Abstract
Hsp90 is a promising therapeutic target for the development of anti-cancer agents due to its integral role in the stability and function of proteins associated with all ten hallmarks of cancer. Novobiocin, a coumarin antibiotic, was the first natural product identified that targeted the Hsp90 C-terminal domain and manifested anti-proliferative activity (SKBr3 IC50 ~ 700 μM). Subsequent structural investigations on novobiocin led to analogues with significantly improved anti-proliferative activity against multiple cancer cell lines. In an effort to develop more efficacious and diverse analogues, it was recently found that the coumarin ring of novobiocin could be replaced with the biphenyl core without compromising activity. Based on these prior studies, a series of alkylamino biphenylamides was designed, synthesized and evaluated for anti-proliferative activity against two breast cancer cell lines. SAR studies demonstrated that the incorporation of an alkylamino side chain onto the biphenyl core improved anti-proliferative activity and resulted in compounds that exhibit sub-micromolar to mid-nanomolar activity through Hsp90 inhibition. Importantly, these studies indicate the presence of a hydrophilic region about the central core that can be exploited for the design of new inhibitors.
Keywords: Heat shock protein 90, Hsp90 C-terminal inhibitors, Alkylamino biphenylamides, Structure-activity relationship, Breast cancer
Graphical Abstract
1. Introduction
Heat shock protein 90 (Hsp90) is a highly conserved molecular chaperone that plays a critical role in protein homeostasis.1 Hsp90 regulates the conformational maturation, activation and stability of more than 300 client proteins, including cytosolic signaling proteins, transcription factors, and kinases.2 Several of these clients (eg. Her2, CDK6, Raf1, Akt, survivin, telomerase) have been implicated in the development and progression of cancer and are directly associated with all ten hallmarks of cancer.3–5 Moreover, in cancer cells, Hsp90 is overexpressed to promote cellular growth and/or survival under the hostile micro-environment characterized by proteotoxic stresses, such as imbalanced protein production, accumulation of mutated proteins, and increased protein damage due to oxidative stress.6, 7 Consequently, Hsp90 has emerged as a promising target for the development of anticancer chemotherapeutics.8, 9 In fact, 17 small molecules that target the Hsp90 N-terminus have been investigated in clinical trials for the treatment of various cancers, highlighting the therapeutic potential of Hsp90 inhibitors as anticancer agents.10 Although these investigational drugs have provided proof-of-concept for Hsp90 inhibitors in cancer, their clinical translation has been hampered by concomitant induction of the pro-survival heat shock response (HSR), cardiac arrhythmia and hepatotoxicity, and thus necessitate alternative methods to modulate Hsp90 for cancer treatment.11, 12
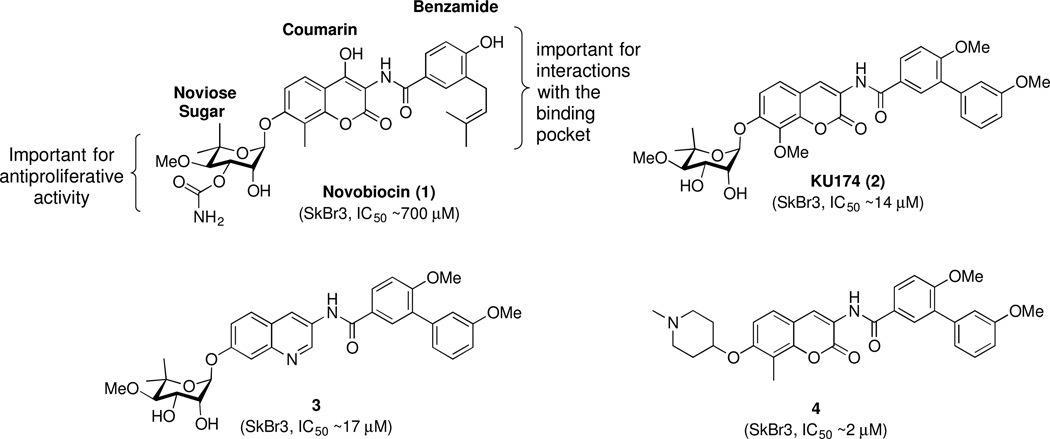
Prior studies identified small molecules that bind the Hsp90 C-terminal domain and allosterically modulate Hsp90 function.13–15 Novobiocin (1), a coumarin antibiotic, was the first natural product identified as an Hsp90 C-terminal inhibitor.13 Importantly, novobiocin did not induce the HSR, which is a major detriment resulting from Hsp90 N-terminal inhibition. Unfortunately, novobiocin exhibits poor efficacy against cancer cells (SKBr3 IC50 ~ 700 μM), and consequently, offers limited therapeutic potential. Recent studies on novobiocin led to the elucidation of some structure-activity relationships (SAR) and identified key structural elements required for Hsp90 inhibition (Figure 1).16–23 SAR studies revealed that the benzamide side chain is critical to manifest anti-proliferative activity and modifications to this moiety produced molecules that exhibited increased anti-proliferative activity against multiple cancer cell lines.18, 20–24 In addition, it was found that the noviose sugar could be replaced with ionizable amines, which resulted in analogues that showed improved potency and solubility.21–23, 25 In contrast, studies suggested that the coumarin core serves only as a backbone to orient the benzamide and sugar side chains in the binding pocket.19 Subsequent studies determined that the coumarin ring could be replaced with other aromatic and heteroaromatic scaffolds without compromising activity.19, 26
Figure 1.
HSP90 C-terminal inhibitors.
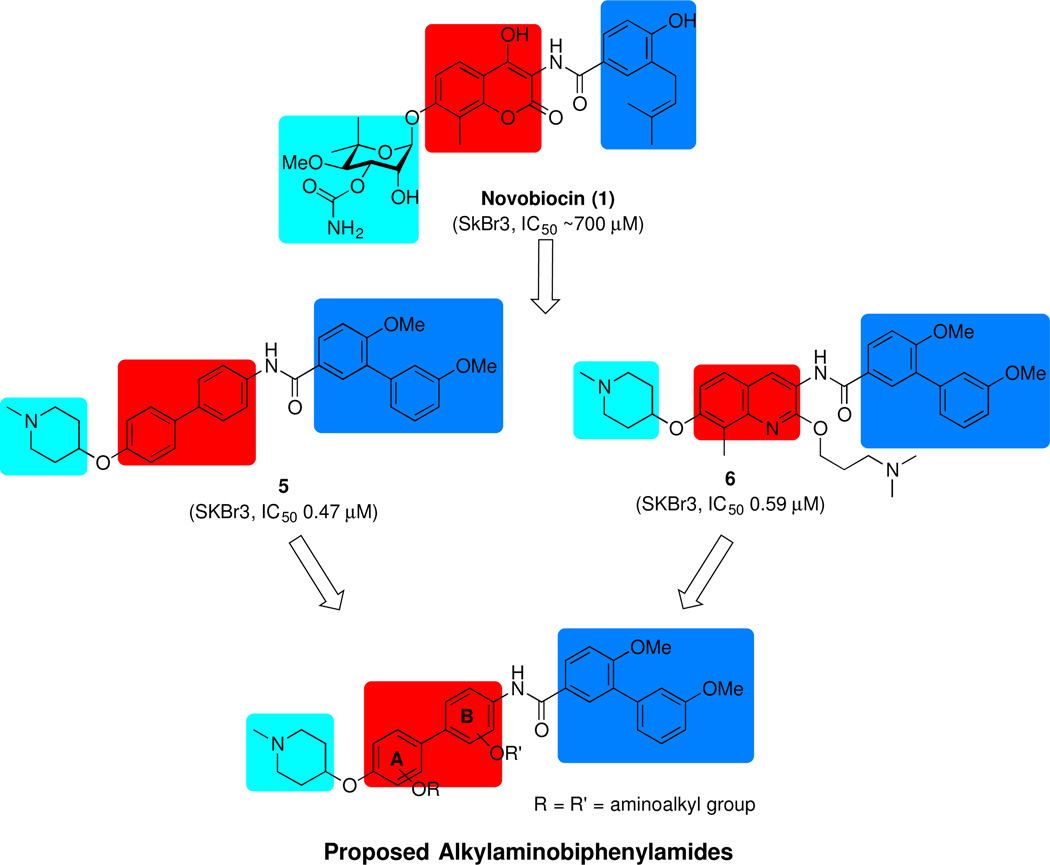
More recently, it was demonstrated that the coumarin core could be replaced with a biaryl moiety, which resulted in increased anti-proliferative activity.26, 27 As a flexible structure, the biphenyl ring system can adopt multiple conformations within the binding pocket and potentially, provide additional interactions with the protein. Indeed, structure-activity relationship (SAR) studies on the biaryl moiety led to the development of several efficacious inhibitors, such as KU820 (5) (Figure 2), which exhibited improved anti-proliferative activity. In addition, a library of quinoline derivatives that contain an alkylamino side chain at the 2-position were explored and led to identification of molecule, 6 (Figure 2), which showed sub-micromolar anti-proliferative activity against several cancer cell lines.28 Importantly, the SAR studies suggested the potential for a previously unexplored hydrophilic region about the central core. Since incorporation of the alkylamino side chain onto the central core improved anti-proliferative activity by providing additional interactions with this region, it was proposed that placing this side chain onto the biphenyl core could also result in similar effects. Therefore, a series of alkylamino biphenylamides was synthesized and evaluated in an effort to develop more efficacious inhibitors.
Figure 2.
Rationale for proposed biphenylamide analogues.
2. Results and Discussion
2.1 Design of Alkylamino biphenylamides
Early studies on novobiocin and related compounds revealed that the benzamide side chain and the sugar are important for anti-proliferative activity, while the central core appears to serve as an anchor to orient these appendages within the binding pocket.19, 26 Therefore, a series of alkylamino biphenylamides (Figure 2) was designed to contain the optimized biaryl side chain and the sugar surrogate, N-methyl-4-piperidine, which are present in compound 5 in an effort to obtain additional interactions with the surrounding binding region as observed with 6. The alkylamino side chain was incorporated into all four positions of the biphenyl core to identify the location that showed optimal anti-proliferative activity. Futhermore, prior studies on alkylamino quinolines suggested the propylamino linker was most efficacious.28 Since the biaryl core can adopt multiple conformations, the linker length was varied to contain either a 2-carbon or 3-carbon linker. Molecular overlay studies29 with compounds 5 and 6 (Figure 3), suggested the alkylamino side chain should be incorporated onto the B-ring of the biphenyl core. Therefore, the alkylamino biphenylamides with B-ring modifications were pursued first.
Figure 3.
Molecular overlay of energy minimized compounds 5 (green) and 6 (grey).
2.2 Synthesis and biological evaluation of alkylamino biphenylamides
As shown in Scheme 2, the preparation of biphenylamides containing B-ring modifications began by a Suzuki coupling between 4-hydoxyphenylbronic acid (7) and bromides 8a–b, which were obtained through MOM-protection of the corresponding and commercially available phenols. Mitsunobu etherification of the nitrophenols (12a–b) with 1-methyl-4-hydroxypiperidine (13) afforded nitro aromatics 14a–b, which were then reduced with Pd/C under a hydrogen atmosphere to yield the anilines, 15a–b. Amide coupling of anilines 15a–b, with acid chloride 16a provided the corresponding amides, 17a–b, which were then MOM-deprotected and subjected to Mitsunobu etherification to give the final biphenylamides, 20a–d, in moderate yields.
Scheme 2.
Synthesis of alkylamino biphenylamides with B-ring modifications.
Reagaents and condtions: a Pd(PPh3)4, 2M K2CO3, 1,4-dioxane, 100 °C, 12 h, 58% ~ 62%; b PPh3, DIAD, THF, 0 °C to rt, 12 h, 56% ~ 60%; c 10% Pd/C, H2, MeOH/THF, rt, 12 h, ~100%; d Et3N, DCM, 0 °C to rt, 12 h, 68% ~ 88%; e p-TSA, MeOH, rt, 12 h, 77% ~ 82%; f Aminoalkyl alcohol, TMAD, PBu3, benzene, 80 °C, 12 h, 31% ~ 54%.
Upon synthesis of these alkylamino biphenylamides, they were evaluated for anti-proliferative activity against SKBr3 (Her2 overexpressing breast cancer cells) and MCF-7 (estrogen receptor positive breast cancer cells) cell lines. As shown in table 1, biphenylamides that contain modifications to the B-ring manifested comparable activity to the unsubstituted analogue, 5. A phenol at either the C-2 or C-3 position of the B-ring produced compounds that were less potent than the unsubstituted analogue (18a, 18b vs 5). Surprisingly, introduction of alkylamino substituents at the 2-position of the B-ring (20a, 20b vs 18a) did not affect anti-proliferative activity. However, introduction of alkylamino substituents at the 3-position improved potency, as analogues (20c, 20d) exhibited ~5 fold greater anti-proliferative activity than 18b. These data suggested that the alkylamino side chain is beneficial for anti-proliferative activity, however it may not provide optimal interactions with the surrounding region as was observed with the quinolines derivatives. These results encouraged investigation of alkylamino substitutions onto the A ring of the biphenylamide derivatives as well.
Table 1.
Anti-proliferative activity of biphenylamides with B ring modifications.
 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | SKBr3 (IC50, µM) |
MCF-7 (IC50, µM) |
| 5 | H | H | 0.47 ± 0.06 a | 0.71 ± 0.02 |
| 18a | OH | H | 0.91 ± 0.02 | 0.71 ± 0.06 |
| 18b | H | OH | 3.43 ± 0.18 | 1.80 ± 0.11 |
| 20a | H | 0.84 ± 0.06 | 0.81 ± 0.18 | |
| 20b | H | 0.69 ± 0.05 | 0.75 ± 0.02 | |
| 20c | H | 0.65 ± 0.01 | 0.79 ± 0.01 | |
| 20d | H | 0.55 ± 0.05 | 0.67 ± 0.02 | |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate.
As shown in Scheme 3, a series of biphenyl derivatives containing a modified A-ring was synthesized for comparison with B-ring modifications. Synthesis of biphenyl derivatives was initiated via a Suzuki coupling of nitrophenylboronic acid (11) with previously reported bromophenols,30 10a–b, to afford nitrophenols 12c–d, which then underwent Mitsunobu etherification with 1-methyl-4-hydroxypiperidine (13) to give the corresponding nitro aromatics, 14c–d. Reduction of 14c–d, followed by amide coupling with acid chloride 16a afforded the amides 17c–d. Acid-catalyzed MOM-deprotection of 17c–d, followed by Mitsunobu etherification gave the desired biphenylamides (20e–f) in modest to low yields.
Scheme 3.
Synthesis of alkylamino biphenylamides with A-ring modifications.
Reagaents and condtions: a Pd(PPh3)4, 2M Na2CO3, toluene/EtOH, 120 °C, 12 h, 60% ~ 73%; c PPh3, DIAD, THF, 0 °C to rt, 12 h, 89% ~ 92%; d 10% Pd/C, H2 CH3COOH, MeOH/THF, rt, 12 h, ~100%; e Et3N, THF, 0 °C to rt, 12 h, 65% ~ 67%; f 2N HCl, MeOH, rt, 12 h, 82% ~ 87%; g Aminoalkyl alcohol, TMAD, PBu3, benzene, 80 °C, 12 h, 30% ~ 45%.
Upon their preparation, the biphenylamides with modifications to the A-ring were evaluated for their anti-proliferative activity against SKBr3 and MCF-7 breast cancer cell lines (Table-2). In general, biphenylamides containing modifications to the A-ring were more potent than the biphenyl derivatives with B-ring modifications. It appears that substitution on the A-ring of the biphenyl core (18c, 18d, 17c or 17d vs 6) is less favorable, which may be explained by suboptimal conformations of the biphenyl linker that leads to diminished interactions with the binding pocket. Similar to the trend observed with the B-ring modifications, incorporation of the alkylamino side chain onto the A-ring improved anti-proliferative activity, as analogues (20e–h) were 5~10 fold more potent than 18c or 18d. The data suggests that incorporation of an alkylamino side chain onto the 3’-position of the A-ring results in compounds that show good anti-proliferative activity (20e, 20f vs 20a-d, 20g, 20h). Furthermore, analogues containing a 3-carbon linker exhibited slightly improved activity over the corresponding 2-carbon tethered biphenylamide (20f vs 20e).
Table 2.
Anti-proliferative activity of biphenylamides with A ring modifications.
Overall, preliminary structure-activity relationships indicate that the propylamino side chain is important for enhanced anti-proliferative activity. In addition, the 3’ position of the A-ring represents the best location for further investigation of the side chain. To investigate the optimal linker length and substituent effect, a concise library of the biphenylamides containing rigid, hydrophobic and non-ionizable moieties was synthesized as shown in scheme 4.
Scheme 4.
Synthesis of biphenylamides with varied alkyl side chains.
Reagaents and condtions: a i. Pd(PPh3)4, 2M Na2CO3, toluene/EtOH, 120 °C, 12 h, 79%, ii. 10% Pd/C, H2, THF/MeOH, rt, 12 h, ~100% ; b EDCI•HCl, HOBt, Et3N, DCM, 0 °C to rt, 12 h, 68%; c i. 3.2 N KOH, EtOH, 90 °C, 3 h, 68%, ii. BnBr, K2CO3, acetone, 65 °C, 12, 85%; d 2N HCl, MeOH, rt, 12 h, 85%; e i. TMAD, PBu3, benzene, 80 °C, 12 h, 56% or R-Cl, K2CO3, DMF, 0 °C to rt, 12 h, 50% ~70%; ii. 10% Pd/C, H2, MeOH/THF, rt, 12 h, ~100%; iii. DIAD, PPh3, THF, 0 °C to rt, 12 h, 23% ~ 50%; h K2CO3, MeOH, rt, 4 h, 65%.
The anti-proliferation activity exhibited by these analogues is reported in Table 3. The results indicated that the 3-carbon linker is optimal, as further homologation of the linker length resulted in reduced activity (25a vs 20f). Incorporation of a cyclic amine decreased anti-proliferative activity (25b), suggesting the need for a flexible substituent. In addition, introduction of steric bulk (25c) diminished activity, indicating there is limited space in the surrounding binding pocket. Incorporation of the aliphatic substituent produced a less active compound (25d vs 20f), which suggests that improved anti-proliferative activity may result from either hydrogen-bonding or ionic interactions with the binding region. Surprisingly, incorporation of both a hydrogen-bond donor and/or acceptor (25e, 25f) reduced activity, indicating that the charged ammonium group is important to increase anti-proliferative activity.
Table 3.
Anti-proliferative activity of biphenylamides.
The antiproliferative activity produced by these biphenylamides was confirmed to result from Hsp90 inhibition by a luciferase-refolding assay. As shown in Figure 4A, 20e and 20f inhibited the Hsp90-dependent refolding of thermally denatured luciferase, confirming that these analogues exhibit their cellular activity through modulation of the Hsp90 protein folding machinery. Western blot analysis was performed with MCF-7 cell lysates treated with the most active compound, 20f to provide additional evidence of Hsp90 inhibition. As shown in figure 4B, incubation with 20f induced the degradation of Hsp90-dependent client proteins, Her2, Raf-1, and Akt in a concentration-dependent manner, without affecting actin levels. In addition, Hsp90 levels remained constant at all concentrations, which is a hallmark resulting from Hsp90 C-terminal inhibition.
Figure 4.
A. Luciferase refolding activity of Hsp90 in PC3- MM2 cells with vehicle (DMSO), 20e, 20f and 5 (positive control). The concentrations used were 0.1−100 μM. B. Western blot analyses of Hsp90-dependent client proteins (Her2, Raf-1 and ER) using lysate from MCF-7 breast cancer cells treated with 20f for 24 h. Concentrations (in μM) were indicated above each lane. Geldanamycin (G, 0.5 μM) and dimethylsulfoxide (D, 100%) were employed as positive and negative controls respectively.
3. Conclusions
In summary, a series of alkylamino biphenylamides was designed, synthesized and evaluated for anti-proliferative activity against MCF-7 and SKBr3 breast cancer cell lines. The incorporation of an alkylamino side chain onto the biphenyl core resulted in compounds that manifest sub-micromolar to mid-nanomolar inhibitory activity. Both, luciferase refolding assays and Western blot analyses confirmed these compounds exhibit their anti-proliferative activity through Hsp90 inhibition. SAR studies revealed that amines attached to a three-carbon linker gave the most potent biphenylamides analogues.
4. Experimental section
4.1 Anti-proliferation assays
Cells were maintained in a 1:1 mixture of Advanced DMEM/F12 (Gibco) supplemented with non-essential amino acids, L-glutamine (2 mM), streptomycin (500 μg/mL), penicillin (100 units/mL), and 10% FBS. Cells were grown to confluence in a humidified atmosphere (37° C, 5% CO2), seeded (2000/well, 100 μL) in 96-well plates, and allowed to attach overnight. Compound or GDA at varying concentrations in DMSO (1% DMSO final concentration) was added, and cells were returned to the incubator for 72 h. At 72 h, the number of viable cells was determined using an MTS/PMS cell proliferation kit (Promega) per the manufacturer’s instructions. Cells incubated in 1% DMSO were used at 100% proliferation, and values were adjusted accordingly. IC50 values were calculated from separate experiments performed in triplicate using GraphPad Prism.
4.2 Western blot Analyses
MCF-7 cells were cultured as described above and treated with various concentrations of drug, GDA in DMSO (1% DMSO final concentration), or vehicle (DMSO) for 24 h. Cells were harvested in cold PBS and lysed in RIPA lysis buffer containing 1 mM PMSF, 2 mM sodium orthovanadate, and protease inhibitors on ice for 1 h. Lysates were clarified at 14000g for 10 min at 4° C. Protein concentrations were determined using the Pierce BCA protein assay kit per the manufacturer’s instructions. Equal amounts of protein (20 μg) were electrophoresed under reducing conditions, transferred to a nitrocellulose membrane, and immunoblotted with the corresponding specific antibodies. Membranes were incubated with an appropriate horseradish peroxidase-labeled secondary antibody, developed with a chemiluminescent substrate, and visualized.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support of this project by the NIH/NCI (CA120458). The NMR support for this project was provided by NSF (9512331). The authors are greatly thankful to Frontier Scientific, Inc. for providing high quality boronic acids for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Taipale M, Jarosz DF, Lindquist S. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 2.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover Kenneth D, Karras Georgios I, Lindquist S. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg Robert A. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Garg G, Khandelwal A, Blagg BS. Adv. Cancer Re.s. 2016;129:51–88. doi: 10.1016/bs.acr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai C, Dai S, Cao J. J. Cell. Physio. 2012;227:2982–2987. doi: 10.1002/jcp.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephanie CB, Joseph AB, Brian SJB. Curr. Cancer Drug Targets. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhury S, Welch TR, Blagg BSJ. ChemMedChem. 2006;1:1331–1340. doi: 10.1002/cmdc.200600112. [DOI] [PubMed] [Google Scholar]
- 9.Bishop SC, Burlison JA, Blagg BS. Curr. Cancer Drug Targets. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 10.Neckers L, Workman P. Hsp90. Clin. Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitesell L, Lindquist SL. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 12.Brandt GE, Blagg BS. Curr. Top. Med. Chem. 2009;9:1447–1461. doi: 10.2174/156802609789895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcu MG, Schulte TW, Neckers L. J. Natl. Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 14.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly A, Blagg BS. Curr. Med. Chem. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu XM, Shen G, Neckers L, Blake H, Holzbeierlein J, Cronk B, Blagg BSJ. J. Am. Chem. Soc. 2005;127:12778–12779. doi: 10.1021/ja0535864. [DOI] [PubMed] [Google Scholar]
- 17.Burlison JA, Neckers L, Smith AB, Maxwell A, Blagg BSJ. J. Am. Chem. Soc. 2006;128:15529–15536. doi: 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- 18.Burlison JA, Avila C, Vielhauer G, Lubbers DJ, Holzbeierlein J, Blagg BSJ. J. Org. Chem. 2008;73:2130–2137. doi: 10.1021/jo702191a. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly AC, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, Blagg BSJ. J. Org. Chem. 2008;73:8901–8920. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskew JD, Sadikot T, Morales P, Duren A, Dunwiddie I, Swink M, Zhang X, Hembruff S, Donnelly A, Rajewski RA, Blagg BS, Manjarrez JR, Matts RL, Holzbeierlein JM, Vielhauer GA. BMC Cancer. 2011;11:468. doi: 10.1186/1471-2407-11-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Donnelly AC, Kusuma BR, Brandt GEL, Brown D, Rajewski RA, Vielhauer G, Holzbeierlein J, Cohen MS, Blagg BSJ. J. Med. Chem. 2011;54:3839–3853. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Blagg BSJ. Bioorg. Med. Chem. Lett. 2013;23:552–557. doi: 10.1016/j.bmcl.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg G, Zhao H, Blagg BSJ. ACS Med. Chem. Lett. 2014 doi: 10.1021/ml5004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh S, Liu Y, Garg G, Anyika M, McPherson NT, Ma J, Dobrowsky RT, Blagg BSJ. ACS Med. Chem. Lett. 2016;7:813–818. doi: 10.1021/acsmedchemlett.6b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Reddy Kusuma B, Blagg BSJ. ACS Med. Chem. Lett. 2010;1:311–315. doi: 10.1021/ml100070r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Garg G, Zhao J, Moroni E, Girgis A, Franco LS, Singh S, Colombo G, Blagg BSJ. Eur. J. Med. Chem. 2015;89:442–466. doi: 10.1016/j.ejmech.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Moroni E, Colombo G, Blagg BSJ. ACS Med. Chem. Lett. 2013;5:84–88. doi: 10.1021/ml400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusuma BR, Khandelwal A, Gu W, Brown D, Liu W, Vielhauer G, Holzbeierlein J, Blagg BSJ. Bioorg. Med. Chem. Lett. 2014;22:1441–1449. doi: 10.1016/j.bmc.2013.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matts RL, Dixit A, Peterson LB, Sun L, Voruganti S, Kalyanaraman P, Hartson SD, Verkhivker GM, Blagg BSJ. ACS Chem. Biol. 2011;6:800–807. doi: 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimomura K, Ikai T, Kanoh S, Yashima E, Maeda K. Nat. Chem. 2014;6:429–434. doi: 10.1038/nchem.1916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.