuronal activities via these receptors (Johnson et al., 2011). NE acting through α1A-adrenergic receptors facilitates GABAergic activity in the BLA and repeated stress impairs this response (Braga et al., 2004). Excessive NE release in the BLA due to repeated stress exposure may desensitize the α1- adrenergic receptors, thus contributing to the hyper-excitability of the amygdala in stress-induced anxiety disorders (Braga et al., 2004). Considering that BLA sends GABAergic afferents to the CeA (Perez de la Mora et al., 2008), dysregulation of GABAergic transmission in the BLA may in turn adversely affect CeA activity. Similarly to BLA, α1-adrenergic receptors are abundantly distributed in the CeA (Day et al., 1997, Domyancic and Morilak, 1997). NE exerts its effects in the CeA via the α1-adrenergic receptors that are involved in the behavioral response to stressors (Pacak et al., 1993, Khoshbouei et al., 2002). The CeA is also enriched in α2- adrenergic receptors which are involved in the autonomic response to stressful stimuli (Glass et al., 2002). Similar to α1-adrenergic receptors, β-adrenergic receptors in the amygdala have been implicated in stress-induced behavioral response (Porterfield et al., 2012, Camp and Johnson, 2015). Although β-adrenergic receptors are not as abundant in the CeA, they have been found to be co-localized with CRF neurons and functionally related (Rudoy et al. 2009). Administration of a β1-adrenergic receptor antagonist attenuates heightened CRF expression levels produced by cocaine withdrawal in a rat model of chronic cocaine exposure (Rudoy et al. 2009).
The results from the present anatomical study suggest that DOPR might reside on presynaptic NE afferents in the amygdala, particularly in the CeA. As DOPR activation has been shown to inhibit NE release in a variety of tissues and species including humans (degli Uberti et al., 1993, Yilmaz and Gilmore, 1999, Berger et al., 2006, Hudzik et al., 2011, Schulte et al., 2011), this mechanism could be an important contributor to the anxiolytic effects of DOPR agonists. This relationship was tested functionally in the present study by examining the effects of a DOPR agonist, SNC80, on anxiety produced by yohimbine. Yohimbine is an α2-adrenergic antagonist that is used as a pharmacological stressor. It increases noradrenergic transmission in the amygdala (Khoshbouei et al., 2002, Crespi, 2009) and increases anxiety-like behaviors (Park et al., 2013, Yeung et al., 2013). Yohimbine also increases CRF mRNA in the amygdala of the rat which contributes to its actions as a pharmacological stressor (Funk et al., 2006). It is shown here that SNC80 administered prior to yohimbine blocked the anxiogenic effects of yohimbine as measured on the elevated zero maze. It is possible that DOPR located on NE terminals inhibited NE release and thus reduced the pro-anxiety effects of yohimbine. An alternative or perhaps additional mechanism is the potential blockade of yohimbine-induced CRF induction by SNC80 which would dampen the stress response. Further studies are needed to address these hypotheses.
Functional implications
The present results suggest an interaction between limbic CRF, NE, and DOPR systems that may play a significant role in the modulation of stress response in the amygdala. The LC, a noradrenergic nucleus that projects to almost all levels of neuraxis (Foote et al., 1983; Aston-Jones, et al., 1984), receives CRF and dynorphin (DYN) afferents from the amygdala (Van Bockstaele et al., 1996a; Van Bockstaele et al., 1996b; Van Bockstaele et al., 1998; Reyes et al., 2007; Reyes et al., 2008; Reyes et al., 2011). Furthermore, the CRF and DYN amygdalar efferents directly target LC noradrenergic dendrites (Van Bockstaele et al., 1996a; Reyes et al., 2008; Reyes et al., 2011). Given that limbic NE is critical in activating CRF neurons (Raber et al., 1995), the present results indicate that amygdalar CRF neurons are under noradrenergic control and are poised to regulate the LC noradrenergic system. In the presence of stressors, activation of the CRF, NE, and DOPR systems elicits physiological and behavioral responses allowing the human body to respond appropriately and cope with the challenge. The immediate response to stress is adaptive; however, chronic or prolonged stress exposure may exacerbate the system that could lead to maladaptive responses. NE released into the CeA would activate CRF neurons, which in turn activate LC neurons. However, the localization of DOPR on CRF neurons may reduce CRF activity and therefore preclude CeA activation that regulates LC neurons. In addition, the BLA sends GABAergic projections to the CeA which could modulate its activity (Perez de la Mora et al., 2008). The colocalization of CRF and DOPR in the CeA that are targeted by NE suggests noradrenergic modulation of the limbic CRF and DOPR system that could impact global NE release, as we previously showed that CRF forms asymmetric synapses onto LC noradrenergic neurons (Van Bockstaele et al., 1998, Reyes et al., 2008, Reyes et al., 2011). Anatomical and electrophysiological evidence suggest CRF as an excitatory neurotransmitter in the LC that translates to enhanced norepinephrine release that impact cortical targets (Curtis et al., 1997, Page and Abercrombie, 1999, Curtis et al., 2002). The amplification of CRF and NE release may disturb allostasis, an essential component of maintaining homeostasis (McEwen, 2006) which involves adaptation in the face of potentially stressful challenges that activates neural, neuroendocrine and neuroendocrine-immune mechanisms (Sterling and Eyer, 1988). Given the intricate interactions of CRF, NE and DOPR systems, dysregulation of one system may succumb to dysfunction in another.
In summary, the findings presented herein indicate the complex relationship between the CRF, NE, and DOPR systems in two regions of the amygdala that are critically important to stress responses and emotional homeostasis. Recent reports indicate a rising number of stress-related psychiatric disorders that are resistant to standard pharmacological treatments (Jasovic-Gasic, 2015). Targeting multiple neuromodulatory systems (Hopkins, 2007) including CRF, NE, and DOPR systems may be a relevant therapeutic approach to restore altered neuronal activity and treat stress-related disorders.
Acknowledgments
This project was supported by the National Institutes of Health grants DA009082 to E.J.V.B. and R01 DA018326 and T32 DA07237 to E.M.U.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta physiologica Scandinavica. 1986;128:201–207. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- Ambrose-Lanci LM, Peiris NB, Unterwald EM, Van Bockstaele EJ. Cocaine withdrawal-induced trafficking of delta-opioid receptors in rat nucleus accumbens. Brain research. 2008;1210:92–102. doi: 10.1016/j.brainres.2008.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, Bannister TD, Almli L, Stevens JS, Bradley B, Binder EB, Wahlestedt C, Ressler KJ. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Science translational medicine. 2013;5:188ra173. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. The Journal of endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Bloom FE. Anatomy and physiology of locus coeruleus neurons: functional implications. In: Ziegler M, Lake CR, editors. Norepinephrine (Frontiers of Clinical Neuroscience) Vol. 2. Baltimore: Williams and Wilkins; 1984. pp. 92–116. [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Advances in anatomy, embryology, and cell biology. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Altamirano AV, Jones DJ, Sanchez TA, Gould GG, Pardon MC, Morilak DA, Frazer A. Regulation of the norepinephrine transporter by chronic administration of antidepressants. Biological psychiatry. 2004;55:313–316. doi: 10.1016/s0006-3223(03)00676-0. [DOI] [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. British journal of pharmacology. 2006;148:795–806. doi: 10.1038/sj.bjp.0706782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube P, Laforest S, Bhatnagar S, Drolet G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiology & behavior. 2013;122:237–245. doi: 10.1016/j.physbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacology, biochemistry, and behavior. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Nobrega JN, Erb S. Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behavioural brain research. 2011;217:472–476. doi: 10.1016/j.bbr.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PloS one. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Bakalli H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience. 2012;223:200–208. doi: 10.1016/j.neuroscience.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RM, Johnson JD. Repeated stressor exposure enhances contextual fear memory in a beta-adrenergic receptor-dependent process and increases impulsivity in a non-beta receptor-dependent fashion. Physiology & behavior. 2015;150:64–68. doi: 10.1016/j.physbeh.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. The European journal of neuroscience. 2010;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain research. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. The Journal of comparative neurology. 1989;281:320–333. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. The Journal of comparative neurology. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:363–372. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Barson JR, Liang SC, Leibowitz SF. Common effects of fat, ethanol, and nicotine on enkephalin in discrete areas of the brain. Neuroscience. 2014;277:665–678. doi: 10.1016/j.neuroscience.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. The Journal of comparative neurology. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Collin E, Mauborgne A, Bourgoin S, Chantrel D, Hamon M, Cesselin F. In vivo tonic inhibition of spinal substance P (-like material) release by endogenous opioid(s) acting at delta receptors. Neuroscience. 1991;44:725–731. doi: 10.1016/0306-4522(91)90091-2. [DOI] [PubMed] [Google Scholar]
- Crespi F. Anxiolytics antagonize yohimbine-induced central noradrenergic activity: a concomitant in vivo voltammetry-electrophysiology model of anxiety. Journal of neuroscience methods. 2009;180:97–105. doi: 10.1016/j.jneumeth.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Criado JR, Morales M. Acute ethanol induction of c-Fos immunoreactivity in pre-pro-enkephalin expressing neurons of the central nucleus of the amygdala. Brain research. 2000;861:173–177. doi: 10.1016/s0006-8993(99)02468-3. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ. Evidence for functional release of endogenous opioids in the locus ceruleus during stress termination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:RC152. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connolly KR, Valentino RJ. Corticotropin-releasing factor neurones of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. Journal of neuroendocrinology. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. The Journal of pharmacology and experimental therapeutics. 1997;281:163–172. [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. Journal of chemical neuroanatomy. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- degli Uberti EC, Ambrosio MR, Vergnani L, Portaluppi F, Bondanelli M, Trasforini G, Margutti A, Salvadori S. Stress-induced activation of sympathetic nervous system is attenuated by the delta-opioid receptor agonist deltorphin in healthy man. The Journal of clinical endocrinology and metabolism. 1993;77:1490–1494. doi: 10.1210/jcem.77.6.8263131. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. The Journal of comparative neurology. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Progress in neuro-psychopharmacology & biological psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Farb CR, Ledoux JE. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse. 1999;33:218–229. doi: 10.1002/(SICI)1098-2396(19990901)33:3<218::AID-SYN6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Farley IJ, Hornykiewicz O. Noradrenaline distribution insubcortical areas of the human brain. Brain research. 1977;126:53–62. doi: 10.1016/0006-8993(77)90214-1. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Involvement of basolateral amygdala alpha2-adrenoceptors in modulating consolidation of inhibitory avoidance memory. Learn Mem. 2008;15:238–243. doi: 10.1101/lm.760908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nature genetics. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biological psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of learning and memory. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Colago EE, Pickel VM. Alpha-2A-adrenergic receptors are present on neurons in the central nucleus of the amygdala that project to the dorsal vagal complex in the rat. Synapse. 2002;46:258–268. doi: 10.1002/syn.10136. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. Journal of neurochemistry. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Gray EG. Axosomatic and axo-dendritic synapses of the cerebral cortex: an electron microscopic study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Critical reviews in neurobiology. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, Koob GF. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1994;11:179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain research. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Brain research Molecular brain research. 2004;131:17–25. doi: 10.1016/j.molbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology. Nature biotechnology. 2007;25:1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, Coupal M, Adam L, Payza K, Griffin A, Smagin G, Song D, Swedberg MD, Brown W. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. The Journal of pharmacology and experimental therapeutics. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, Cottone P. CRF-CRF1 receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2456–2466. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Jaremko KM, Thompson NL, Jr, Reyes BA, Jin J, Ebersole B, Jenney CB, Grigson PS, Levenson R, Berrettini WH, Van Bockstaele EJ. Morphine-induced trafficking of a mu-opioid receptor interacting protein in rat locus coeruleus neurons. Progress in neuro-psychopharmacology & biological psychiatry. 2014;50:53–65. doi: 10.1016/j.pnpbp.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasovic-Gasic M. Is treatment-resistance in psychiatric disorders a trap for polypharmacy? Psychiatria Danubina. 2015;27:308–313. [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn-Schmiedeberg’s archives of pharmacology. 2009;379:40–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, Hou M, Prager EM, Ledoux JE. Regulation of the Fear Network by Mediators of Stress: Norepinephrine Alters the Balance between Cortical and Subcortical Afferent Excitation of the Lateral Amygdala. Frontiers in behavioral neuroscience. 2011;5:23. doi: 10.3389/fnbeh.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Wee S, Edwards S, Whitfield TW, Jr, Oleata CS, Luu G, Schmeichel BE, Koob GF, Roberto M. Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biological psychiatry. 2013;74:520–528. doi: 10.1016/j.biopsych.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Arnkoff DB, Glass CR, Tinsley R, Geraci M, Hernandez E, Luckenbaugh D, Drevets WC, Carlson PJ. Avoidant coping in panic disorder: a yohimbine biological challenge study. Anxiety, stress, and coping. 2012;25:425–442. doi: 10.1080/10615806.2011.609587. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Alexander NJ, McCool BA, Weiner JL, Jones SR. Chronic social isolation during adolescence augments catecholamine response to acute ethanol in the basolateral amygdala. Synapse. 2015;69:385–395. doi: 10.1002/syn.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacology, biochemistry, and behavior. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA., Jr Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biological psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Annals of the New York Academy of Sciences. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Kravets JL, Reyes BA, Unterwald EM, Van Bockstaele EJ. Direct targeting of peptidergic amygdalar neurons by noradrenergic afferents: linking stress-integrative circuitry. Brain structure & function. 2015;220:541–558. doi: 10.1007/s00429-013-0674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Gianoulakis C. Effects of acute ethanol on corticotropin-releasing hormone and beta-endorphin systems at the level of the rat central amygdala. Psychopharmacology. 2011;218:229–239. doi: 10.1007/s00213-011-2337-x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neuroscience letters. 1991;134:139–144. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic preembedding double-labeling methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tracing methods. 1. Vol. 2. New York: Plenum Press; 1989. pp. 129–172. [Google Scholar]
- Levitt ES, Purington LC, Traynor JR. Gi/o-coupled receptors compete for signaling to adenylyl cyclase in SH-SY5Y cells and reduce opioid-mediated cAMP overshoot. Molecular pharmacology. 2011;79:461–471. doi: 10.1124/mol.110.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Hormones and behavior. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamalaki E, Kvetnansky R, Brady LS, Gold PW, Herkenham M. Repeated immobilization stress alters tyrosine hydroxylase, corticotropin-releasing hormone and corticosteroid receptor messenger ribonucleic Acid levels in rat brain. Journal of neuroendocrinology. 1992;4:689–699. doi: 10.1111/j.1365-2826.1992.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ., Jr Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in neurosciences. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron. 2015;87:605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain research. 2005;1035:32–40. doi: 10.1016/j.brainres.2004.11.052. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Vol. 840. New York: Wiley & Sons; 2006. pp. 33–44. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiology of learning and memory. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology. 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Pal SN, Marriott LK, Gold PE. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FR, Milligan G. Delta-opioid-receptor-mediated inhibition of adenylate cyclase is transduced specifically by the guanine-nucleotide-binding protein Gi2. The Biochemical journal. 1990;267:391–398. doi: 10.1042/bj2670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. The Journal of comparative neurology. 1990;295:662–682. doi: 10.1002/cne.902950409. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Baker NL, Ramakrishnan V, Brady KT, McRae-Clark A. Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. The American journal of drug and alcohol abuse. 2015;41:146–152. doi: 10.3109/00952990.2014.945590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Onur OA, Walter H, Schlaepfer TE, Rehme AK, Schmidt C, Keysers C, Maier W, Hurlemann R. Noradrenergic enhancement of amygdala responses to fear. Social cognitive and affective neuroscience. 2009;4:119–126. doi: 10.1093/scan/nsn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kvetnansky R, Fukuhara K, Armando I, Kopin IJ, Goldstein DS. Effects of single or repeated immobilization on release of norepinephrine and its metabolites in the central nucleus of the amygdala in conscious rats. Neuroendocrinology. 1993;57:626–633. doi: 10.1159/000126417. [DOI] [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF. Corticotropin-releasing factor (CRF) and alpha 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int J Neuropsychopharmacol. 2013;16:1867–1875. doi: 10.1017/S1461145713000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Perez de la Mora M, Jacobsen KX, Crespo-Ramirez M, Flores-Gracia C, Fuxe K. Wiring and volume transmission in rat amygdala. Implications for fear and anxiety. Neurochemical research. 2008;33:1618–1633. doi: 10.1007/s11064-008-9722-9. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. British journal of pharmacology. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain research Brain research reviews. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, Muller JF, McDonald AJ. Limited convergence of rhinal cortical and dopaminergic inputs in the rat basolateral amygdala: an ultrastructural analysis. Brain research. 2010;1332:48–56. doi: 10.1016/j.brainres.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton J, editor. The amygdala: A functional analysis. New York: Oxford Univ Press; 2000. pp. 31–115. [Google Scholar]
- Pitkanen A, Savander M, Nurminen N, Ylinen A. Intrinsic synaptic circuitry of the amygdala. Annals of the New York Academy of Sciences. 2003;985:34–49. doi: 10.1111/j.1749-6632.2003.tb07069.x. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, Janak PH, George O, Rice KC, Messing RO. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Frontiers in neuroscience. 2015;9:487. doi: 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield VM, Gabella KM, Simmons MA, Johnson JD. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1beta production. Brain, behavior, and immunity. 2012;26:1249–1255. doi: 10.1016/j.bbi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. The Journal of comparative neurology. 2006;496:859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II: The amygdaloid complex. Elsevier Science; New York: 1987a. [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II: The amygdaloid complex. In: Bjorklund A, et al., editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier Science; 1987b. pp. 279–386. [Google Scholar]
- Primeaux SD, Wilson SP, McDonald AJ, Mascagni F, Wilson MA. The role of delta opioid receptors in the anxiolytic actions of benzodiazepines. Pharmacology, biochemistry, and behavior. 2006;85:545–554. doi: 10.1016/j.pbb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain research. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Raber J, Koob GF, Bloom FE. Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling; comparison with the hypothalamic response. The Journal of pharmacology and experimental therapeutics. 1995;272:815–824. [PubMed] [Google Scholar]
- Radley J, Morilak D, Viau V, Campeau S. Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience and biobehavioral reviews. 2015 doi: 10.1016/j.neubiorev.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari AK, Baldo BA, Bakshi VP. Predator Stress-Induced CRF Release Causes Enduring Sensitization of Basolateral Amygdala Norepinephrine Systems that Promote PTSD-Like Startle Abnormalities. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:14270–14285. doi: 10.1523/JNEUROSCI.5080-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology. 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retson TA, Hoek JB, Sterling RC, Van Bockstaele EJ. Amygdalar neuronal plasticity and the interactions of alcohol, sex, and stress. Brain structure & function. 2014 doi: 10.1007/s00429-014-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Carvalho AF, Vakharia K, Van Bockstaele EJ. Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Experimental neurology. 2011;230:96–105. doi: 10.1016/j.expneurol.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Drolet G, Van Bockstaele EJ. Dynorphin and stress-related peptides in rat locus coeruleus: contribution of amygdalar efferents. The Journal of comparative neurology. 2008;508:663–675. doi: 10.1002/cne.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Glaser JD, Magtoto R, Van Bockstaele EJ. Pro-opiomelanocortin colocalizes with corticotropin- releasing factor in axon terminals of the noradrenergic nucleus locus coeruleus. The European journal of neuroscience. 2006;23:2067–2077. doi: 10.1111/j.1460-9568.2006.04744.x. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Johnson AD, Glaser JD, Commons KG, Van Bockstaele EJ. Dynorphin-containing axons directly innervate noradrenergic neurons in the rat nucleus locus coeruleus. Neuroscience. 2007;145:1077–1086. doi: 10.1016/j.neuroscience.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Vakharia K, Ferraro TN, Levenson R, Berrettini WH, Van Bockstaele EJ. Opiate agonist-induced re-distribution of Wntless, a mu-opioid receptor interacting protein, in rat striatal neurons. Experimental neurology. 2012;233:205–213. doi: 10.1016/j.expneurol.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. Cocaine effects on norepinephrine in the amygdala: Cocaine withdrawal-related anxiety and stress-related relapse. Cell Sci Rev. 2005:2. [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sah P, Lopez De Armentia M. Excitatory synaptic transmission in the lateral and central amygdala. Annals of the New York Academy of Sciences. 2003;985:67–77. doi: 10.1111/j.1749-6632.2003.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. Journal of pharmacological sciences. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology. 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain research. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Salman S, Buttigieg J, Zhang M, Nurse CA. Chronic exposure of neonatal rat adrenomedullary chromaffin cells to opioids in vitro blunts both hypoxia and hypercapnia chemosensitivity. The Journal of physiology. 2013;591:515–529. doi: 10.1113/jphysiol.2012.243477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman S, Holloway AC, Nurse CA. Chronic opioids regulate KATP channel subunit Kir6.2 and carbonic anhydrase I and II expression in rat adrenal chromaffin cells via HIF-2alpha and protein kinase A. American journal of physiology Cell physiology. 2014;307:C266–277. doi: 10.1152/ajpcell.00135.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Fukui Y, Hawkes R. Spatial distribution of corticotropin-releasing factor immunopositive climbing fibers in the mouse cerebellum: analysis by whole mount immunohistochemistry. Brain research. 2008;1222:106–117. doi: 10.1016/j.brainres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. The Journal of comparative neurology. 2000;420:211–232. [PubMed] [Google Scholar]
- Schulte K, Kumar M, Zajac JM, Schlicker E. Noradrenaline release in rodent tissues is inhibited by interleukin-1beta but is not affected by urotensin II, MCH, NPW and NPFF. Pharmacological reports : PR. 2011;63:102–111. doi: 10.1016/s1734-1140(11)70404-2. [DOI] [PubMed] [Google Scholar]
- Seguela P, Watkins KC, Geffard M, Descarries L. Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neuroscience. 1990;35:249–264. doi: 10.1016/0306-4522(90)90079-j. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Hughes J. Discrete mapping of brain Mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10:499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Smith ME, Flynn FW. Distribution of Fos-like immunoreactivity within the rat brain following intraventricular injection of the selective NK(3) receptor agonist senktide. The Journal of comparative neurology. 2000;426:413–428. doi: 10.1002/1096-9861(20001023)426:3<413::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Ethanol and corticotropin releasing factor receptor modulation of central amygdala neurocircuitry: An update and future directions. Alcohol. 2015;49:179–184. doi: 10.1016/j.alcohol.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends in neurosciences. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- Stone EA, Zhang Y, Hiller JM, Simon EJ, Hillman DE. Activation of fos in mouse amygdala by local infusion of norepinephrine or atipamezole. Brain research. 1997;778:1–5. doi: 10.1016/s0006-8993(97)00667-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in neurosciences. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain research. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tsuda A, Yokoo H, Yoshida M, Mizoguchi K, Shimizu T. Psychological stress-induced increases in noradrenaline release in rat brain regions are attenuated by diazepam, but not by morphine. Pharmacology, biochemistry, and behavior. 1991a;39:191–195. doi: 10.1016/0091-3057(91)90420-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yokoo H, Mizoguchi K, Yoshida M, Tsuda A, Tanaka M. Noradrenaline release in the rat amygdala is increased by stress: studies with intracerebral microdialysis. Brain research. 1991b;544:174–176. doi: 10.1016/0006-8993(91)90902-8. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM. [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. The Journal of pharmacology and experimental therapeutics. 1992;260:427–436. [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nature reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Chan J, Pickel VM. Input from central nucleus of the amygdala efferents to pericoerulear dendrites, some of which contain tyrosine hydroxylase immunoreactivity. Journal of neuroscience research. 1996a;45:289–302. doi: 10.1002/(SICI)1097-4547(19960801)45:3<289::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. The Journal of comparative neurology. 1996b;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. Journal of neuroendocrinology. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain research. 2010;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain research. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cotten SW, Dominguez HM, Lane JE, Shelton K, Su ZI, Ettenberg A. Noradrenergic beta-receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3467–3474. doi: 10.1523/JNEUROSCI.3861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Experimental neurology. 2011;230:186–196. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Milner TA. Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience. 2011;179:9–22. doi: 10.1016/j.neuroscience.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GP, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8193–8197. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang H, Du X, Ma Q, Song J, Chen M, Dong Y, Ma L, Zheng P. Morphine and DAMGO produce an opposite effect on presynaptic glutamate release via different downstream pathways of mu opioid receptors in the basolateral amygdala. Neuropharmacology. 2014;86:353–361. doi: 10.1016/j.neuropharm.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Yeung M, Lu L, Hughes AM, Treit D, Dickson CT. FG7142, yohimbine, and betaCCE produce anxiogenic-like effects in the elevated plus-maze but do not affect brainstem activated hippocampal theta. Neuropharmacology. 2013;75:47–52. doi: 10.1016/j.neuropharm.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Yilmaz B, Gilmore DP. Effects of mu, kappa, and delta opioid receptor agonists and antagonists on rat hypothalamic noradrenergic neurotransmission. Brain research bulletin. 1999;48:491–495. doi: 10.1016/s0361-9230(99)00020-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, McDonald AJ. Light and electron microscopic analysis of enkephalin-like immunoreactivity in the basolateral amygdala, including evidence for convergence of enkephalin-containing axon terminals and norepinephrine transporter-containing axon terminals onto common targets. Brain research. 2016;1636:62–73. doi: 10.1016/j.brainres.2016.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Muller JF, McDonald AJ. Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience. 2013;228:395–408. doi: 10.1016/j.neuroscience.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]