Abstract
Stroke is the leading cause of disability in adults. Drug treatments that target stroke-induced pathological mechanisms and promote recovery are desperately needed. In the brain, an ischemic event triggers major inflammatory responses that are mediated by the resident microglial cells. In this review, we focus on the microglia activation after ischemic brain injury as a target of immunomodulatory therapeutics. We divide the microglia-mediated events following ischemic stroke into three categories: acute, subacute, and long-term events. This division encompasses the spatial and temporal dynamics of microglia as they participate in the pathophysiological changes that contribute to the symptoms and sequela of a stroke. The importance of Toll-like receptor (TLR) signaling in the outcomes of these pathophysiological changes is highlighted. Increasing evidence shows that microglia have a complex role in stroke pathophysiology and they mediate both detrimental and beneficial effects on stroke outcome. So far, most of the pharmacological studies in experimental models of stroke have focused on neuroprotective strategies which are impractical for clinical applications. Post-ischemic inflammation is long lasting and thus, could provide a therapeutic target for novel delayed drug treatment. However, more studies are needed to elucidate the role of microglia in the recovery process from an ischemic stroke and to evaluate the therapeutic potential of modulating post-ischemic inflammation to promote functional recovery.
Keywords: inflammation, TLR, NF-κB, nuclear factor kappa B
1. INTRODUCTION
Stroke is the fifth leading cause of death in the United States of America (Mozaffarian et al., 2016) and a leading cause of disability in adults. Approximately 10 million patients survive stroke annually. In the USA alone, the direct and indirect annual costs in 2012 were $33 billion for the acute and chronic treatment of stroke (Mozaffarian et al., 2016). The main risk factor for stroke is age, and as life span increases the number of people suffering from stroke is rising. Slow and incomplete recovery is associated with a reduction in quality of life, which is compounded by the lack of drug treatments to facilitate the recovery process. Acute care relies on thrombolytic treatment using tissue plasminogen activator (TPA), but it can be administered only to a small fraction of patients. Furthermore, even when TPA is given within the therapeutic window (4.5 hours) it has inconsistent efficacy.
Focal ischemic stroke is caused by an arterial thrombus that blocks blood flow to the brain and leads to a loss of oxygen and glucose supply to the downstream tissue. Anaerobic conditions result in protein and lipid modification, dysfunction of ionic pumps, loss of membrane potential, Ca2+ dysregulation, mitochondrial dysfunction, endoplasmic reticulum (ER) stress and apoptosis (Dirnagl et al., 1999). The region that is fully dependent on the vasculature immediately downstream of the thrombosis can start to die within minutes, and forms the core of the infarct. The area surrounding the infarct, the penumbra, has a partial loss of blood flow in addition to an extracellular milieu that is dominated by chemicals diffusing from necrotic cells within the infarct area. The initial injury also includes an excessive and uncontrolled release of neurotransmitters, such as glutamate, from stressed cells that triggers excitotoxicity in local cells. The peri-infarct region may be minimally impacted by the diminished blood flow directly (if spatially distant from the thrombus), but cells in this region must cope with indirect effects such as excitotoxicity. Restoring blood flow leads to deoxygenation and the formation of reactive oxygen species that contribute to reperfusion damage (Nour et al., 2013). These neurodegenerative processes in the ischemic brain result in disruption of neural circuits such as those that control motoric, sensory and cognitive functions.
The treatment strategies for stroke can be divided into four different categories: prophylaxis, neuroprotection, thrombolysis and promotion of recovery. Prophylactic approaches include healthy life style, and treatment of high blood pressure and cholesterol levels. Many of the failed clinical trials for stroke were focused on neuroprotection. Most experimental stroke therapies are designed to intervene within a narrow time-window after the ischemic event in order to prevent the stressed cells in the peri-infarct area from dying, and it has been shown that neuroprotective efficacy is highly dependent on the time of administration (Belayev et al., 2001). The thrombolysis approach given within 4.5 h is the only one that has shown efficacy, although it is also limited, and can lead to reperfusion injury. The inability to translate the preclinical experiments to the clinic has led to proposals that next generation therapeutic strategies should focus on outcomes other than lesion size (Dirnagl, 2012). Instead, the goal should be to target the pathophysiological mechanisms that dominate during the days and weeks following the initial ischemic event (Dirnagl, 2012). For example, new therapies could be developed that target microglia-mediated inflammation following stroke, which would extend the therapeutic window from hours to days.
Microglia are the resident macrophages in the central nervous system and regulate tissue homeostasis throughout life. The proportion of glial cells in the mouse brain that are microglia is estimated at 5–12% (Lawson et al., 1990). Microglia are a distinct subtype of mononuclear macrophages, which is a class that also includes peripheral tissue macrophages, dendritic cells and monocyte-derived cells (Butovsky et al., 2012; Gautier et al., 2012; Prinz and Priller, 2014). The complex inflammatory response in the brain following a stroke includes activation of microglia and infiltration of peripheral immune cells into the brain parenchyma. In stroke, an immune response is triggered by proteins released rapidly from dead and damaged cells. Toll-like receptors (TLRs) are a key class of receptors that regulate microglia activation. TLRs are pattern recognition receptors, imperative to the immune system for the recognition of pathogen-associated molecular patterns (PAMPs) and endogenous-derived danger-associated molecular patterns (DAMPs). They are expressed among a variety of cells, spanning the periphery and immune-privileged brain. There are a variety of TLRs (nomenclature ranging 1–11 in humans), that differ in PAMP/DAMP recognition and location (Akira and Takeda, 2004). For example, TLR4 and TLR2 are localized to the cell surface and become activated upon the recognition of lipopolysaccharide (LPS). Other TLRs, such as TLR9, are localized intracellularly within endosomal membranes and primarily recognize nucleic acids. Some TLRs, TLR4 and TLR2 in particular, can recognize DAMPs from ischemic stroke events such as heat shock proteins, fibrinogen, RNA and methylated DNA (Fadakar et al., 2014). While the expression of all TLR orthologues has been reported in mouse microglia, TLR4 and TLR2 are the most predominantly expressed TLRs in the human brain (Nishimura and Naito, 2005; Olson and Miller, 2004).
2. MICROGLIA IN ISCHEMIC STROKE
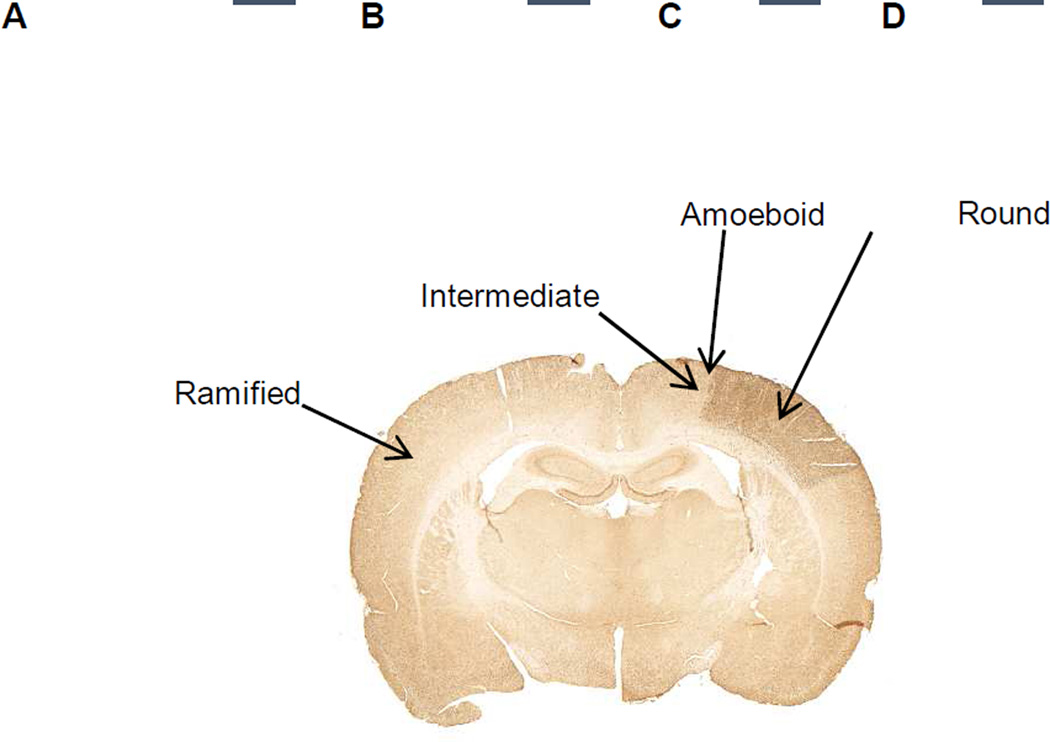
In ischemic injury, microglial cells undergo changes in morphology and gene expression that are known collectively as microglial activation (Kettenmann et al., 2011). In healthy tissue, microglia have a ramified structure of highly motile processes that are actively sensing and maintaining homeostatic conditions within the area surrounding that individual microglial cell. In ischemic injury, microglial cell retracts finer processes and may exhibit chemotaxis to DAMPs released from the dead and dying cells. When activated after stroke, microglial cells progress through four morphological states that are indicative of increasing activation (Figure 1): ramified, intermediate, amoeboid and round (Lehrmann et al., 1997; Thored et al., 2009). Ramified microglia are in the resting state, with small cell body and long processes and can be found in the contralateral side of the ischemic brain and in distal areas in the ipsilateral side. The intermediate state of activated microglia is characterized by enlarged cell body and short processes. Amoeboid microglial cells have very short or no processes and an amoeboid shape. Intermediate and amoeboid types of cells can be found in the peri-infarct region (Anttila and Airavaara, 2016 unpublished results; Lehrmann et al., 1997). Round microglia are macrophage-like and the most activated form of microglia. Round cells are found in the infarct core region (Anttila and Airavaara, 2016 unpublished results; Lehrmann et al., 1997). While following the same morphological progression, the intracellular dynamics and protein production of microglial cells can be categorized as either M1-like (‘classical’) or M2-like (‘alternative’) (Tang and Le, 2016). M1 type is pro-inflammatory and characterized by inducible nitric oxide synthase and nuclear factor kappa B (NF-κB) activation, and production and release of nitric oxide and pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β). M2 type is often anti-inflammatory and characterized by production of molecules that support anti-inflammation and tissue repair such as insulinlike growth factor 1 (IGF1) and arginase 1. Though, it has been suggested that in many cases the cellular phenotypes overlap (Boche et al., 2013). Moreover, the categorization to M1 and M2 phenotypes can be clear under in vitro conditions, but it is not well known how applicable this categorization is in vivo.
Figure 1. Microglia can be classified into four different phenotypes based on the morphology of the cell: A) ramified, B) intermediate, C) amoeboid and D) round type.
90 minutes transient MCAo was induced in adult male Sprague Dawley rats as described earlier (Airavaara et al., 2009). Rat brains were perfusion-fixed 7 days post-stroke, embedded in paraffin, sectioned, and immunostained for Iba1. Ramified microglial cells are found in the contralateral side of the brain. Intermediate and amoeboid type cells are found in the peri-infarct region. Round type cells are found in the ischemic core region (dark brown). Categorization of microglia morphology is adopted from Lehrmann et al., 1997; Thored et al., 2009. Scale bar is 10 µm.
Several recent review articles have discussed microglia and stroke (Liguz-Lecznar and Kossut, 2013; Ma et al., 2016; Pedata et al., 2015). Here, we provide a different point of view by dividing the microglia-mediated events in ischemic stroke into three phases: acute, subacute, and long-term events (Figure 2). Separating microglial responses by location and time, we can better understand the phenotypes of microglia in spatiotemporal manner of post-ischemic processes. Furthermore, we propose that next generation therapeutic design must consider the dynamics of microglia activation since the phenotypes of activated microglia at the time of treatment can be drastically different in different areas of the brain.
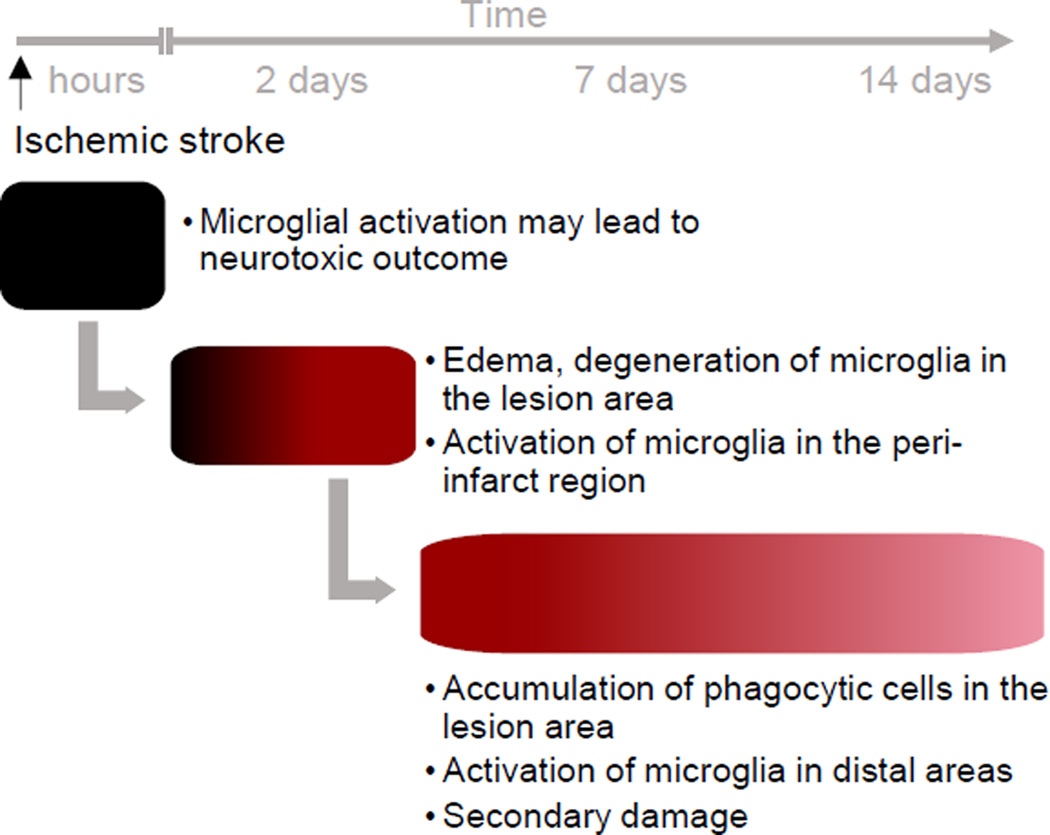
Figure 2. Time-scale of microglia-mediated events after ischemic stroke.
The acute ischemic damage is induced within minutes to hours in the ischemic core region. The microglial responses include release of pro-inflammatory cytokines, chemokines and neurotransmitters. Using protective molecules targeted to microglia, the lesion size can be decreased. In the subacute phase the lesion continues to develop. There is edema and degeneration of microglial cells in the lesion area. In the peri-infarct region microglia polarize and get activated. There are no morphological changes in areas distal to the ischemic core region. The subacute phase also includes infiltration of inflammatory cells. Long-term changes include accumulation of phagocytic microglia to the core area and clearance of dead tissue. Microglial activation can be observed also in the distal areas and there is formation of secondary damage. Schematic time-line is modified from studies of Anttila and Airavaara, 2016 unpublished results; Ito et al., 2001; Kato et al., 1996; Lehrmann et al., 1997; Perego et al., 2011; Ritzel et al., 2015; Rupalla et al., 1998; Thored et al., 2009.
2.1. Acute microglial response
Similar to any other cell in the ischemic brain, microglial cells go through apoptotic and necrotic death as a result of loss of oxygen and glucose in the infarct core region. Microglia have been reported to start to degenerate in the ischemic area at 4 hours after transient MCAo (Kato et al., 1996). Excitotoxicity from the release of glutamate is a trigger for microglia in the infarct area and a defining feature for the peri-infarct region (Murugan et al., 2013). Microglial cells are stimulated by the lack of oxygen and the precipitous rise in extracellular neurotransmitters from the affected neurons. Microglial activation in the peri-infarct area, defined by morphological change, has been observed 3.5 hours after transient filament MCAo in rat (Ito et al., 2001) and as early as 30 minutes after ischemia onset in a permanent MCAo model in mice (Rupalla et al., 1998). The acute responses of microglia to ischemia include nonspecific release and secretion of pro-inflammatory cytokines, chemokines and neurotransmitters into the extracellular milieu. Similar to neurons in the ischemic core region, the release can be uncontrolled and massive. Blocking of inflammatory processes is beneficial in experimental stroke models and the neuroprotective effects of various anti-inflammatory strategies are discussed in sections 3 and 4.
2.2. Subacute microglial response
The lesion continues to develop during the subacute phase in the hours and few days after transient ischemic stroke, and is associated with edema and infiltration of peripheral leukocytes. The subacute inflammatory response of microglia after ischemic injury can be characterized by changes in the morphology and polarization state of microglial cells in the peri-infarct region as microglia respond to DAMPs (Kigerl et al., 2014; Lehrmann et al., 1997; Neher et al., 2013). DAMPs tend to be intracellular proteins that are not typically found in the extracellular space in sufficient quantities to be stimulating. However, in stroke, many DAMPs are released into the extracellular space from damaged native cells (Neher et al., 2013). Subacutely activated microglial cells that are triggered after stroke onset are responding to a particular subset of DAMPs in the peri-infarct region. Activated microglia of mostly intermediate type and few phagocytic CD68+ cells can be detected in the penumbra area starting from 1 day post-stroke after transient or permanent ischemia (Lehrmann et al., 1997; Perego et al., 2011). In severe ischemia, the infarct core area is depleted of microglia due to the lack of oxygen-providing blood flow, and microglial activation occurs mainly in the penumbra area at the subacute phase, but round microglia and macrophages have been reported to re-enter the core area starting from 1 day post-stroke (Ito et al., 2001; Lehrmann et al., 1997; Ritzel et al., 2015).
2.3. Long-term microglial response and secondary damage
The long-term effects of microglial response include microglial activation in the peri-infarct region as well as in distal areas, accumulation of phagocytic cells in the lesion area and secondary damage. Numbers of the round microglia and macrophages are highest in the core area between 3 and 7 days post-stroke which we consider to be already the long-term phase (Kato et al., 1996; Lehrmann et al., 1997; Perego et al., 2011). Morphological changes in microglia can also be found distal to the initial injury. Microglia in the long-term phase are responding to a fundamentally different milieu than microglial cells that have been activated by the initial ischemic event (Figure 2). As such, the long-term phase of activation may have distinctly different characteristics depending on how, when and where microglia was activated relative to the injury as well as the onset of therapeutic intervention.
The neuroprotective M2 phenotype is the predominant form of microglia during the first days after stroke, but diminishes by day 7 post-stroke when the neurodegenerative M1 phenotype starts to dominate (Hu et al., 2012; Perego et al., 2011). It is suggested that the polarization of microglia and macrophages first to M2 type after ischemic injury may represent an attempt to restrict the damage by cleaning the necrotic tissue. Thus, a therapeutic approach may be to change the polarization dynamics in order to delay the M2 phenotype after ischemia (Hu et al., 2012). After the ischemic lesion has fully developed, immune cells accumulate to the ischemic core to clear the necrotic tissue. Phagocytic monocytes/macrophages infiltrate the ischemic brain between 3 and 6 days post-stroke and aggregate at the ischemic core area (Kato et al., 1996; Schroeter et al., 1997). The peak number of microglia and macrophages within the peri-infarct and core infarct area occurs at the same time, between 4 and 7 days post-stroke in transient MCAo, and decreases by 14–21 days post-stroke (Ito et al., 2001; Lehrmann et al., 1997). According to Lehrmann et al., at 3 months post-stroke, microglia are no longer activated (Lehrmann et al., 1997). However, microglial activation in the stroke brain may be more persistent than was once thought. Activated microglia are present in the subventricular zone (SVZ) and striatum for up to 4 months after transient filament MCAo (Thored et al., 2009). In the same model, activated microglia could be detected as long as 6 months post-stroke in the thalamus (Justicia et al., 2008). In patients, microglial activation has been reported in the stroke brain up to 6 months after stroke using 11C-PK11195 PET imaging (Thiel et al., 2010).
Neuronal degeneration after focal ischemic stroke occurs also in distal brain regions that receive inputs from neurons in the infarct area (Block et al., 2005). After cortical damage, neurons in the thalamus and substantia nigra pars compacta degenerate retrogradely and anterogradely, and microglial activation in these remote areas has been linked to the secondary neurodegeneration (Block et al., 2005). Secondary thalamic atrophy and microglial activation has been described in patients suffering from middle cerebral artery infarction (Pappata et al., 2000; Tamura et al., 1991). The integrity of thalamic circuitry seems to be important for motor recovery of patients (Binkofski et al., 1996) and dampening long-term microglial activation in thalamus may improve recovery from stroke. After transient MCAo, neuronal damage in substantia nigra pars reticulata can be first detected at 7 days post-stroke and it progresses until 14 days post-stroke (Dihne and Block, 2001; Nagasawa and Kogure, 1990). In substantia nigra pars reticulata, microglial activation has been shown to precede neurodegeneration (Dihne and Block, 2001; Korematsu et al., 1995) and persist for at least a month after stroke (Korematsu et al., 1995). Besides clearing damaged neurons it has been recently suggested that phagocytosis of viable neurons also occurs in the ischemic brain, and the phenomenon is named phagoptosis (Neher et al., 2013). Phagoptosis occurs when neurons expose phosphatidylserine on their cell membrane as a response to stressful, sublethal stimuli and phosphatidylserine functions as an ‘eat-me’ signal for microglia and macrophages (Fricker et al., 2012; Neher et al., 2011). In mutant mice and rats with decreased phagocytic activity of microglia, the delayed neuronal loss after ischemia was prevented and behavioral recovery was promoted compared to wild-type animals (Neher et al., 2013). Together, the data suggests that inflammation may have a significant role in secondary neurodegeneration.
2.4. Microglia-mediated plasticity after cerebral ischemia
Although the recovery from stroke is slow and often incomplete, in most cases there is still spontaneous recovery observed during post-stroke months (Rossini et al., 2003). The role of microglia in spontaneous recovery is not well studied. During postnatal development, microglia are involved in synaptic pruning by engulfing developing synapses in an activity-dependent manner (Paolicelli et al., 2011; Schafer et al., 2012). Using in vivo two-photon imaging it has been shown that in the resting state, microglial processes are in contact with neuronal synapses about 5 minutes at a time, approximately once in an hour (Wake et al., 2009). When neuronal activity is reduced, contact frequency between microglia and synapses decreases. Half an hour after ischemia onset, the microglia contact time with synapses in the penumbra area increases dramatically to about 80 minutes at a time (Wake et al., 2009). Recently, it has been postulated that in non-injury state, microglia play an active part in forming connections between neurons (Paolicelli et al., 2011). There is also evidence that NF-κB, a key transcriptional regulator of inflammatory genes, has a role in regulating synaptic plasticity (Mattson and Camandola, 2001). Moreover, cytokines may be vital to maintain neuroplasticity at low levels but over-expression after cerebral ischemia may impair synaptic plasticity and therefore increase neurodegeneration (Kriz and Lalancette-Hebert, 2009). By using [18F]PBR06 PET imaging of microglia and immunohistochemistry, it has been shown that there is a positive correlation between microglial activation and impaired motor function in transient MCAo model in mice (Lartey et al., 2014).
However, inflammation can have both detrimental and beneficial effects on post-stroke plasticity. Proliferating resident microglial cells confer neuroprotection within the first 3 days after ischemia by producing neurotrophic factors such as IGF1 (Lalancette-Hebert et al., 2007). Using transgenic mice, it was shown that selective ablation of proliferating resident microglia after transient MCAo results in substantial increase of the lesion size and the amount of apoptotic neurons, simultaneously with a decrease in the levels of IGF1 (Lalancette-Hebert et al., 2007). Also, transplantation of exogenous microglia after or during focal ischemic stroke improves behavioral recovery. When human or newborn rat microglia were transplanted into rats 48 hours after or during transient MCAo, respectively, functional recovery was improved (Kitamura et al., 2005; Narantuya et al., 2010). Transplanted microglia migrated to the infarct region and was shown to upregulate neurotrophic factors such as glial cell-line derived neurotrophic factor (GDNF) and brain derived neurotrophic factor (BDNF) (Kitamura et al., 2005; Narantuya et al., 2010). Narantuya et al. found fewer apoptotic and CD68+ cells in the infarct core and peri-infarct region at 7 and 14 days after stroke (Narantuya et al., 2010). In addition, gene expression of anti-inflammatory cytokines was upregulated (Narantuya et al., 2010). However, after permanent MCAo, there was no improvement in recovery when microglia from newborn rats was transplanted 24 hours after MCAo induction, though the study did not include quantification of molecules produced by the transplanted microglia, such as neurotrophic factors or cytokines (Jiang et al., 2013).
Collectively, data suggests that microglia have both negative and positive consequences on plasticity. Future studies identifying and regulating these properties of microglia may yield therapeutic approaches to promoting plasticity and repair of damaged neuronal circuits following stroke.
3. TOLL-LIKE RECEPTORS
TLRs are a receptor family mediating innate immunity and particularly TLR2 and TLR4 have been suggested to be master regulators of microglia activation. They are part of a receptor superfamily defined by the presence of a Toll-IL-1 receptor (TIR) domain, which includes the interleukin-1 receptors. TLRs are type-I transmembrane proteins consisting of three structural domains: C-terminal leucine-rich repeat (LRR) ligand recognition domain, central transmembrane domain, and an N-terminal cytoplasmic tail that facilitates downstream signaling (Fang and Hu, 2011). The LRRs differ in size and abundance among the various receptors and play a major role in ligand binding. The LRR family members share a highly conserved 11-residue tandem repeat sequence, LxxLxLxxNxL that forms a horseshoe-like structure. The “x” residues are found within the horseshoe curve and play a major role in receptor ligand interactions (Bell et al., 2003; Fang and Hu, 2011; Medzhitov and Janeway, 2000). On the cytoplasmic tail, TLRs contain the TIR signaling domain; a site for adaptor protein recruitment and oligomerization (Jiang et al., 2006). Upon ligand binding, receptors form homo- or heterodimers and elicit transduction signals through distinct intracellular pathways that often lead to the activation of transcription factors and inflammatory responses.
3.1. Expression in ischemic injury
After focal cerebral ischemia in mice, the number of TLR4 positive cells increased in a time-dependent manner from 1 hour to 22 hours post-stroke (Hyakkoku et al., 2010). Also, TLR2 mRNA expression gradually increased over the first 4 days in the ischemic brain (Lehnardt et al., 2007; Ziegler et al., 2007). This increase is also observed in the hippocampus of neonatal rats following artery ligature (Zhang et al., 2015). When TLR2 expression levels were compared to TLR4 and TLR9 expression levels, it was found that ishemic insult increases TLR2 mRNA level more than the others (Ziegler et al., 2007). Using a reporter protein under murine TLR2 promoter (Lalancette-Hebert et al., 2009), it was found that after the initial increase in TLR2 expression after photothrombotic stroke, there is a subsequent decline in the TLR2 expression levels over the 14 day period monitored (Quattromani et al., 2014). Both TLR2 and TLR4 expression has been associated with microglial cells after ischemia (Hyakkoku et al., 2010; Lehnardt et al., 2007; Ziegler et al., 2007). Another study showed that TLR2 and TLR4 are found mainly in neurons and that there is a delayed expression in microglia after ischemic injury (Tang et al., 2007). Interestingly, in a study where TLR3, TLR7, TLR8 and TLR9 mRNA levels were measured from blood of 110 stroke patients, it was found that TLR8 levels correlated with infarct volumes and that TLR7 and TLR8 at different time points were associated with poor outcome from ischemic stroke (Brea et al., 2011). Collectively, there is ample evidence the TLR4 receptors are increased in the brain following stroke and may be a viable target for pharmacological manipulations.
3.2. TLR signaling
TLR activation involves the recruitment of various TIR-domain containing adaptor proteins, including myeloid differentiation primary response gene 88 (MyD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), MyD88 adaptor-like protein (Mal) and TRIF-related adaptor molecule (TRAM) (O’Neill and Bowie, 2007). TLR4 is the most versatile due to the association with multiple second messenger cascades through each of the four TLR-associated adaptor proteins. These four adaptor proteins trigger one of the two distinct intracellular signaling cascades: the MyD88-dependent pathway and TRIF-dependent pathway both of which converge to activate NF-κB (Figure 3).
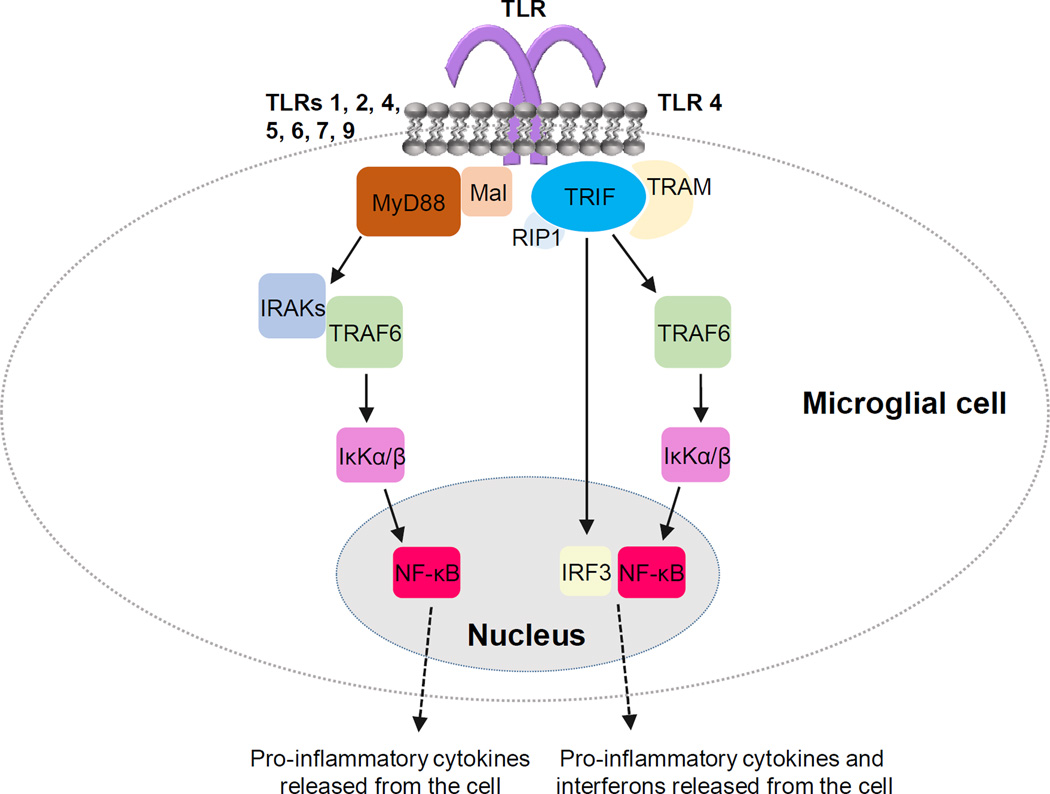
Figure 3. Toll-like receptor (TLR) signaling in microglial cell.
Activation of TLR leads to signaling via two principal pathways: MyD88-dependent pathway and TRIF-dependent pathway. TLRs 1, 2, 4, 5, 6, 7 and 9 (Akira and Takeda, 2004) signal via MyD88-dependent pathway where myeloid differentiation primary response gene 88 (MyD88) and MyD88 adaptor-like protein (Mal) recruitment leads to activation of IL-1 receptor-associated kinases (IRAKs) and tumor necrosis factor receptor-associated factor 6 (TRAF6). Phosphorylation of the inhibitory unit of the transcription factor NF-κB complex, κKα/β, leads to nuclear translocation and activation of NF-κB and production of proinflammatory cytokines. TLR4 signals also via TRIF-dependent pathway. TIR-domain-containing adapter-inducing interferon-β (TRIF) and TRIF-related adaptor molecule (TRAM) recruitment leads to activation of the transcription factor interferon regulatory factor 3 (IRF3) and production of interferons. TRIF interacts also with receptor interacting protein 1 (RIP1) and TRAF6 leading to activation of NF-κB as in MyD88-dependent pathway.
The MyD88-dependent pathways (MyD88 and Mal initiated) have been described previously (Deng et al., 2000). Briefly, the cascade begins with MyD88 phosphorylating IL-1 receptor-associated kinases (IRAKs), which activate tumor necrosis factor receptor-associated factor 6 (TRAF6) to form a TRAF6 complex that activates mitogen-activated protein kinase (MAPK) kinase kinase (MAP3K). MAPK3K has a number of targets but only two pathways are known to be relevant to microglial response to stroke: activation of the transcription factor NF-κB and activation of mitogen-activated protein kinases (MAPKs). In NF-κB activation, TRAF6 phosphorylates the inhibitory unit of the NF-κB complex, IκKα/β, allowing for NF-κB nuclear translocation and transcription of pro-inflammatory cytokines (Wang et al., 2013). MyD88 knockout (KO) mice demonstrate no response to the TLR4 agonist LPS as measured by pro-inflammatory cytokine production, B cell proliferation and endotoxin shock response (Kawai et al., 1999). Likewise, no observable response was reported with the addition of TLR2 and TLR9 agonists, peptidoglycan and bacterial CpG-DNA, respectively, in absence of MyD88 in in vivo and in vitro studies (Häcker et al., 2000; Takeuchi et al., 2000).
Interestingly, the MyD88-dependent pathway is known to have properties of auto-regulation that prevents prolonged activation of this pathway (Broad et al., 2007). The autoregulation is critical for understanding how microglial cells respond differently to the same stimuli in the acute, subacute and long-term stages of ischemic injury. In the acute phase, microglial activation proceeds as outlined above. However, in the subacute phase, there is a basal level of MyD88-pathway activation through TLRs as well as from activation of IL-1 receptors. In the long-term phase of microglial activation, this pathway can be expected to be maximally repressed. As new microglia are recruited into the peri-infarct region in the later stages of ischemia, IL-1 levels throughout the brain are elevated and microglial cells migrating into the peri-infarct area are likely to encounter TLR activating DAMPs when the IRAK pathway has been activated. Acutely activated microglial cells, on the other hand, are more likely to be activated by the presence of DAMPs without prior IRAKs activation. Microglial cells that are activated by subacute stimuli may encounter TLR4-activating DAMPs that could have a prophylactic effect on stroke injuries (Marsh et al., 2009).
The TRIF-dependent pathway leads to production of interferons through activation of the transcription factor interferon regulatory factor 3 (IRF3) (Hennessy et al., 2010) and activation of NF-κB through TRAF6, but independently of MyD88 and IRAKs (Jiang et al., 2004). Signaling through the TRIF-dependent pathway entails TRIF interacting with TRAF6 and receptor interacting protein 1 (RIP1), which then similarly activate NF-κB as described above. However, it is not clear that the TRIF pathway plays a significant role in ischemia-induced injury (Hua et al., 2009). TLR4 signal transduction can occur in a TRIF-dependent and MyD88-dependent fashion, whereas the majority of TLRs tend to be MyD88-dependent (Akira and Takeda, 2004; Lu et al., 2008). Pathway activation is usually the result of the type of ligand produced in response to ischemic injury.
The role of TLRs in cerebral ischemic injury has gained more focus in past years. Dying neurons release a variety of DAMPs which can stimulate TLR signaling, resulting in inflammation, and thus perpetuating the cycle of damage. Microglial activation, pro-inflammatory cytokine production, upregulation of immune receptors and subsequent tissue damage following cerebral ischemia as a result of TLR2 and TLR4 activation has been reported (Kilic et al., 2008; Lambertsen et al., 2005; Lehnardt et al., 2007; Lehnardt et al., 2003). Additionally, others have shown that an increase in TLR4+ peripheral monocytes correlates with increased cytokine levels in stroke patients, suggesting that peripheral cells pass through the compromised blood-brain barrier and exacerbate damage (Yang et al., 2008). Microglia, activated in a TLR9-dependent manner, release neurotoxic cytokine, IL-17, that in turn can activate T cells (Derkow et al., 2015). Thus, it is important to clarify the role of these signaling pathways for drug targeting and therapeutic potential, and this is discussed in detail below.
3.3. TLR agonists
Much of the attention on experimental stroke treatments has been focused on a prophylactic approach. In regards to TLRs, this entails preconditioning using low level activation prior to an ischemic event. Preconditioning of TLRs that signal via the MyD88-dependent pathway [TLRs 1, 2, 4, 5, 6, 7, 9 (Akira and Takeda, 2004)] may trigger compensatory measures that, in effect, inhibit this pathway from being maximally activated and repress NF-κB activation (Broad et al., 2007). Several studies have shown that preconditioning with TLR4 agonists is neuroprotective against ischemic injury (Rosenzweig et al., 2004; Stevens et al., 2008; Tasaki et al., 1997). Administration of LPS before MCAo in rats has been shown to be protective (Tasaki et al., 1997) and these results indicate that preconditioning effects of TLR4 activation mediate neuroprotection. Follow-up studies showed that administration of LPS (0.2mg/kg or 0.8mg/kg) in mice prior to MCAo resulted in an upregulation of anti-inflammatory genes following ischemia (Vartanian et al., 2011). Pro-inflammatory cytokines, however, did not seem to be affected (Vartanian et al., 2011). In addition, pretreatment with TLR8 agonist before focal ischemic reperfusion injury has been shown to have detrimental effects on the stroke outcome (Tang et al., 2013). Administration of TLR2 agonist, PAC3CSK4, prior to arterial occlusion resulted in smaller infarct size, reduced mortality, smaller neuronal damage, and decreased level of NF-κB and Bax, protein involved in cell death signaling (Hua et al., 2008; Lu et al., 2011). However, there are no published reports with experimental animal stroke models describing how manipulation of TLR receptor activity affects the functional recovery following stoke. If TLR4 activation leads to increases of anti-inflammatory genes post-stroke, it would be important to know whether this leads to faster recovery and reduction in post-stroke inflammation complications. Clinical trials have set forth to examine the efficacy of preconditioning with TLR agonists, although the main focus is cardiac ischemic injury (Veighey and Macallister, 2012).
3.4. TLR antagonists
Alternative approaches have focused on modulating TLR-mediated activation of NF-κB. It is widely accepted that NF-κB plays an essential role during cerebral ischemia, not only in the context of TLR signaling but as a major contributor to other inflammatory signal mechanisms. For instance, NF-κB nuclear translocation and DNA binding was apparent within 30 minutes of reperfusion following 2 hours artery ligation, as determined by an NF-κB-driven reporter transgene (Schneider et al., 1999). Adenoviral delivery of a dominant negative form of IκKα/β injected prior to MCAo reduced infarct size (Xu et al., 2002). Pharmacological inhibition of NF-κB is a rational therapeutic target; however, one must approach this with caution. Deficits in NF-κB function have been associated with susceptibility to infection and reduced immune cell viability (Ridder and Schwaninger, 2009).
Ideally, pharmacological modulation would involve dampening NF-κB induction in a TLR-dependent manner. TAK-242, an antagonist of TLR4, when given intraperitoneally 3 mg/kg 1 hour after ischemia has been shown to decrease infarction volume and neurological deficits in mice (Hua et al., 2015). The neuroprotective effect of TAK-242 was associated with decrease in the expression of inflammatory cytokines (Hua et al., 2015). In addition, intracerebroventricular injection of TAK-242 decreased infarction volume and neurological deficits when measured 22 hours after MCAo in mice, and inhibited TLR4-nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) signaling (Suzuki et al., 2012). Eritoran, which has been shown to inhibit TLR4 signaling at nanomolar concentrations (Mullarkey et al., 2003) has not been studied in experimental rodent stroke models, but it has been shown to decrease myocardial ischemic infarction damage (Mullarkey et al., 2003), as well as to decrease NF-κB nuclear localization (Mullarkey et al., 2003).
One controversial candidate is the blood-brain barrier permeable, TLR4 antagonist, naloxone. Naloxone was initially reported to dose-dependently diminish LPS-induced TLR4 activation (Hutchinson et al., 2008), but three, independent commercial laboratories failed to replicate the result (Skolnick et al., 2014). Also, Wang et al. found that naloxone inhibits superoxide production through inactivation of NADPH oxidase 2 (NOX2) which may be critical for the anti-inflammatory effect on microglia (Wang et al., 2012).
Nevertheless, the different effects in TLR4 KO mice indicated that TLR4 or its signaling pathways could be mediators of naloxone effects (Hutchinson et al., 2010). However, the compensatory changes that arise from development may cause the effect not to be direct. Moreover, this drug has been used minimally in experimental models of cerebral ischemia, despite its TLR4 specificity and varying claims regarding downstream effects. Chen et al. reported that intracerebroventricular infusions of (−)-naloxone prior to and following artery occlusion decreased infarct area and inflammation, as assayed by presence of inflammatory cells (Chen et al., 2001). Likewise, naloxone has been shown to be neuroprotective against beta-amyloid peptide 1–42 (Aβ 1–42) through the reduction of microglia-produced superoxide (Liu et al., 2002). It is clear that naloxone decreases activation and expression of microglia and likely the effects are mediated with pathways shared with TLR4 signaling. Most encouraging data for TLR-antagonism as a potential treatment comes from case-reports of stroke patients showing that naloxone was able to reverse neurological deficits (Baskin and Hosobuchi, 1981).
3.5. Genetically modified mice: role of TLRs in acute and subacute events
Mice lacking TLR receptors and mice with dominant negative mutations in TLRs in stroke experiments are summarized in Table 1. First, we discuss the effects of TLRs during the acute and subacute microglia-related events in various gene modified (GM) TLR mouse lines. In a study where both TLR2 and TLR4 KO mice were used, it was found that both KO mouse lines have reduced lesion size after focal ischemic injury (Tang et al., 2007). Similar acute neuroprotective effect with TLR4 KO mice has been also observed in a study with permanent MCAo, and the smaller lesion damage was associated with decreased inflammatory response (Caso et al., 2007). In studies where mice had dominant negative TLR4 mutation, the lesion size was decreased after transient focal ischemic injury (Cao et al., 2007; Kilic et al., 2008) and mice had increased number of neurons, decreased number of cells with fragmented DNA and decreased number of activated caspase-3 positive cells in the striatum (Kilic et al., 2008). In a study by Hyakkoku and colleagues, where TLR3, TLR4 and TLR9 KO mice were studied in parallel, it was found that only mice lacking TLR4 receptors had decreased infarction volume when measured 24 hours after transient MCAo (Hyakkoku et al., 2010). Moreover, it has been shown that NF-κB translocation is reduced in TLR4 KO mice, but not in TLR3 or TL9 KO mice (Hyakkoku et al., 2010). Blocking of TLR4 signaling by anti-TLR4-MD2 antibody also reduced infarction size in mouse focal stroke (Andresen et al., 2016), and although this study was not done via gene manipulation, it is in line with the results from TLR4 KO mice. TLR4 has been suggested to be involved in mediating ischemic preconditioning also in KO studies since ischemic preconditioning induced less neuroprotection in TLR4 KO mice than in wild-type mice (Pradillo et al., 2009). Similar to TLR4 KO mice, also TLR2 KO mice have smaller lesion size after focal MCAo in the filament model (Lehnardt et al., 2007; Ziegler et al., 2007). In a study where TRIF-dependent signaling of TLR receptors was studied, it was found that TRIF KO mice did not have altered lesion size or neurological deficits as compared to wild-type mice at 24 hours after transient ischemic injury, and TRIF deficiency did not alter stroke-induced NF-κB activity (Hua et al., 2009). Collectively, the data support that loss of TLR4 is beneficial against ischemic brain injury.
Table 1.
Effects of TLR receptor knockout on ischemic injury outcome.
| GM mouse | Outcome in stroke | Reference |
|---|---|---|
| TLR2 | Reduced lesion size at 1–3 days after stroke |
(Lehnardt et al., 2007; Tang et al., 2007; Ziegler et al., 2007) |
| Reduced lesion size at day 3 post-stroke, but increased sizes on days 7 and 14, this was associated with increased apoptosis |
(Bohacek et al., 2012) | |
| TLR3 | No effect on lesion size | (Hyakkoku et al., 2010) |
| TLR4 | Reduced lesion size at 1–3 days after stroke |
(Cao et al., 2007; Caso et al., 2007; Hyakkoku et al., 2010; Kilic et al., 2008; Tang et al., 2007) |
| Reduced lesion size associated with better neurological score when measured at post-stroke day 7 |
(Caso et al., 2007) | |
| TLR4 KO mice have bigger lesion size than wild-type controls in ischemic preconditiong-induced neuroprotection |
(Pradillo et al., 2009) | |
| TLR9 | No effect on lesion size | (Hyakkoku et al., 2010) |
3.6. Genetically modified mice: role of TLRs in long-term events
When acute versus long-term consequences after ischemic stroke are compared, they are somewhat opposite in TLR4 and TLR2 KO mice. In both TLR4 and TLR2 KO mice there is decreased lesion size when measured 1 day after initiation of ischemic insult (Caso et al., 2007; Lehnardt et al., 2007). In TLR4 KO mice the smaller lesion sizes can be observed also in long-term studies. In a 7 days follow-up study a focal permanent MCAo injury was induced in TLR4 KO mice and it was found that the smaller lesion size persisted when measured at 7 days post-stroke and it was accompanied with better performance in neurological tests (Caso et al., 2007). However, it would be interesting to carry out experiments with time points beyond 7 days after ischemic injury. The long-term results from TLR2 KO mice are quite the opposite. In a study where TLR2 KO mice were subjected to focal ischemic stroke with different reperfusion times, it was reported that lack of TLR2 led to reduced proliferation of brain resident microglial cells and late augmentation in neuronal apoptosis (Bohacek et al., 2012). Although the lesion size was reduced on day 3 after stroke in TLR2 KO mice, it was found to be increased at 7 and 14 days post-stroke (Bohacek et al., 2012), indicating delayed exacerbation of the damaged area (Bohacek et al., 2012). Subsequent studies have revealed that inflammatory and apoptotic pathways, when measured on mRNA level, are altered even one month after ischemic stroke (Winters et al., 2013). Indeed, the study that implemented a reporter under murine TLR2 promoter showed that after ischemic injury there is a bi-phasic response, and the TLR2 signal first peaks at 48 hours after stroke and then slowly declines (Lalancette-Hebert et al., 2009). A second peak in TLR2 signal occurs at 28–30 days post-stroke.
4. OTHER PHARMACOLOGICAL MODULATIONS OF INFLAMMATORY SIGNALING IN ISCHEMIC STROKE
In addition to modulating TLRs, modulating other aspects of the inflammatory response from microglia, and other immune cells contributing to the pathophysiological progression of an ischemic brain injury can be neuroprotective. For example, there is evidence that antagonizing two major pro-inflammatory cytokines, TNF-α and IL-1β, is beneficial in ischemic stroke models and increased levels of TNF-α and IL-1β exacerbate the ischemic damage (Barone et al., 1997; Dawson et al., 1996; Loddick and Rothwell, 1996; Yamasaki et al., 1995; Yang et al., 1998). Furthermore, administration of IL-1 receptor antagonist within 6 hours after symptom onset resulted in better neurological outcome at 3 months post-stroke in patients with cortical infarcts (Emsley et al., 2005). In contrast, mice lacking TNF-α receptors exhibit an increase in ischemic damage after focal ischemic stroke indicating that TNF-α may have some neuroprotective role as well (Bruce et al., 1996). Where antagonizing IL-8 reduced infarct size in rabbits (Matsumoto et al., 1997), exogenous IL-6 administration decreased infarct size in rats (Loddick et al., 1998). Inhibition of adhesion molecules improves stroke outcome in experimental animal models as adhesion molecules can facilitate leukocyte adhesion to endothelial cells. Intercellular adhesion molecule 1 (ICAM-1) neutralizing antibody is neuroprotective in transient MCAo model as shown by reduced infarct size and a correlative decrease in the amount of leukocytes in the lesion area (Zhang et al., 1994). However, in clinical trials, enlimomab, a murine anti-ICAM-1 antibody, failed to improve stroke outcome when it was given within 6 hours of stroke onset for 5 days, and, surprisingly, worsened the stroke outcome (Enlimomab Acute Stroke Trial, 2001).
Microglia are also activated through purinergic receptors that interact with ATP (Koizumi et al., 2013) released by neurons in response to oxygen deprivation (Volonte et al., 2003). Although many purinergic receptors have a role in post-injury inflammation, the P2X7 receptor (P2X7R) has a well-established mechanism (Di Virgilio, 2015) that makes it a strong candidate as a therapeutic target (De Marchi et al., 2016). P2X7 receptors are upregulated in microglial cells at the infarct and peri-infarct region 1 and 4 days after permanent MCAo (Franke et al., 2004; Melani et al., 2006). P2X7 receptor antagonism at the onset or within 30 minutes after ischemia onset is neuroprotective in permanent and transient MCAo model, respectively, by reducing infarction volume and neurological deficits (Arbeloa et al., 2012; Cisneros-Mejorado et al., 2015; Melani et al., 2006). There is no evidence to support any positive effect of administration outside of the acute phase of microglial activation. P2X7R has been shown to have a role in ischemic preconditioning as well. Ischemic preconditioning fails to be neuroprotective in P2X7R KO mice whereas wild-type mice have significantly smaller lesions in transient MCAo model after ischemic preconditioning (Hirayama et al., 2015). As TLRs, P2X7R is capable of activating NF-κB through the auto-regulated MyD88 pathway (Liu et al., 2011) which may explain the effects of P2X7R in ischemic preconditioning. Further studies are needed to find out the potential of targeting P2X7R at subacute and long-term phases of microglial activation.
Minocycline, a tetracycline antibiotic derivative and microglial activation inhibitor, has been shown to be neuroprotective in focal cerebral ischemia in the acute and long-term phases of microglial activation. When minocycline was given systemically to rats either 12 hours before or 4 hours after the onset of ischemia, it reduced the infarction size (Yrjanheikki et al., 1999). Long term minocycline treatment for 42 days has been shown to improve functional recovery when it was combined with rehabilitative training, and the combination increased functional recovery more than the rehabilitative training alone at 14 and 28 days post-stroke although minocycline had no effect on the infarct volume (Liebigt et al., 2012). Minocycline decreased the proliferation of microglial cells and increased the amount of doublecortin-positive cells in peri-infarct region at 14 days after stroke and it also increased the survival of proliferating astrocytes at 42 days post-stroke (Liebigt et al., 2012). In addition, delayed administration of minocycline in the long-term phase of microglial activation, starting from 4 days post-stroke, has been shown to be beneficial (Liu et al., 2007). Post-stroke treatment with minocycline for 4 weeks did not affect infarct size but it reduced microglial activation, promoted functional recovery of the rats and enhanced neurogenesis in hippocampus (Liu et al., 2007). Minocycline treatment within 24 hours after stroke onset has been shown to be effective also in some clinical studies. Minocycline is safe and well-tolerated in patients also in combination with TPA which is used for thrombolysis (Fagan et al., 2010). In an open-label, evaluator-blinded study minocycline improved stroke outcome when it was given 6–24 hours after the onset of stroke symptoms for 5 days (Lampl et al., 2007). In another study with similar design, minocycline improved neurological function only in male stroke patients (Amiri-Nikpour et al., 2015). Also in mice, minocycline has been reported to be inefficient at reducing infarction volume and neurological deficits in females (Li and McCullough, 2009). The inefficiency of minocycline in females could be explained by differences in the post-stroke inflammatory response between females and males. Male mice have more activated microglia/macrophages in the brain after ischemic stroke and less anti-inflammatory IL-10 producing suppressor T-cells (Banerjee et al., 2013). There is evidence that estrogen affects the levels of key inflammatory mediators such as TNF-α and NF-κB (Koellhoffer and McCullough, 2013). In general, estrogen is neuroprotective in ischemic stroke (Hurn and Macrae, 2000) and premenopausal women have lower stroke incidence and suffer from smaller cerebral infarcts than age-matched men. Similar to humans, female mice and rats have smaller infarct volume compared to males (Alkayed et al., 1998; Banerjee et al., 2013; Brait et al., 2010). More clinical studies would be needed to determine the efficacy of minocycline in human stroke especially in female patients.
Cyclosporine A is one of the most widely used immunosuppressive drugs in neural cell and organ transplant recipients, and it has been shown to have neuroprotective effects in experimental stroke models. High dose (10 mg/kg) of cyclosporine A given 3h, 24h, 48h and 78h after reperfusion decreased the lesion size and neurological deficits in focal rat stroke model (Yu et al., 2004). In another study where cyclosporine A (10 mg/kg) was given to rats 20 minutes before MCAo or immediately after reperfusion it was found that only the pretreatment decreased lesion size measured with magnetic resonance imaging (Cho et al., 2013). The studies were focused on measuring lesion sizes and neurological deficits and the effects on microglia were not specifically examined. However, in a long-term study in global ischemia rat model cyclosporine A decreased the number of microglia/macrophages at 7, 14 and 30 days after permanent ligation of the common carotid arteries (Wakita et al., 1995). However, a clinical trial with cyclosporine did not find altered lesion volume in stroke patients when it was given intravenously within 5 hours after symptom onset (Nighoghossian et al., 2015).
Glucocorticoids are a class of steroid hormones that bind to glucocorticoid receptors, and are used to reduce inflammation in diseases caused by overactive immune system such as asthma, allergies and autoimmune diseases. Blood cortisol levels are increased after stroke and the increase persists at least for 7 days (Fassbender et al., 1994). Moreover, cortisol levels correlate with the lesion volume and neurological outcome (Fassbender et al., 1994).Similar to many other studies, glucocorticoids have been studied mostly with the focus on neuroprotection, and there is limited data on how the treatment would affect long-term inflammatory consequences. In many studies glucocorticoids have been ineffective in reducing infarction volume or to improve neurological outcome in experimental animal models of stroke (Donley and Sundt, 1973; Lee et al., 1974). Moreover, in a study where organotypic cultures from neonatal pups were used with oxygen-glucose deprivation, it was found that corticosterone and dexamethasone potentiate the toxic effect (Mulholland et al., 2005). However, high dose of dexamethasone (3 mg/kg intraperitoneally) decreases the levels of TNF-α in the cortex and reduces the infarction volume in a rat model of permanent MCAo when dexamethasone is given 10 minutes after the occlusion (Bertorelli et al., 1998). Administration of high doses of methylprednisolone (105 mg/kg intra-arterially) resulted in mild neuroprotection, 20% reduction in lesion volume, only in transient MCAo model but not in permanent occlusion (Slivka and Murphy, 2001). When methylprednisolone (30 mg/kg intraperitoneally) was given to rats 3h, 12h and 24h after MCAo, it was found that the levels of TNF-α and IL-6 were decreased and apoptosis was reduced in ipsilateral striatum 3 days after stroke (Jing et al., 2012).
NSAIDs are a group of drugs that have both analgesic and anti-inflammatory effects. Most NSAIDs inhibit the activity of cyclooxygenase (COX)-1 and −2, and downstream synthesis of prostaglandins. It has been observed that COX-2 is increased in the blood of ischemic stroke patients (Ferronato et al., 2011). Also in the case of NSAIDs most studied have been focused on neuroprotection and the acute and subacute phases of microglia-mediated events. High dose of ibuprofen pretreatment has been shown to reduce infarction volume in rats and the neuroprotective effect was associated with decreased levels of ICAM-1 in the striatum (Antezana et al., 2003). Ibuprofen has been shown to be protective only in the transient MCAo and to worsen the outcome in permanent MCAo, indicating that COX is activated during reperfusion (Cole et al., 1993). However, selective inhibition of COX-2 with NS-398 given at 6 hours after the induction of permanent or transient MCAo reduced infarction volume in mice and rats (Nagayama et al., 1999; Nogawa et al., 1997). The mRNA and protein levels of COX-2 are upregulated in the ischemic brain starting from 6 hours after transient ischemia induced with MCAo and the upregulation is most prominent in the cells at the peri-infarct region (Nogawa et al., 1997). COX-2 expression was increased also in a photothrombotic stroke model in the rat barrel cortex and ibuprofen pretreatment decreased the expression of COX-2 (Jablonka et al., 2012). In a long-term follow-up, it was found also that chronic treatment with ibuprofen restored plasticity in the barrel cortex (Jablonka et al., 2012). However, when ibuprofen was administered long term for 27 days after stroke, it was found that ibuprofen treatment did not alter neurological outcome nor did it alter the secondary pathology (β-amyloid or calcium load) in the thalamus (Lipsanen et al., 2011). Similar to ibuprofen, acute treatment with nimesulide has been shown to reduce infarction volume and decrease neurological deficits in dose-dependent manner (Candelario-Jalil et al., 2004), and the neuroprotective effect was associated with decreased levels of prostaglandin E2 in the stroke brain. In another study, it was found that nimesulide reduced infarction volume and promoted behavioral recovery when the first dose was given within 2 hours after permanent MCAo in rats (Candelario-Jalil et al., 2005). Moreover, in a study where meloxicam was given 1 hour before surgery and then again after 24 hours it was found that it reduced infarction volume in permanent MCAo model in mice (Jacobsen et al., 2013). Unlike the NSAIDs described above, indomethacin failed to show neuroprotection when given 5 minutes before ischemic insult in a forebrain ischemia model in rats (Sutherland et al., 1988). Indomethacin did not alter the number of CD11b+ cells when followed over 4 weeks after stroke, but it reduced the amount of phagocytic CD68+ cells (Hoehn et al., 2005).
The complexity of inflammatory proteins and their effects makes drug design difficult or may require complex cocktails of inflammatory modulators. Modulating inflammatory pathways have resulted in neuroprotection but have been difficult to translate into effective clinical treatments. The clinical studies on anti-inflammatory agents in ischemic stroke patients and the outcome are summarized in Table 2.
Table 2.
Effects of anti-inflammatory treatment in ischemic stroke patients.
| Treatment | Outcome in stroke | Reference |
|---|---|---|
| IL-1 receptor antagonist |
Better neurological outcome 3 months after cortical stroke when given within 6 hours of symptom onset |
(Emsley et al., 2005) |
| Anti-ICAM-1 antibody |
Worsened stroke outcome when given within 6 hours of symptom onset |
(Enlimomab Acute Stroke Trial, 2001) |
| Naloxone | Reversed neurological deficits after brain ischemia (case- report) |
(Baskin and Hosobuchi, 1981) |
| Minocycline | Improved neurological function when given 6–24 hours after the onset of stroke symptoms |
(Amiri-Nikpour et al., 2015; Lampl et al., 2007) |
| Cyclosporine | No change in lesion volume when given within 5 hours of symptom onset |
(Nighoghossian et al., 2015) |
5. CONCLUSIONS
The immune response in the brain following a stroke is mediated through resident microglial cells and invading peripheral immune cells. Although the time course of microglial activation and leukocyte infiltration is dependent on the type and duration of ischemic injury, the inflammatory response has major effects in stroke pathophysiology. More studies are needed to evaluate how modulation of the inflammatory response influences functional recovery to ultimately improve stroke outcome. Many of the studies described in this review were focused on early neuroprotective effects and few studies longitudinally monitored the animals’ recovery or measured microglial activation and its consequences. Administration of immunomodulatory drugs may have beneficial or detrimental effects based on the phase of immune response to the ischemic injury. Future studies will also need to consider the age and sex of animals when evaluating immunomodulating drugs. Most of the studies have been conducted in young male animals, and very little is known how the inflammatory events are altered in aged brain, which is most prominently affected by stroke in humans. Also the sex seems to affect inflammatory response after stroke and may have an impact on the efficacy of the treatment.
In this review, we took an approach for microglial signaling by dividing the microglial responses in time and location to acute, subacute and long-term responses. Although, for many studies this is difficult to implement, taking this approach and looking at microglial function in specific time and location can lead for better understanding of microglial signaling and function. When the post-ischemic inflammatory responses of microglia are divided into three categories, we can also investigate novel therapeutics and their effectiveness on specific outcome measures. The stroke field has been predominantly focused on finding therapeutics that decrease the lesion volume and several clinical studies have shown that this approach does not result in effective therapeutics. By studying other outcome measures such as functional recovery of impaired motor or cognitive functions we can find therapeutics for novel drug targets and find out whether modulating the inflammatory response improves recovery from stroke.
Highlights.
Post-ischemic microglial responses are divided to acute, subacute and long-term phases.
Inflammatory response has major role in the pathophysiology of ischemic stroke.
TLRs are a receptor family mediating innate immunity.
More studies focusing on the long-term microglial activation after stroke are needed.
Acknowledgments
J.E.A. and M.A. were funded by Academy of Finland #250275, #256398, #281394. M.A. was funded also by and GLORIA EU FP7 (http://gloria.helsinki.fi). B.K.H. and E.S.W. were supported by the Intramural Research Program at the National Institute on Drug Abuse. K.W.W. was supported by the US Army Research Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Airavaara M, Shen H, Kuo CC, Peranen J, Saarma M, Hoffer B, Wang Y. Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J Comp Neurol. 2009;515:116–124. doi: 10.1002/cne.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, Rezaei Y. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand. 2015;131:45–50. doi: 10.1111/ane.12296. [DOI] [PubMed] [Google Scholar]
- Andresen L, Theodorou K, Grunewald S, Czech-Zechmeister B, Konnecke B, Luhder F, Trendelenburg G. Evaluation of the Therapeutic Potential of Anti-TLR4-Antibody MTS510 in Experimental Stroke and Significance of Different Routes of Application. PLoS One. 2016;11:e0148428. doi: 10.1371/journal.pone.0148428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antezana DF, Clatterbuck RE, Alkayed NJ, Murphy SJ, Anderson LG, Frazier J, Hurn PD, Traystman RJ, Tamargo RJ. High-dose ibuprofen for reduction of striatal infarcts during middle cerebral artery occlusion in rats. J Neurosurg. 2003;98:860–866. doi: 10.3171/jns.2003.98.4.0860. [DOI] [PubMed] [Google Scholar]
- Anttila JE, Airavaara M. Expression of Iba1+ and ED1+ cells in rat cortex, striatum and thalamus after cortical stroke: time course study until 4 months. Poster in the 9th International Symposium on Neuroprotection and Neurorepair; Leipzig, Germany. 2016. unpublished results 4/19-4/22/2016. [Google Scholar]
- Arbeloa J, Perez-Samartin A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Baskin DS, Hosobuchi Y. Naloxone reversal of ischaemic neurological deficits in man. Lancet. 1981;2:272–275. doi: 10.1016/s0140-6736(81)90524-9. [DOI] [PubMed] [Google Scholar]
- Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett. 1998;246:41–44. doi: 10.1016/s0304-3940(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1996;39:460–470. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- Block F, Dihne M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75:342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Bohacek I, Cordeau P, Lalancette-Hebert M, Gorup D, Weng YC, Gajovic S, Kriz J. Tolllike receptor 2 deficiency leads to delayed exacerbation of ischemic injury. J Neuroinflammation. 2012;9:191. doi: 10.1186/1742-2094-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea D, Sobrino T, Rodriguez-Yanez M, Ramos-Cabrer P, Agulla J, Rodriguez-Gonzalez R, Campos F, Blanco M, Castillo J. Toll-like receptors 7 and 8 expression is associated with poor outcome and greater inflammatory response in acute ischemic stroke. Clin Immunol. 2011;139:193–198. doi: 10.1016/j.clim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Broad A, Kirby JA, Jones DE, Applied I and Transplantation Research, G. Toll-like receptor interactions: tolerance of MyD88-dependent cytokines but enhancement of MyD88-independent interferon-beta production. Immunology. 2007;120:103–111. doi: 10.1111/j.1365-2567.2006.02485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Wide therapeutic time window for nimesulide neuroprotection in a model of transient focal cerebral ischemia in the rat. Brain Res. 2004;1007:98–108. doi: 10.1016/j.brainres.2004.01.078. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Mhadu NH, Gonzalez-Falcon A, Garcia-Cabrera M, Munoz E, Leon OS, Fiebich BL. Effects of the cyclooxygenase-2 inhibitor nimesulide on cerebral infarction and neurological deficits induced by permanent middle cerebral artery occlusion in the rat. J Neuroinflammation. 2005;2:3. doi: 10.1186/1742-2094-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liao SL, Chen WY, Hong JS, Kuo JS. Cerebral ischemia/reperfusion injury in rat brain: effects of naloxone. Neuroreport. 2001;12:1245–1249. doi: 10.1097/00001756-200105080-00038. [DOI] [PubMed] [Google Scholar]
- Cho TH, Aguettaz P, Campuzano O, Charriaut-Marlangue C, Riou A, Berthezene Y, Nighoghossian N, Ovize M, Wiart M, Chauveau F. Pre- and post-treatment with cyclosporine A in a rat model of transient focal cerebral ischaemia with multimodal MRI screening. Int J Stroke. 2013;8:669–674. doi: 10.1111/j.1747-4949.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- Cisneros-Mejorado A, Gottlieb M, Cavaliere F, Magnus T, Koch-Nolte F, Scemes E, Perez-Samartin A, Matute C. Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab. 2015;35:843–850. doi: 10.1038/jcbfm.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DJ, Patel PM, Reynolds L, Drummond JC, Marcantonio S. Temporary focal cerebral ischemia in spontaneously hypertensive rats: the effect of ibuprofen on infarct volume. J Pharmacol Exp Ther. 1993;266:1713–1717. [PubMed] [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- De Marchi E, Orioli E, Dal Ben D, Adinolfi E. P2X7 Receptor as a Therapeutic Target. Advances in Protein Chemistry and Structural Biology, Volume 104 Ion Channels as Therapeutic Targets, Part B. 2016 doi: 10.1016/bs.apcsb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Derkow K, Krüger C, Dembny P, Lehnardt S. Microglia Induce Neurotoxic IL-17+ γδ T Cells Dependent on TLR2, TLR4, and TLR9 Activation. PLoS One. 2015;10:e0135898. doi: 10.1371/journal.pone.0135898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. P2X receptors and inflammation. Curr Med Chem. 2015;22:866–877. doi: 10.2174/0929867322666141210155311. [DOI] [PubMed] [Google Scholar]
- Dihne M, Block F. Focal ischemia induces transient expression of IL-6 in the substantia nigra pars reticulata. Brain Res. 2001;889:165–173. doi: 10.1016/s0006-8993(00)03129-2. [DOI] [PubMed] [Google Scholar]
- Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–25. doi: 10.1111/j.1749-6632.2012.06691.x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Donley RF, Sundt TM., Jr The effect of dexamethasone on the edema of focal cerebral ischemia. Stroke. 1973;4:148–155. doi: 10.1161/01.str.4.2.148. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ, Acute Stroke I. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlimomab Acute Stroke Trial, I. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Fadakar K, Dadkhahfar S, Esmaeili A, Rezaei N. The role of Toll-like receptors (TLRs) in stroke. Rev Neurosci. 2014;25:699–712. doi: 10.1515/revneuro-2013-0069. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Hu J. Toll-like receptor and its roles in myocardial ischemic/reperfusion injury. Med Sci Monit. 2011;17:RA100–RA109. doi: 10.12659/MSM.881709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Schmidt R, Mossner R, Daffertshofer M, Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke. 1994;25:1105–1108. doi: 10.1161/01.str.25.6.1105. [DOI] [PubMed] [Google Scholar]
- Ferronato S, Lira MG, Olivato S, Scuro A, Veraldi GF, Romanelli MG, Patuzzo C, Malerba G, Pignatti PF, Mazzucco S. Upregulated expression of Toll-like receptor 4 in peripheral blood of ischaemic stroke patients correlates with cyclooxygenase 2 expression. Eur J Vasc Endovasc Surg. 2011;41:358–363. doi: 10.1016/j.ejvs.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- Fricker M, Neher JJ, Zhao JW, Thery C, Tolkovsky AM, Brown GC. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci. 2012;32:2657–2666. doi: 10.1523/JNEUROSCI.4837-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000;192:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- Hirayama Y, Ikeda-Matsuo Y, Notomi S, Enaida H, Kinouchi H, Koizumi S. Astrocyte-mediated ischemic tolerance. J Neurosci. 2015;35:3794–3805. doi: 10.1523/JNEUROSCI.4218-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, Stein DG. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab. 2015;35:536–542. doi: 10.1038/jcbfm.2014.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Wang J, Sayeed I, Ishrat T, Atif F, Stein DG. The TRIF-dependent signaling pathway is not required for acute cerebral ischemia/reperfusion injury in mice. Biochem Biophys Res Commun. 2009;390:678–683. doi: 10.1016/j.bbrc.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Jablonka JA, Kossut M, Witte OW, Liguz-Lecznar M. Experience-dependent brain plasticity after stroke: effect of ibuprofen and poststroke delay. Eur J Neurosci. 2012;36:2632–2639. doi: 10.1111/j.1460-9568.2012.08174.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen KR, Fauerby N, Raida Z, Kalliokoski O, Hau J, Johansen FF, Abelson KS. Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp Med. 2013;63:105–113. [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Wang J, Yu L, Ou C, Liu X, Zhao X, Wang J. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav Brain Res. 2013;250:222–229. doi: 10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Li C, Choe J, Crozat K, Rutschmann S, Du X, Bigby T, Mudd S, Sovath S, et al. Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis. Proc Natl Acad Sci U S A. 2006;103:10961–10966. doi: 10.1073/pnas.0603804103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A. 2004;101:3533–3538. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Hou Y, Song Y, Yin J. Methylprednisolone improves the survival of new neurons following transient cerebral ischemia in rats. Acta Neurobiol Exp (Wars) 2012;72:240–252. doi: 10.55782/ane-2012-1897. [DOI] [PubMed] [Google Scholar]
- Justicia C, Ramos-Cabrer P, Hoehn M. MRI detection of secondary damage after stroke: chronic iron accumulation in the thalamus of the rat brain. Stroke. 2008;39:1541–1547. doi: 10.1161/STROKEAHA.107.503565. [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K, Liu XH, Araki T, Itoyama Y. Progressive expression of immunomolecules on activated microglia and invading leukocytes following focal cerebral ischemia in the rat. Brain Res. 1996;734:203–212. [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Yanagisawa D, Inden M, Takata K, Tsuchiya D, Kawasaki T, Taniguchi T, Shimohama S. Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J Pharmacol Sci. 2005;97:289–293. doi: 10.1254/jphs.sc0040129. [DOI] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Ohsawa K, Inoue K, Kohsaka S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia. 2013;61:47–54. doi: 10.1002/glia.22358. [DOI] [PubMed] [Google Scholar]
- Korematsu K, Goto S, Nagahiro S, Inoue N, Oyama T, Yamada K, Ushio Y. Change of phosphotyrosine immunoreactivity on microglia in the rat substantia nigra following striatal ischemic injury. Glia. 1995;13:147–153. doi: 10.1002/glia.440130208. [DOI] [PubMed] [Google Scholar]
- Kriz J, Lalancette-Hebert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117:497–509. doi: 10.1007/s00401-009-0496-1. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Phaneuf D, Soucy G, Weng YC, Kriz J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain. 2009;132:940–954. doi: 10.1093/brain/awn345. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Meldgaard M, Ladeby R, Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:119–135. doi: 10.1038/sj.jcbfm.9600014. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Lartey FM, Ahn GO, Ali R, Rosenblum S, Miao Z, Arksey N, Shen B, Colomer MV, Rafat M, Liu H, et al. The relationship between serial [(18) F]PBR06 PET imaging of microglial activation and motor function following stroke in mice. Mol Imaging Biol. 2014;16:821–829. doi: 10.1007/s11307-014-0745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee MC, Mastri AR, Waltz AG, Loewenson RB. Ineffectiveness of dexamethasone for treatment of experimental cerebral infarction. Stroke. 1974;5:216–218. doi: 10.1161/01.str.5.2.216. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrmann E, Christensen T, Zimmer J, Diemer NH, Finsen B. Microglial and macrophage reactions mark progressive changes and define the penumbra in the rat neocortex and striatum after transient middle cerebral artery occlusion. J Comp Neurol. 1997;386:461–476. [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebigt S, Schlegel N, Oberland J, Witte OW, Redecker C, Keiner S. Effects of rehabilitative training and anti-inflammatory treatment on functional recovery and cellular reorganization following stroke. Exp Neurol. 2012;233:776–782. doi: 10.1016/j.expneurol.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Kossut M. Influence of inflammation on poststroke plasticity. Neural Plast. 2013;2013:258582. doi: 10.1155/2013/258582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsanen A, Hiltunen M, Jolkkonen J. Chronic ibuprofen treatment does not affect the secondary pathology in the thalamus or improve behavioral outcome in middle cerebral artery occlusion rats. Pharmacol Biochem Behav. 2011;99:468–474. doi: 10.1016/j.pbb.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1–42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002;302:1212–1219. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xiao Y, Li Z. P2X7 receptor positively regulates MyD88-dependent NF-kappaB activation. Cytokine. 2011;55:229–236. doi: 10.1016/j.cyto.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Lu C, Liu L, Chen Y, Ha T, Kelley J, Schweitzer J, Kalbfleisch JH, Kao RL, Williams DL, Li C. TLR2 ligand induces protection against cerebral ischemia/reperfusion injury via activation of phosphoinositide 3-kinase/Akt signaling. J Immunol. 2011;187:1458–1466. doi: 10.4049/jimmunol.1003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Ikeda K, Mukaida N, Harada A, Matsumoto Y, Yamashita J, Matsushima K. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest. 1997;77:119–125. [PubMed] [Google Scholar]
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Stepanyan TD, Self RL, Hensley AK, Harris BR, Kowalski A, Littleton JM, Prendergast MA. Corticosterone and dexamethasone potentiate cytotoxicity associated with oxygen-glucose deprivation in organotypic cerebellar slice cultures. Neuroscience. 2005;136:259–267. doi: 10.1016/j.neuroscience.2005.07.043. [DOI] [PubMed] [Google Scholar]
- Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304:1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- Murugan M, Ling EA, Kaur C. Glutamate receptors in microglia. CNS Neurol Disord Drug Targets. 2013;12:773–784. doi: 10.2174/18715273113126660174. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Kogure K. Exo-focal postischemic neuronal death in the rat brain. Brain Res. 1990;524:196–202. doi: 10.1016/0006-8993(90)90690-d. [DOI] [PubMed] [Google Scholar]
- Nagayama M, Niwa K, Nagayama T, Ross ME, Iadecola C. The cyclooxygenase-2 inhibitor NS-398 ameliorates ischemic brain injury in wild-type mice but not in mice with deletion of the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab. 1999;19:1213–1219. doi: 10.1097/00004647-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, Kim SU. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5:e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Emmrich JV, Fricker M, Mander PK, Thery C, Brown GC. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A. 2013;110:E4098–E4107. doi: 10.1073/pnas.1308679110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- Nighoghossian N, Berthezene Y, Mechtouff L, Derex L, Cho TH, Ritzenthaler T, Rheims S, Chauveau F, Bejot Y, Jacquin A, et al. Cyclosporine in acute ischemic stroke. Neurology. 2015;84:2216–2223. doi: 10.1212/WNL.0000000000001639. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–199. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55:1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Pedata F, Dettori I, Coppi E, Melani A, Fusco I, Corradetti R, Pugliese AM. Purinergic signalling in brain ischemia. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem. 2009;109:287–294. doi: 10.1111/j.1471-4159.2009.05972.x. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Quattromani MJ, Cordeau P, Ruscher K, Kriz J, Wieloch T. Enriched housing down-regulates the Toll-like receptor 2 response in the mouse brain after experimental stroke. Neurobiol Dis. 2014;66:66–73. doi: 10.1016/j.nbd.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation. 2015;12:106. doi: 10.1186/s12974-015-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Rupalla K, Allegrini PR, Sauer D, Wiessner C. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol. 1998;96:172–178. doi: 10.1007/s004010050878. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28:382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Davis H, Arnelle D, Deaver D. Translational potential of naloxone and naltrexone as TLR4 antagonists. Trends Pharmacol Sci. 2014;35:431–432. doi: 10.1016/j.tips.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Slivka AP, Murphy EJ. High-dose methylprednisolone treatment in experimental focal cerebral ischemia. Exp Neurol. 2001;167:166–172. doi: 10.1006/exnr.2000.7532. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G, Lesiuk H, Bose R, Sima AA. Effect of mannitol, nimodipine, and indomethacin singly or in combination on cerebral ischemia in rats. Stroke. 1988;19:571–578. doi: 10.1161/01.str.19.5.571. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Hattori K, Hamanaka J, Murase T, Egashira Y, Mishiro K, Ishiguro M, Tsuruma K, Hirose Y, Tanaka H, et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci Rep. 2012;2:896. doi: 10.1038/srep00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol. 2000;12:113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- Tamura A, Tahira Y, Nagashima H, Kirino T, Gotoh O, Hojo S, Sano K. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke. 1991;22:615–618. doi: 10.1161/01.str.22.5.615. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Yeh SJ, Li YI, Wang YC, Baik SH, Santro T, Widiapradja A, Manzanero S, Sobey CG, Jo DG, et al. Evidence for a detrimental role of TLR8 in ischemic stroke. Exp Neurol. 2013;250:341–347. doi: 10.1016/j.expneurol.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Thiel A, Radlinska BA, Paquette C, Sidel M, Soucy JP, Schirrmacher R, Minuk J. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. J Nucl Med. 2010;51:1404–1412. doi: 10.2967/jnumed.110.076612. [DOI] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Vartanian KB, Stevens SL, Marsh BJ, Williams-Karnesky R, Lessov NS, Stenzel-Poore MP. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J Neuroinflammation. 2011;8:140. doi: 10.1186/1742-2094-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veighey K, Macallister RJ. Clinical applications of remote ischemic preconditioning. Cardiol Res Pract. 2012;2012:620681. doi: 10.1155/2012/620681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte C, Amadio S, Cavaliere F, D’Ambrosi N, Vacca F, Bernardi G. Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:403–412. doi: 10.2174/1568007033482643. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Kimura J. Protective effect of cyclosporin A on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke. 1995;26:1415–1422. doi: 10.1161/01.str.26.8.1415. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhou H, Gao H, Chen SH, Chu CH, Wilson B, Hong JS. Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J Neuroinflammation. 2012;9:32. doi: 10.1186/1742-2094-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ge P, Zhu Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm. 2013;2013:124614. doi: 10.1155/2013/124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters L, Winters T, Gorup D, Mitrecic D, Curlin M, Kriz J, Gajovic S. Expression analysis of genes involved in TLR2-related signaling pathway: Inflammation and apoptosis after ischemic brain injury. Neuroscience. 2013;238:87–96. doi: 10.1016/j.neuroscience.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhan Y, Wang Y, Feuerstein GZ, Wang X. Recombinant adenoviral expression of dominant negative IkappaBalpha protects brain from cerebral ischemic injury. Biochem Biophys Res Commun. 2002;299:14–17. doi: 10.1016/s0006-291x(02)02573-1. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- Yang GY, Gong C, Qin Z, Ye W, Mao Y, Bertz AL. Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport. 1998;9:2131–2134. doi: 10.1097/00001756-199806220-00041. [DOI] [PubMed] [Google Scholar]
- Yang QW, Li JC, Lu FL, Wen AQ, Xiang J, Zhang LL, Huang ZY, Wang JZ. Upregulated expression of toll-like receptor 4 in monocytes correlates with severity of acute cerebral infarction. J Cereb Blood Flow Metab. 2008;28:1588–1596. doi: 10.1038/jcbfm.2008.50. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Hess DC, Borlongan CV. Combined cyclosporine-A and methylprednisolone treatment exerts partial and transient neuroprotection against ischemic stroke. Brain Res. 2004;1018:32–37. doi: 10.1016/j.brainres.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Zhang P, Cheng G, Chen L, Zhou W, Sun J. Cerebral hypoxia-ischemia increases toll-like receptor 2 and 4 expression in the hippocampus of neonatal rats. Brain Dev. 2015;37:747–752. doi: 10.1016/j.braindev.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, Miyasaka M, Ward PA. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]