Abstract
Every year in the United States, millions of individuals incur ischemic brain injury from stroke, cardiac arrest, or traumatic brain injury (TBI). These forms of acquired brain injury can lead to death, or in many cases long-term neurologic and neuropsychological impairments. The mechanisms of ischemic and traumatic brain injuries that lead to these deficiencies result from a complex interplay of multiple interdependent molecular pathways that include excitotoxicity, acidotoxicity, ionic imbalance, oxidative stress, inflammation, and apoptosis. This article briefly reviews several of the traditional, well-known mechanisms of brain injury and then discusses more recent developments and newer mechanisms. Although much is known concerning mechanisms of injury and the manipulation of these mechanisms to result in protection of neurons and increased behavioral performance in animal models of injury, it has been difficult to translate these effects to humans. Attention is given to why this is so and newer outcome measures of injury are discussed.
Keywords: Mechanisms of ischemic brain injury, Excitotoxicity, Oxidative stress, Neuroinflammation, Apoptosis, Synaptic plasticity, Neurovascular unit, Translation
MECHANISMS OF INJURY FOLLOWING BRAIN ISCHEMIA
Excitotoxicity
The glutamate excitotoxicity hypothesis of ischemic cell damage suggests that injury is triggered by glutamate, an excitatory amino acid, released during ischemia from the intracellular compartment into the extracellular environment.1 Glutamate is a major transmitter in the nervous system and, in addition to being required for rapid synaptic transmission for neuron-to-neuron communication, glutamate plays important roles in neuronal growth and axon guidance, brain development and maturation, and synaptic plasticity. Under normal physiologic conditions, the presence of glutamate in the synapse is regulated by active ATP-dependent transporters in neurons and glia. However, if these uptake mechanisms are impaired by metabolic disturbances brought about by ischemia, glutamate excessively accumulates, stimulating sodium (Na+) and calcium (Ca2+) fluxes into the cell through glutamate receptors, thereby injuring or killing the cell. Glutamate activates different types of ion channel–forming receptors (ionotropic) and G-protein–coupled receptors (metabotropic) that have an important role in brain function. The major ionotropic receptors activated by glutamate are commonly referred to as the N-methyl-D-aspartic acid (NMDA), alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA), and kainic acid receptors. The ionotropic receptors are ligand-gated ion channels permeable to various cations. Overactivation of these receptors leads to an increase in intracellular Ca2+ load and catabolic enzyme activity, which can trigger a cascade of events leading to apoptosis and necrosis. These events can include membrane depolarization, production of oxygen free radicals, and cellular toxicity. NMDA and AMPA receptor antagonists showed great promise for providing neuroprotection in animal models, but have failed to translate clinically. One complication with this approach is the unwanted side effects associated with blocking receptors that are critical for normal brain function. Alternative approaches are now being considered, such as using partial NMDA antagonists such as memantine2 or blocking receptor interactions with postsynaptic scaffolding molecules postsynaptic density protein 93/95.3, 4 Therapies targeting molecules downstream of NMDA receptors, such as calcium-calmodulin–dependent protein kinase (CAMKII) and death-associated protein kinase (DAPK) may also hold promise for limiting the excitotoxic cascade. In contrast, metabotropic receptors (metabotropic glutamate receptors [mGluRs]) are G-protein–coupled receptors that have been subdivided into 3 groups, based on sequence similarity, pharmacology, and intracellular signaling mechanisms. The role of mGluRs in brain injury is complex; however, most of the evidence points to a neuroprotective role, likely via antiapoptotic signaling and decreased excitability countering excitotoxicity.
Acidotoxicity
Metabolic acidosis can occur as a result of lactate accumulation during and following ischemia, or when mitochondrial respiration is dysfunctional. Acid-sensing ion channels (ASICs) represent a group of ion channels activated by protons, and act as sensors of tissue pH.2 They belong to the epithelial sodium family of amiloride-sensitive cation channels and allow Na+ and Ca2+ entry into neurons. There have been at least 6 ASIC subunits cloned and ASIC1a, ASIC2a, and ASIC2b are expressed in the brain and spinal cord. ASIC1a and ASIC2s are found in brain regions with high synaptic density and facilitate excitatory transmission. ASICs have been shown to be activated in ischemia and their activation contributes to neuronal cell death through Ca2+, Na+, and Zn2+ influx into the cell. Remarkably, inhibition of ASIC1a following stroke has a therapeutic window of 5 hours in experimental stroke models,5 which is beyond that of the currently available treatment, tissue plasminogen activator (tPA).
Oxidative Stress
During normal mitochondrial respiration, cytochrome c is involved in a 4-electron transfer to reduce oxygen to water without the production of oxygen radicals.6, 7 During ischemia, when oxygen supply is limited, the electron transport chain becomes highly reduced and oxygen radicals can be produced. This process can be exacerbated by the mitochondrial Ca2+ accumulation that occurs during and following ischemia resulting in mitochondrial dysfunction, which can result in the formation of reactive oxygen species. Several oxygen radical species can be produced, including superoxide, perhydroxyl, hydrogen peroxide, and hydroxyl radicals. Another pathway for forming hydroxyl radical is through the reaction of superoxide and nitric oxide to form peroxynitrite. All of these reactive oxygen species, especially peroxynitrite and superoxide, can bind directly to DNA, changing its structure and causing cell injury and enhancement of apoptosis. An antioxidant/antiinflammatory agent, edaravone, is currently in use to treat acute ischemic stroke in Japan. However, this and other antioxidant compounds (NXY-059; SAINT trials8) have failed to improve outcome following stroke in the United States. Thus, new targeted approaches to preventing reactive oxygen species damage are needed. Targeting downstream mediators of oxidative stress are emerging therapies, such as inhibition of poly(ADP ribose) polymerase-1 or transient receptor potential melastatin-2 channels. Further research into the biological consequences following oxidative stress may lead to new directed therapies to reduce ischemic injury.
Inflammation
Inflammation plays an important role in the pathogenesis of ischemic brain injury.9, 10 The brain responds to ischemic injury with an acute and prolonged inflammatory process that is characterized by rapid activation of resident cells (microglia), production of proinflammatory mediators, and infiltration of various types of inflammatory cells, such as neutrophils, different types of T cells, macrophages, and other cells, into the ischemic brain tissue. Cytokines and chemokines contribute to ischemic brain injury and, during ischemia, cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor alpha, transforming growth factor beta, and chemokines such as cytokine-induced neutrophil chemoattractant and monocyte chemoattractant protein-1 are produced by a variety of different activated cell types, such as endothelial cells, microglia, neurons, platelets, leukocytes, and fibroblasts. The inflammatory response in brain may have various consequences on outcome, depending on the degree of inflammatory response and when it occurs. Although acute inflammatory events may be involved in secondary injury processes, more delayed inflammatory events may be reparative. However, it is difficult to elucidate the precise mechanisms of the inflammatory responses following ischemia because inflammation is a complex series of interactions between inflammatory cells and molecules, all of which could be either detrimental or beneficial. Not surprisingly, acute broad-spectrum inhibitors of inflammation reduce injury in experimental models of cerebral ischemia. The lack of success in clinical translation points to the need for a deeper understanding regarding the role of the various cellular and molecular contributors to postischemic inflammation. There is also the need to better understand the complex temporal profile of the various inflammatory mediators.
Apoptosis
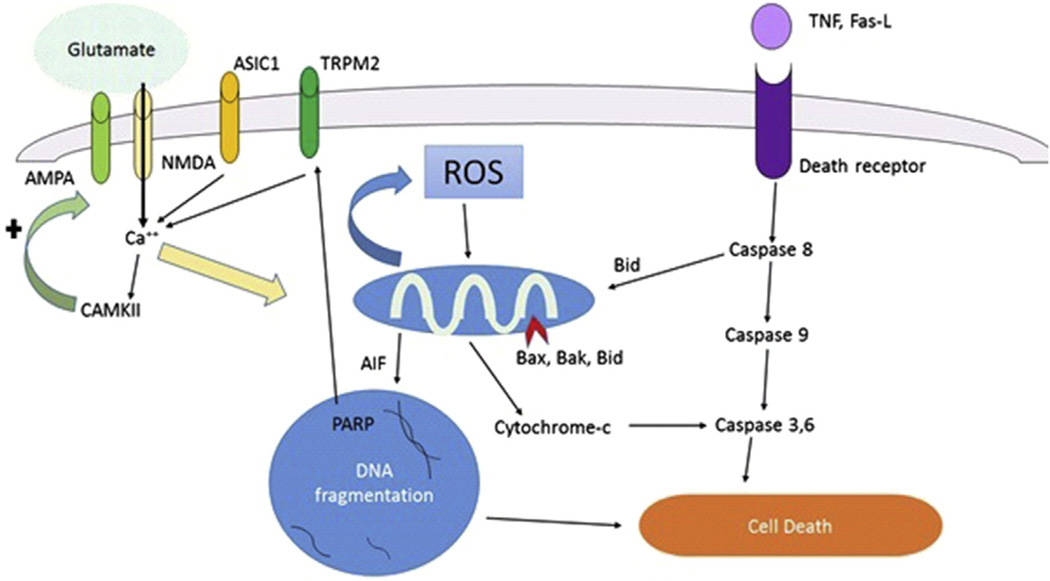
Apoptosis is a genetically controlled mechanism of cell death involved in the regulation of tissue homeostasis.11, 12 Biochemical events lead to characteristic cell changes (morphology) and cell death. These changes include cell blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation, and messenger RNA decay. Triggers of apoptosis include oxygen free radicals, death receptor ligation, DNA damage, protease activation, and ionic imbalance. The 2 major pathways of apoptosis are the extrinsic (Fas and other tumor necrosis factor receptor superfamily members and ligands) and the intrinsic (mitochondria-associated) pathways, both of which are found in the cytoplasm. The extrinsic pathway is triggered by death receptor engagement, which initiates a signaling cascade mediated by caspase-8 activation, whereas the intrinsic pathway is engaged when various apoptotic stimuli trigger the release of cytochrome c from mitochondria independently of caspase-8 activation. Both pathways ultimately cause caspase-3 activation, resulting in the degradation of cellular proteins necessary to maintain cell survival and integrity. In addition, there is a complex interplay of the Bcl-2 family of proteins, which either promote (Bax, Bak, Bad, Bim, Bid) or prevent (Bcl-2, Bcl-xL, Bcl-w) injury. Bcl-2 and its family member, Bcl-xL, are among the most powerful death-suppressing proteins and inhibit both caspase-dependent and caspase-independent cell death. Among the caspase-independent apoptotic cell death pathways is apoptosis-inducing factor (AIF). AIF is stored within the same mitochondrial compartment as cytochrome c. DNA damage (via PARP activation) and oxidative or excitotoxic stress release AIF, which is translocated to the nucleus to induce apoptosis. Fig. 1 shows these pathways.
Fig. 1.
Glutamate, reactive oxygen species, and apoptotic cell death pathways. Fas-L, Fas ligand; ROS, reactive oxygen species.
Translation of these injury mechanisms to neuroprotection in humans
Translation from basic science cerebral ischemia research to treatment of patients with ischemia remains a difficult challenge. Despite hundreds of compounds and interventions that provide benefit in experimental models of cerebral ischemia, efficacy in humans remains to be demonstrated. The reasons for failure to translate have been the focus of discussion for years.13, 14 Some clinicians attribute the failure to flaws in clinical trial design, others question the predictive value of current animal models, and some question the quality of preclinical data. It is likely that a combination of all these shortcomings is to blame. All of the mechanisms of ischemic injury mentioned earlier have been subject to interventions to block or inhibit the injury in preclinical animal models. Investigators have used excitatory amino acid inhibitors, ion channel blockers, free radical scavengers, antiapoptotic agents, and antiinflammatory agents with great success in ameliorating the injury from ischemia in animal models. However, none of these agents have met with success in human clinical trials. Thus, new approaches are needed that consider the complex interplay among various cell types within the brain as well as placing emphasis on examination of long-term functional recovery and plasticity.
INTERCELLULAR INTERACTIONS AND REPAIR
Neurovascular Unit
Extensive research has focused on the intracellular signaling cascades triggered by ischemic brain injury and TBI that lead to cell death. However, despite advances in the understanding of these intracellular pathways, neuroprotective strategies have failed to translate into acute treatments for ischemic stroke, cardiac arrest, or TBI. The only successful treatment to improve stroke outcome is timely revascularization (restoration of blood flow) either mechanically15, 16 or pharmacologically, with tPA.17 A decade ago researchers began to describe the microvasculature and surrounding parenchyma in terms of multiple cellular components, including neurons, microvascular endothelial cells, astrocytes, and pericytes. The ability of astrocytes to communicate with neurons and at the same time be in close proximity to endothelial cells provided the template for the concept of the neurovascular unit.18–20 The neurovascular unit has emerged as a convenient model to conceptualize the intercellular crosstalk in the brain regulating blood flow, integrity of the blood-brain barrier, and ultimately outcomes following injury. More recently, myelinating oligodendrocytes have been added to the picture, as cells that not only maintain myelin integrity but provide trophic support for the underlying axons and signal to surrounding neurons, astrocytes, and other components of the neurovascular unit. Thus, it is clear that emerging new research needs to focus on intercellular signaling in order to understand the mechanisms of ischemic injury in hopes of ultimately enhancing functional recovery.
Successes using revascularization strategies or therapeutic hypothermia are tempered by the short therapeutic time window, severely limiting the number of individuals eligible for such interventions. Therefore, it is imperative that new therapeutic strategies are designed to repair and restore brain function following injury. The focus here is on the role of the components of the neurovascular unit in protection and repair, independent of their role in cerebral blood flow control. Endogenous repair of injured brain involves several intertwined regenerative processes, including neurogenesis, angiogenesis, oligodendrogenesis, and synaptogenesis. In the past 2 decades, promising research has shown that stroke promotes ongoing neurogenesis in the adult and aging brain. Adult neurogenesis is the process of producing new neurons from endogenous neural stem cells residing in either the subventricular zone (SVZ) or dentate gyrus of the hippocampus. Neurogenesis following brain injury requires proliferation of endogenous stem cells, migration to the site of injury, and differentiation into mature neurons. There is evidence of injury-induced neurogenesis following various injuries, including stroke, intracranial hemorrhage, global cerebral ischemia, and TBI. However, recent data have shown limited neuronal replacement following injury, showing that neurogenesis alone is not sufficient to produce full functional recovery. Another major consideration is angiogenesis, which is the formation of new microvessels. As with neurogenesis, angiogenesis has been observed in the penumbra following ischemic stroke and emerging evidence indicates that angiogenesis enhances neurogenesis, thus indicating that these processes may be coupled and should be considered together to enhance recovery. For example, migration of neural stem cells from the SVZ has been observed to be via new microvessels.21, 22 Despite experimental strategies to enhance angiogenesis and/or neurogenesis, it seems that the injured adult brain provides an environment that is not conducive to repopulation of functional neurons. Injury causes loss of cells, which are then replaced with extracellular matrix, termed the glial or fibrotic scar. For decades, this was thought to be produced by surviving astrocytes migrating to the location of injury. However, recent work has implicated nonvascular pericytes as contributors to scar formation in the injured brain.23, 24 Recent work has shown that alteration in the composition of the scar following injury can improve the environment for repair. Thus, future research will require consideration of various cell types and their responses to injury and therapeutic interventions as a means to optimize recovery. In addition to neuronal and vascular injury, white matter injury is a major component of ischemic stroke and brain injury. Injury to myelinated axons is a consequence of oligodendrocyte disorder. In addition to demyelination, injury to oligodendrocytes also likely alters vascular integrity, as shown by recent work demonstrating oligodendrocyte-endothelial interactions in culture and during vascular remodeling.25 Thus, new strategies to enhance cellular crosstalk during the recovery phase following brain injury are needed to enhance the repair and restoration of neuronal, vascular, and white matter function.
Neuroinflammation
In addition to oligovascular and neurovascular coupling, this entire unit interacts with the immune system. Inflammation is a major player in the outcome following all forms of brain injury. The central nervous system (CNS) inflammatory response is thought to be triggered by injured neurons. However, several recent studies have shown that all cells are susceptible to ischemic injury, with neurons being the most sensitive to direct injury, followed by oligodendrocytes, astrocytes, microglia, and endothelial cells being particularly resistant to injury. The initial immune response seems to be driven by astrocyte and microglial responses to the release of danger-associated molecular pattern (DAMP) from injured neurons. For decades it had been assumed that massive inflammatory responses drive ongoing injury. However, more recent studies have confused the issue, indicating inflammation both in injury and repair. The complex orchestrated response of the array of immune cells makes this an important and ever-changing area of research. Microglia are brain-specific macrophages that rapidly respond to injury and classic microgliosis produces a proinflammatory environment that contributes to cellular injury. Recently, an alternative or M2 activation of microglia and macrophages has been described, which produce antiinflammatory cytokines, such as IL-10, and are likely protective. It seems that strategies that enhance the alternative activation pathway reduce neuronal injury and that many cells of the immune system have subsets that have both detrimental and protective capacities. For example, brain injury causes the influx of both proinflammatory T cells (Th1, Th17) and antiinflammatory Treg cells, the balance of which ultimately contributes to the magnitude of injury and repair. Similarly, infiltrating B cells have the detrimental capacity to produce antibodies against local antigens, and also antiinflammatory cytokines such as IL-10. To further complicate the matter, emerging evidence indicates that injury-induced alterations in vascular endothelial cells play a major regulatory role in immune cell infiltration into the injured brain, particularly through blood-brain barrier dysfunction. Therefore, future studies are needed to carefully dissect the relative contributions of infiltrating and resident immune cells after injury; broad-spectrum immunotherapies are not likely to provide benefit because, in addition to blocking detrimental proinflammatory responses, treatments may block beneficial immune responses that are critical for long-term functional recovery.
FUNCTIONAL PLASTICITY
A potential issue with animal studies is an overly narrow focus on acute histologic outcome, rather than long-term functional outcomes, which are ultimately the criteria for success in humans. Pharmacologic therapies targeting histologic protection have a narrow therapeutic window, whereas neurorestorative strategies aimed at more chronic processes are likely to have a broader window for intervention. Acute brain injuries alter neuronal networks by causing neuronal cell death and alterations in excitability and synaptic contacts of surviving neurons. A better understanding of how acute brain injuries alter injured and noninjured networks could provide a means to improve function independent of neuroprotection.
Excitation/Inhibition Imbalance
A delicate balance between excitation and inhibition exists in the mammalian brain and alterations in this balance result in dysfunctional brain activity and ultimately impaired cognitive and behavioral outcomes. Changes in neuronal excitability following an acute brain injury can be the result of alterations in excitatory and/or inhibitory signaling in brain. Glutamate and gamma-aminobutyric acid (GABA) are the dominant excitatory and inhibitory neurotransmitters in the CNS, respectively. There is a growing body of literature to suggest that acute brain injury results in an imbalance between excitation and inhibition in brain. In the early stages following an acquired brain injury the balance is shifted toward excitatory transmission with increases in glutamate signaling and a downregulation of GABAergic signaling.1, 26 This imbalance can result in excitotoxicity that produces activation of cell death mechanisms. In the subacute and chronic phases following brain injury the balance shifts to favor inhibitory GABAergic signaling. GABA receptor activation can produce phasic inhibition that is rapid and mediated by synaptic GABA receptors and tonic inhibition, which is more long-lasting and associated with activation of extrasynaptic GABA receptors. Following experimental stroke there is an increase in tonic inhibition in the cortex that is evident at 1 week following injury and prevents functional recovery. When this tonic inhibition is reversed with an inhibitor of GABAA α5 signaling at delayed time points there is an improvement in functional recovery of motor and sensorimotor function that is not the result of altering histologic outcome.27, 28 Similar increases in tonic inhibition have been observed in the dentate gyrus following controlled cortical impact injury that is reversed with the GABAA α4 or δ antagonist.29–31 Modulation of tonic inhibition may provide a novel strategy to regulate neuronal excitability and promote plasticity in networks that are altered following ischemic brain injury or TBI. Functional imaging in patients with stroke supports the hypothesis that there is overinhibition of the ipsilateral cortex, particularly from the contralateral hemisphere.32
An alternative to reducing inhibitory signaling to restore the excitation/inhibition balance and potentially improve functional recovery is to enhance excitatory transmission. Although this may seem counterintuitive given the excitotoxicity mechanisms that occur during the acute phase, there is evidence that increasing glutamatergic signaling in the chronic phase can be beneficial for functional plasticity and recovery. Inhibition of the glutamate transporter to enhance glutamatergic transmission in acute slices restored excitatory transmission to control levels in animals that had TBI. Positive allosteric modulators of AMPA receptor function administered in the subacute phase following stroke resulted in improved motor function through enhancement of brain-derived neurotrophic factor function.33 Similarly, administration of d-cycloserine, which enhances NMDA receptor activation, has been shown to improve functional recovery and synaptic plasticity following injury.34 These results suggest that the imbalance between excitation and inhibition prevents structural and synaptic plasticity in the brain and restoring the balance by antagonizing GABA receptor activity or enhancing glutamate receptor activity may serve as a promising therapy to enhance functional recovery following brain injury. This emerging area of research will lead to both new therapeutic strategies as well as a new and more complex understanding of altered signaling in the injured brain.
Synaptic Plasticity
Another potential target for therapies to improve functional recovery is synaptic plasticity, which is an experience-dependent modification of synaptic strength. Synaptic plasticity deficits are a commonly observed consequence of acute brain injury in animal models. A well-characterized form of synaptic plasticity, long-term potentiation (LTP) of CA1 pyramidal cells, is an increase in synaptic strength that is a cellular correlate of learning and memory. Impairments in hippocampal LTP have been observed in most animal models of acute brain injury including stroke, cardiac arrest, and TBI.35–37 The loss of LTP is sustained for at least 1 month, which is well beyond the time of neuronal cell death.38, 39 This finding implies that, in addition to cell death, these forms of brain injury can produce long-lasting changes in synaptic function of surviving neurons, which likely contributes to a lack of recovery of function. The mechanisms contributing to LTP deficits remain to be elucidated, but likely candidates include excitatory/inhibitory imbalance, alterations of intracellular signaling, and prolonged neuroinflammation. LTP is initiated by activation of NMDA receptors during high-frequency stimulation and a subsequent Ca2+-dependent increase in postsynaptic AMPA receptor function. Studies examining NMDA receptor function in injured brain are mixed, with reports of reduced or unchanged expression and function. Enhancing NMDA receptor function is capable of restoring hippocampal LTP following TBI.40 Delayed administration of low doses of kainate 48 hours after ischemia also promotes the restoration of LTP in the postischemic brain.41 Inhibition of B lymphocytes with an anti-CD20 antibody can also promote recovery of plasticity, suggesting that the loss of LTP may involve multicellular processes.42 Targeting of synaptic plasticity deficits in the hippocampus independent of neuroprotection may serve as a promising therapy to improve cognitive function at delayed times after an acute brain injury. The hippocampus has been an important research target because of its well-established role in memory deficits following injury; however, it is likely that similar phenomena are occurring in other brain regions. Future studies are needed to assess alterations in synaptic plasticity in various brain regions not directly injured by ischemia.
Structural Plasticity
Early changes in dendritic morphology have been observed in periinfarct regions following stroke. Reduced spine density and dendritic swelling are observed during the first 24 hours following stroke onset. Although recovery of dendritic spines is observed in the subacute period following stroke, there is little evidence for this recovery following TBI.43 This finding may have important implications for differences in therapies targeting synaptic function to improve function following different acquired brain injuries. Axonal damage and degeneration occur during the acute and subacute periods following stroke and TBI.44, 45 Recovery from stroke requires formation of new connections in injured and noninjured tissues that depends on axonal sprouting. There are many trophic and intercellular signaling processes that mediate axonal regeneration and can be targeted pharmacologically or with cell-based therapies to enhance stroke recovery.46, 47 Note that aberrant axonal sprouting can contribute to hyperexcitability and posttraumatic epilepsy.48, 49 Therefore, it is important to take care that restorative strategies enhance functionally appropriate synaptic contacts and avoid maladaptive structural plasticity and hyperexcitability. Cell-based and brain stimulation therapies hold promise for providing a cellular environment conducive to promoting structural plasticity.
SUMMARY
A major question in experimental ischemia remains whether neuroprotection observed in young, healthy animals can be extrapolated to the human population, which is generally sick and aging. It is important to consider the impact of comorbidities such as hypertension and diabetes that are highly associated with cerebral ischemia. Various other risk factors have been observed, such as obesity, smoking, and excessive alcohol consumption. In addition to these important modifiable risk factors, it is important to consider age and gender when developing models to test new treatments for cerebral ischemia. Animal models require consideration of various modifiable (hypertension, diabetes, obesity) and nonmodifiable (age, gender, genetics) risk factors, which all interact with each other to add to the complexities of ischemia. Consideration of comorbidities, combined with ongoing research focused on complex intercellular interactions in the injured brain, hold promise for improved preclinical modeling and identification of protective strategies that may ultimately translate to patients.
The narrow focus on brain protection has led to missed research opportunities. It is likely that long-term functional recovery can be enhanced by combining potential protective strategies with therapies designed to allow recovery of function in both injured and uninjured brain regions. Functional plasticity is a mechanism by which neuronal networks that remain following an ischemic brain injury undergo structural and synaptic modifications to restore lost function.50 Recent advances in basic neuroscience research following acquired brain injury has been complemented by advances in functional MRI that show changes in functional connectivity that are associated with motor impairments and spontaneous recovery.51 These changes occur within affected and nonaffected territories and reflect a potential target for improving functional recovery in patients with stroke. Transmagnetic stimulation of affected and nonaffected hemispheres has been shown to be beneficial for recovery of motor function in patients with stroke and was associated with functional connectivity of both hemispheres.52 Other stimulation methods, such as vagus nerve and deep brain stimulation, are also being investigated for their ability to provide functional benefit.53 The mechanisms by which these methods work to improve functional recovery continue to be elucidated and include altered neuronal excitability, enhanced synaptic plasticity, and the release of neurotrophins and other modulatory factors.
KEY POINTS.
Every year in the United States, millions of individuals incur ischemic brain injury from stroke, cardiac arrest, or traumatic brain injury. These forms of acquired brain injury can lead to death, or in many cases long-term neurologic and neuropsychological impairments.
The mechanisms of ischemic and traumatic brain injury that lead to these deficiencies result from a complex interplay of multiple interdependent molecular pathways that include excitotoxicity, acidotoxicity, ionic imbalance, oxidative stress, inflammation, and apoptosis.
This article briefly reviews several of the traditional, well-known mechanisms of brain injury and then discusses more recent developments and newer mechanisms.
Although much is known concerning mechanisms of injury and the manipulation of these mechanisms to result in protection of neurons and increased behavioral performance in animal models of injury, it has been difficult to translate these effects to humans. Attention is given to why this is so and newer outcome measures of injury are discussed.
Footnotes
This work is published in collaboration with the Society for Neuroscience in Anesthesiology and Critical Care.
Disclosures: No disclosures for any authors.
REFERENCES
- 1.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Valdés HE, Clarkson AN, Ao Y, et al. Memantine enhances recovery from stroke. Stroke. 2014;45:2093–2100. doi: 10.1161/STROKEAHA.113.004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 4.Simon R, Xiong Z. Acidotoxicity in brain ischaemia. Biochem Soc Trans. 2006;34:1356–1361. doi: 10.1042/BST0341356. [DOI] [PubMed] [Google Scholar]
- 5.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 7.Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol (1985) 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 8.Diener HC, Lees KR, Lyden P, et al. NXY-059 for treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 9.Tobin MK, Bonds JA, Minshall RD, et al. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C, Mihaela A. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moskowitz MA, Lo EH. Neurogenesis and apoptotic cell death. Stroke. 2003;34:324–326. doi: 10.1161/01.str.0000054047.14853.ad. [DOI] [PubMed] [Google Scholar]
- 13.Herson PS, Traystman RJ. Animal models of stroke: translational potential at present and in 2050. Future Neurol. 2014;9:541–551. doi: 10.2217/fnl.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traystman RJ, Herson PS. Misleading results: translational challenges. Science. 2014;343:369–370. doi: 10.1126/science.343.6169.369. [DOI] [PubMed] [Google Scholar]
- 15.Saver JL, Goyal M, Bonafe A, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 16.Berkhemer OA, van Zwam WH, Dippel DW. Stent-retriever thrombectomy for stroke. N Engl J Med. 2015;373(11):1076–1077. doi: 10.1056/NEJMc1508744. [DOI] [PubMed] [Google Scholar]
- 17.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 18.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23(8):879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 19.Simard M, Arcuino G, Takano T, et al. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Thored P, Wood J, Arvidsson A, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 22.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Klett F, Potas JR, Hilpert D, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab. 2013;33(3):428–439. doi: 10.1038/jcbfm.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Klett F, Priller J. The fibrotic scar in neurological disorders. Brain Pathol. 2014;24(4):404–413. doi: 10.1111/bpa.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham LD, Hayakawa K, Seo JH, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60(6):875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redecker C, Wang W, Fritschy JM, et al. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22(12):1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson AN, Huang BS, Macisaac SE, et al. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lake EM, Chaudhuri J, Thomason L, et al. The effects of delayed reduction of tonic inhibition on ischemic lesion and sensorimotor function. J Cereb Blood Flow Metab. 2015;35(10):1601–1609. doi: 10.1038/jcbfm.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witgen BM, Lifshitz J, Smith ML, et al. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience. 2005;133(1):1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 30.Kharlamov EA, Lepsveridze E, Meparishvili M, et al. Alterations of GABA(A) and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95(1–2):20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Mtchedlishvili Z, Lepsveridze E, Xu H, et al. Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol Dis. 2010;38(3):464–475. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Rehme AK, Eickhoff SB, Wang LE, et al. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55(3):1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson AN, Overman JJ, Zhong S, et al. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31(10):3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhawan J, Benveniste H, Luo Z, et al. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol. 2011;6:823–834. doi: 10.2217/fnl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp Brain Res. 1995;106(2):248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Huang R, Shetty RA, et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis. 2013;59:18–25. doi: 10.1016/j.nbd.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiprianova I, Sandkühler J, Schwab S, et al. Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol. 1999;159(2):511–519. doi: 10.1006/exnr.1999.7109. [DOI] [PubMed] [Google Scholar]
- 38.Sanders MJ, Sick TJ, Perez-Pinzon MA, et al. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861(1):69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- 39.Orfila JE, Shimizu K, Garske AK, et al. Increasing small conductance Ca2+-activated potassium channel activity reverses ischemia-induced impairment of longterm potentiation. Eur J Neurosci. 2014;40(8):3179–3188. doi: 10.1111/ejn.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaka R, Biegon A, Grigoriadis N, et al. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J. 2007;21(9):2033–2041. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]
- 41.Nagy D, Kocsis K, Fuzik J, et al. Kainate postconditioning restores LTP in ischemic hippocampal CA1: onset-dependent second pathophysiological stress. Neuropharmacology. 2011;61(5–6):1026–1032. doi: 10.1016/j.neuropharm.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Doyle KP, Quach LN, Solé M, et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci. 2015;35(5):2133–2145. doi: 10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones TA, Liput DJ, Maresh EL, et al. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma. 2012;29(7):1455–1468. doi: 10.1089/neu.2011.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinman JD. The back and forth of axonal injury and repair after stroke. Curr Opin Neurol. 2014;27:615–623. doi: 10.1097/WCO.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 2014;27:53–60. doi: 10.1016/j.conb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong RC, Mierzwa AJ, Marion CM, et al. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp Neurol. 2015;275(Pt 3):328–333. doi: 10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Prince DA, Parada I, Scalise K, et al. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50(Suppl 2):30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson SM, Xiong W, Wang Y, et al. Prevention of posttraumatic axon sprouting by blocking collapsin response mediator protein 2-mediated neurite outgrowth and tubulin polymerization. Neuroscience. 2012;210:451–466. doi: 10.1016/j.neuroscience.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caleo M. Rehabilitation and plasticity following stroke: Insights from rodent models. Neuroscience. 2015;311:180–194. doi: 10.1016/j.neuroscience.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Thiel A, Vahdat S. Structural and resting-state brain connectivity of motor networks after stroke. Stroke. 2015;46(1):296–301. doi: 10.1161/STROKEAHA.114.006307. [DOI] [PubMed] [Google Scholar]
- 52.Grefkes C, Fink GR. Disruption of motor network connectivity post-stroke and its noninvasive neuromodulation. Curr Opin Neurol. 2012;25(6):670–675. doi: 10.1097/WCO.0b013e3283598473. [DOI] [PubMed] [Google Scholar]
- 53.Cai PY, Bodhit A, Derequito R, et al. Vagus nerve stimulation in ischemic stroke: old wine in a new bottle. Front Neurol. 2014;5:107. doi: 10.3389/fneur.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]