Abstract
Regulation of vascular smooth muscle cell (VSMC) phenotype plays an essential role in many cardiovascular diseases. In the present study, we provide evidence that krüppel-like factor 8 (KLF8) is essential for tumor necrosis factor α (TNFα)-induced phenotypic conversion of VSMC obtained from thoracic aorta from 4-week-old SD rats. Stimulation of the contractile phenotype of VSMCs with TNFα significantly reduced the VSMC marker gene expression and KLF8. The gene expression of KLF8 was blocked by TNFα stimulation in an ERK-dependent manner. The promoter region of KLF8 contained putative Sp1, KLF4, and NFκB binding sites. Myocardin significantly enhanced the promoter activity of KLF4 and KLF8. The ectopic expression of KLF4 strongly enhanced the promoter activity of KLF8. Moreover, silencing of Akt1 significantly attenuated the promoter activity of KLF8; conversely, the overexpression of Akt1 significantly enhanced the promoter activity of KLF8. The promoter activity of SMA, SM22α, and KLF8 was significantly elevated in the contractile phenotype of VSMCs. The ectopic expression of KLF8 markedly enhanced the expression of SMA and SM22α concomitant with morphological changes. The overexpression of KLF8 stimulated the promoter activity of SMA. Stimulation of VSMCs with TNFα enhanced the expression of KLF5, and the promoter activity of KLF5 was markedly suppressed by KLF8 ectopic expression. Finally, the overexpression of KLF5 suppressed the promoter activity of SMA and SM22α, thereby reduced the contractility in response to the stimulation of angiotensin II. These results suggest that cross-regulation of KLF family of transcription factors plays an essential role in the VSMC phenotype.
Keywords: Angiotensin, Krüppel-like factor, Phenotype, Transcription, Vascular smooth muscle cell
INTRODUCTION
The principal role of vascular smooth muscle cells (VSMCs) in blood vessels is to mediate the contractions in response to extracellular stimuli, such as angiotensin II (AngII) [1]. Contractions from AngII stimulation is mainly acquired by maintaining its contractile phenotype [2]. For example, the proliferation of fully differentiated VSMCs stops and the expression of contractile proteins, such as myosin heavy chain (MHC), myosin light chain kinase (MLCK), calponin, smooth muscle actin (SMA) and SM22α, remains at a high level to provide contractility [1]. Although skeletal muscle cells lack the ability to proliferate and migrate, VSMC has plasticity in their phenotypes [1]. The contractile phenotype of VSMC can undergo a phenotypic change, thereby retaining their ability to proliferate in response to environmental stimuli [3]. The significance of phenotypic switch is often attained in many vascular diseases, such as atherosclerosis, arteriosclerosis, restenosis, and vascular aging [3]. Hence, unveiling the mechanism of phenotypic change seems to be a key step in understanding many vascular diseases.
Krüppel-like factors (KLFs) belong to transcription factors containing zinc finger domains, and they regulate a variety of physiologies, such as differentiation, development, and proliferation [4]. Thus far, there have been 17 human KLF proteins. KLFs can be classified into three groups by multiple sequence alignments and phylogenic analyses: Those that are in Group1 (KLF3, KLF8, KLF12) suppresses transcription through the interaction with the carboxy-terminal binding protein (CtBP); those in Group2 (KLF1, KLF2, KLF4, KLF5, KLF6, KLF7) serve as transcriptional activators or repressors; and those in Group3 (KLF9, KLF10, KLF11, KLF13, KLF14, KLF16) have repressor activity through an interaction with transcriptional corepressor Sin3A [5].
Transcriptional activity of KLFs is modulated by a variety of mechanisms. For example, cAMP response element binding protein (CBP) and p300 acetylates KLF4 and KLF5, facilitating transcriptional activity [6,7]. Conversely, histone deacetylase 1 (HDAC1) interacts with KLF5, thereby dissociating KLF5 from p300, which blocks the KLF5-dependent target gene expression [8]. An interaction of KLFs with other proteins is also affected by phosphorylation. For instance, all-trans retinoic acid induces phosphorylation of HDAC2, disrupts the interaction between KLF4 and KLF5, allowing KLF5 to be acetylated and bind to SM22α promoter in VSMCs [9]. The transcriptional activity of KLFs can directly be regulated by phosphorylation. For instance, a stimulation of VSMCs with AngII directly induces the phosphorylation of KLF5, modulating the transcriptional activity of KLF5 [10]. The transcriptional activity of KLFs can also be regulated by modulating the expression levels through ubiquitination and sumoylation [11]. Serum stimulation of HCT116 cancer cells rapidly degrades the KLF4 level through ubiquitination and proteasome pathway [12]. Likewise, it has been reported that expression level of KLF5 is regulated by ubiquitin-dependent proteosomal degradation [13]. In addition to the ubiquitin-dependent regulation of KLF4 and KLF5, expressions of KLF4 and KLF5 are also regulated by sumoylation. KLF4 and KLF5 interact with the SUMO E3 ligase PIAS1 [14]. In case of KLF4, the degradation of KLF4 by sumoylation stimulates the promoter activity of SMA [15]. However, sumoylation of KLF5 stimulates nuclear translocation, which promotes the gene expression of cell cycle regulator [16].
A variety of environmental cues—inflammation, diabetic condition, hypoxic condition, and malnutrition—stimulates VSMCs, which affects vascular integrity [17]. Previously, many reports suggest that tumor necrosis factor α (TNFα) plays a crucial role in vascular remodeling. For example, the loss of TNFα reduces the thickness of vascular walls and the size of atherosclerotic lesions in TNFα/ApoE double knockout mice [18,19]. However, the mechanistic pathway underlying TNFα-induced phenotypic conversion of VSMCs is still ambiguous.
In this present study, we explore the role of KLFs during the TNFα-induced phenotypic conversion of VSMCs and provide evidence that myocardin, KLF4, and Akt1 regulates transcriptional activity of KLF8 which in turn suppresses transcriptional activity of KLF5, thereby enhancing the expression of contractile marker protein of VSMCs.
METHODS
Antibodies against SMA, calponin, and SM22α were obtained from Sigma-Aldrich (St Louis, MO, USA). Anti-actin antibody was purchased from MP Biomedicals (Aurora, OH, USA). Anti-KLF5 antibody was purchased from EMD Millipore (Billerica, MA, USA). AngII and laminin, isolated from the human placenta, were obtained from Sigma-Aldrich (St Louis, MO, USA). Type I collagen was purchased from BD Biosciences (San Jose, CA, USA). Recombinant human TNFα was obtained from KOMA Biotech (Seoul, Korea). IRDye700- or IRDye800-conjugated rabbit or mouse secondary antibodies were obtained from Li-COR Bioscience (Lincoln, NE, USA). Plasmids that encode KLF4 and KLF5 as well as pGL3-KLF5 were kindly provided by Professor Mukesh K. Jain (Case Western Reserve University School of Medicine). FLAG-tagged KLF4, KLF5, KLF8, Akt1, and myocardin were PCR amplified and subcloned into pMIGR2 vector at EcoRI/BamHI. The promoter regions of SM22α and SMA subcloned in pGL3 vector were kind gifts from Dr. Gary K. Owens (University of Virginia). pGL3-KLF8(Δ0) and its deleted mutants (Δ1~Δ4) were kindly provided by Professor Jihe Zhao (Albany Medical College). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated.
Cell culture and phenotypic conversion
VSMCs were isolated from 4-week-old Sprague-Dawley rats, as described previously [20]. In brief, rats were euthanized via intraperitoneal injection of sodium pentobarbital (60 mg/kg) and perfused with PBS for 5 min. The thoracic aorta was aseptically isolated and the surrounding fat and connective tissues were discarded. The vessels were longitudinally cut, and the lumen side was scraped with a razor blade to remove the intima. The vessels were then fragmented into 3~5 mm lengths and explanted with the lumen side down on collagen-coated culture dishes. After seven days of maintenance in Dulbecco's modified Eagle medium (DMEM) containing 10% bovine calf serum and 1% penicillin-streptomycin at 37℃ in a humidified 5% CO2 incubator, the tissue fragments were discarded, and sprouted VSMCs were collected (referred to as P0). Synthetic VSMCs were cultured on gelatin-coated plates at low density (<20%). To acquire the contractile VSMCs, synthetic VSMCs (P0) were cultured on laminin-coated plates at high density (~100%), and passages between P2 and P5 were defined as the contractile phenotype of VSMCs. The phenotype of VSMCs was verified by Western blotting with SMC marker proteins, such as SM22α, calponin, and SMA. To induce the phenotypic conversion of VSMCs, TNFα (50 ng/ml) was supplemented in a culture medium, which was changed on a daily basis, for a total duration of 4 days.
Promoter assay
For the measurement of promoter activity, the dual-luciferase reporter assay system was employed. VSMCs were plated in 12-well plates. The cells were co-transfected with the luciferase reporter constructs and renilla luciferase plasmid, using Lipofectamine 2000 (Invitrogen). Each well contained 0.88 µg of luciferase reporter plasmid, 0.8 µg of expression vector, and 80 ng of renilla luciferase plasmid. The medium was replaced with a fresh medium at 7 h post-transfection. The cells were lysed and assayed for luciferase activity at 24 h post-transfection. Twenty microliters of protein extracts were analyzed in a Glomax™ 20/20 luminometer (Promega, WI, USA).
Collagen gel contraction assay
Collagen gel contraction assay was performed as described previously [20]. VSMCs were starved for 4 h and resuspended in serum-free DMEM (1×106 cells/ml). Cell suspension was mixed with collagen gel solution (8 mg/ml of collagen type I in 2X PBS, pH 8.0) on ice to give 5×105 cells/ml and 4 mg/ml of collagen gel solutions. One hundred microliters of VSMC-collagen gel mixture was added to 12-well plates. The plates were incubated at 37℃ to allow for polymerization. After 30 minutes, the gels were floated in serum-free DMEM; and after 5 hours, AngII was added to initiate a contraction while images were captured using a digital charge-coupled device camera. Collagen gel contraction was measured as a decrease in the gel area using Scion Image software (compliments of Scion Corporation, Frederick, MD; http://www.scioncorp.com). Relative gel area was obtained by dividing the area at each time point by the initial area of the gel.
Constructs and primers
To silence the genes of interest, oligonucleotides tagged with 5′-end AgeI site and 3′-end EcoRI site were designed for Akt1 (5'-CCG GTA ACT TCT CAG TGG CAC AAT GCC TCG AGG CAT TGT GCC ACT GAG AAG TTT TTT TG-3'), and both sense and antisense oligonucleotides were synthesized. Both complementary oligonucleotides were mixed and heated at 98℃ for 5 min, and then cooled to room temperature. Annealed nucleotides were subcloned into the AgeI/EcoRI site of a pLKO.1 lentiviral vector. To measure the expression of KLF8, sense (5′-ACG CCC CAG GTG GAA CCA GT-3′) and anti-sense (5′-TGG CTG CAG GGC TCC CAT CT-3′) oligonucleotides were synthesized and RT-PCR was performed as previously described [20].
Lentiviral knockdown
For gene silencing, HEK293-FT packaging cells were grown to ~70% confluence in 6-well plates. The cells were triple transfected with 5 µg of pLKO.1 lentiviral construct, 1 µg of Δ8.9, and 1 µg of pVSV-G using the calcium phosphate method. The medium was replaced with a fresh medium at 8 h post-transfection. Lentiviral supernatants were harvested at 24 h post-transfection and passed through 0.45 µm filters. Cell-free viral culture supernatants were used to infect the contractile VSMCs in the presence of 8 µg/ml of polybrene. An additional round of infection was done at 48 h and 72 h post-transfection. The infected cells were isolated by 10 µg/ml puromycin for 2 days.
Western blotting
Western blotting was performed as described previously [20].
Statistical analysis
The results are expressed as the mean±S.D. of two independent experiments (n=3 for each experiment). When comparing two groups, unpaired Student's t-test was used to assess the differences. A p value of less than 0.05 was considered statistically significant.
RESULTS
Down regulation of KLF8 during TNFα-induced phenotypic change of VSMCs
Stimulation of contractile type of VSMCs with TNFα (50 ng/ml) significantly reduced the expression of marker proteins, such as SMA, SM22α, calponin (Fig. 1A). In addition, the expression of KLF8 was also significantly reduced by TNFα in a time-dependent manner, as judged by RT-PCR. The promoter activity of KLF8 was also reduced by the pretreatment of TNFα and platelet-derived growth factor (PDGF) (Figs. 1B and 1C), which is known as an inducer of phenotypic change [21]. TNFα-induced down regulation of KLF8 promoter activity was significantly blocked by the inhibition of ERK signaling pathway, whereas the inhibition of p38 MAPK pathway had no effect (Fig. 1D).
Fig. 1. TNFα suppresses the expression of KLF8.
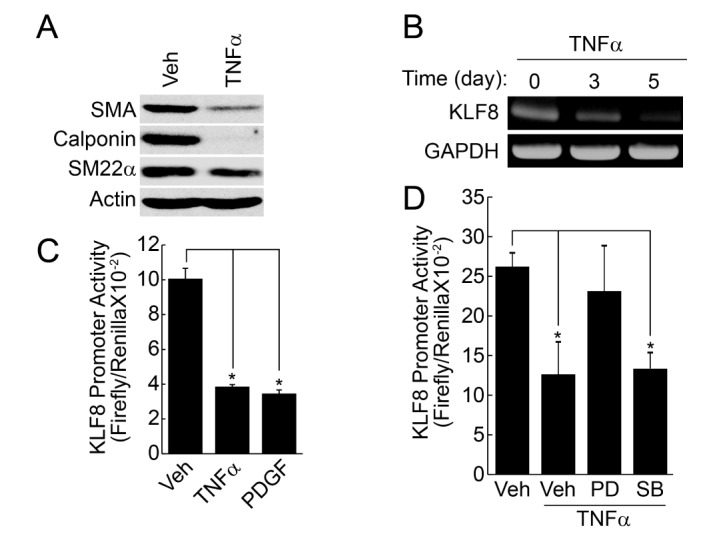
(A) Contractile phenotype of VSMCs were stimulated with TNFα (50 ng/ml) for 4 days. Expression of contractile marker proteins were verified by the indicated antibodies. (B) Contractile cells were treated with TNFα (50 ng/ml) for the indicated times. Expression of KLF8 was verified by RT-PCR. (C) Contractile type of VSMCs were stimulated with TNFα (50 ng/ml) and PDGF (50 ng/ml), and promoter activity of KLF8 was examined as described in the “Materials and Methods” section. (D). Contractile type of VSMCs were pretreated with ERK inhibitor (PD, 10 µM) or p38 MAPK inhibitor (SB, 10 µM), and promoter activity of KLF8 was measured. Data are expressed as the mean±S.D. for three independent experiments (n=3 for each experiment). *Significantly different from values of vehicle-treated group (p<0.05).
Regulation of KLF8 promoter activity by KLF4 and Akt1
The analysis of KLF8 promoter revealed that many cis-acting elements, such as C-Ets-1, CAAT/enhance binding protein β (C/EBPβ), retinoic acid receptor α (RXRα), Ap1, Sp1, KLF4, and NFκB, were located in the promoter region (up to – 1312) (Fig. 2A). Deletion up to –705 markedly enhanced the promoter activity of KLF8, indicating the presence of negative regulatory region between –1312 and –705. Moreover, deletion of last Sp1 binding site (–57, Fig. 1A) completely abolished the promoter activity of KLF8, suggesting that Sp1 transcription factor regulates the basal promoter activity of KLF8. The co-transfection of myocardin markedly enhanced the promoter activity of KLF8, especially in the form of pGL3-Δ2 (contains KLF4 and NFκB binding sites). This indicates that myocardin regulates KLF8 expression through the regulation of KLF4 and/or NFκB (Fig. 2C). Myocardin significantly stimulated the promoter activity of KLF4 (Fig. 2D), and the co-transfection of KLF4 significantly enhanced the promoter activity of KLF8 (Fig. 2E). Finally, silencing or overexpression of Akt1, which has been known as the upstream regulator of NFκB, significantly affected the promoter activity of KLF8 (Figs. 2F and 2G).
Fig. 2. Expression of KLF8 is regulated by myocardin, KLF4 and Akt1.
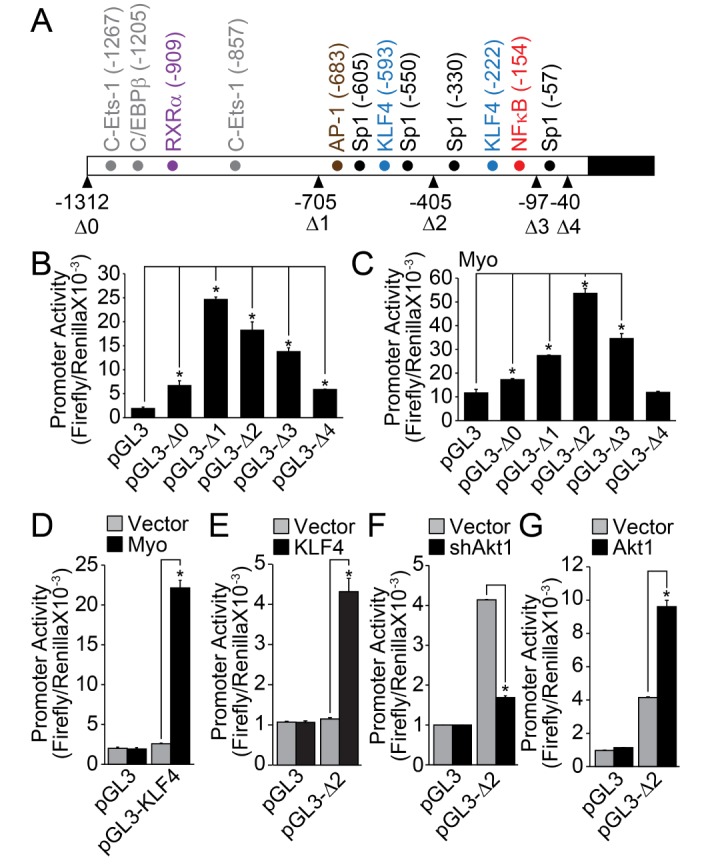
(A) Schematic representation of KLF8 promoter region. Cis-acting elements were indicated at upper figure and deletion mutants were denoted at lower figure. (B) Promoter activity of each deletion mutant was measured as described in the “Materials and Methods” section. (C) Promoter activity of each deletion mutant was measured in the presence of myocardin. (D) Contractile type of VSMCs was co-transfected with myocardin and promoter activity of KLF4 was measured. (E) Contractile type of VSMCs was co-transfected with KLF4 and promoter activity of KLF8 (pGL3-Δ2) was measured. (F, G) Akt1 was either silenced or overexpressed in contractile type of VSMCs, and promoter activity of KLF8 (pGL3-Δ2) was measured. Data are expressed as the mean±S.D. for three independent experiments (n=3 for each experiment). *Significantly different from values of vector group (p<0.05).
Expression of KLF8 significantly upregulates VSMC marker gene expression
The promoter activity of VSMC marker genes, such as SMA and SM22α, was significantly enhanced in the contractile phenotype of VSMCs (Figs. 3A and 3B). Additionally, the promoter activity of KLF8 was also enhanced in the contractile phenotype of VSMC (Fig. 3C). Fibroblast-like morphology of synthetic VSMC was changed into an elongated spindle shape by the overexpression of KLF8 (Fig. 3D). In addition, the expression of contractile marker genes was significantly enhanced by the overexpression of KLF8 (Fig. 3E). Finally, the overexpression of KLF8 strongly enhanced the promoter activity of SMA (Fig. 3F).
Fig. 3. Ectopic expression of KLF8 promotes VSMC differentiation.
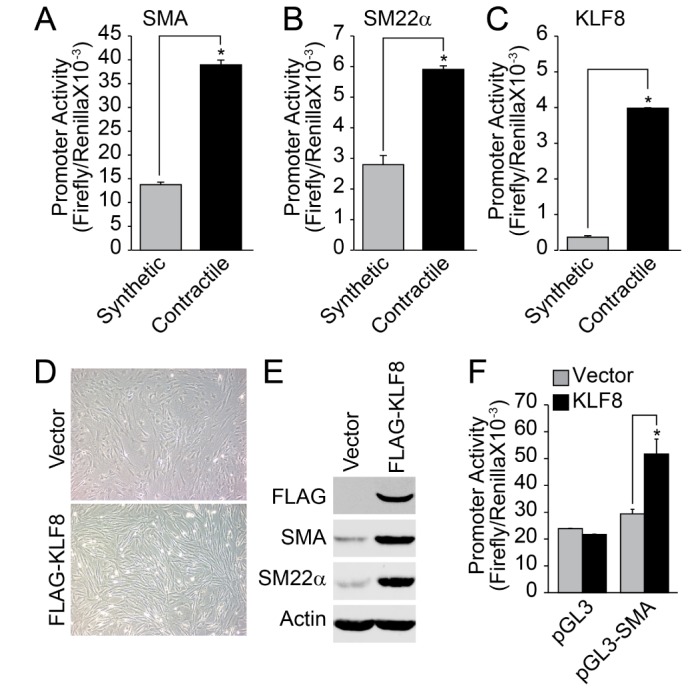
(A~C) Promoter activity of SMA, SM22a, and KLF8 was measured in either synthetic (P0) and contractile (P4) phenotype of VSMCs. (D, E) FLAG-tagged KLF8 was overexpressed in synthetic phenotype of VSMCs, and morphological changes of VMCs (bright field image) as well as expression of contractile marker proteins were examined. (F) Synthetic type of VSMCs were transfected with KLF8 and promoter activity of SMA was measured as described in the “Materials and Methods” section. Data are expressed as the mean±S.D. for three independent experiments (n=3 for each experiment). *Significantly different from values of synthetic or vector group (p<0.05).
KLF8 regulates promoter activity of KLF5
It has been reported that KLF5 plays an essential role in the change of TNFα-induced phenotype of VSMC [22]. Therefore, we examined the effect of KLF8 on the promoter activity of KLF5. The stimulation of contractile type of VSMCs with TNFα markedly enhanced the expression of KLF5, conversely, the expression of VSMC marker genes was down-regulated (Fig. 4A). The co-transfection of KLF8 significantly suppressed the promoter activity of KLF5 (Fig. 4B), whereas the promoter activity of KLF4 was not affected (Fig. 4C). In addition, the co-transfection of KLF5 significantly reduced the promoter activity of both SMA and SM22α (Figs. 4D and 4E). Finally, the overexpression of KLF5 in the contractile phenotype of VSMC abolished the contraction in response to AngII stimulation (Fig. 4F).
Fig. 4. KLF8 suppresses promoter activity of KLF5.
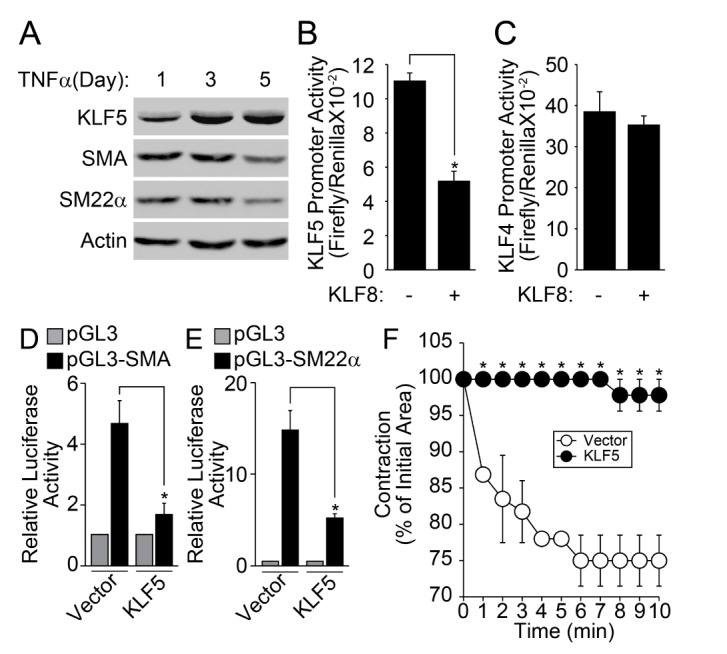
(A) Contractile type of VSMCs were stimulated with TNFα for the indicated times. Expression of KLF5 and contractile marker proteins were verified by western blot analysis. (B, C) Promoter activity of both KLF5 and KLF4 was measured in the presence of KLF8. (D, E) Promoter activity of SMA and SM22α was measured in the presence of KLF5. (F) KLF5 was overexpressed in contractile type of VSMCs, and AngII-induced contraction of VSMCs was measured as described in the “Materials and Methods” section. Data are expressed as the mean±S.D. for three independent experiments (n=3 for each experiment). *Significantly different from values of vector group (p<0.05).
DISCUSSION
The modulation of VSMC plasticity is often found in a variety of vessel-associated diseases, such as atherosclerosis, arteriosclerosis, and peripheral blood vessel diseases [1]. Blood vessel integrity and architecture are destroyed by inappropriate proliferation and migration of VSMC, which results in loss of vessel contractility and occlusion [1]. These pathological responses are mainly caused by a phenotypic conversion of VSMC [1]. Therefore, unveiling the mechanistic pathway fundamental to phenotypic conversion of VSMC would provide useful insight into the pathogenesis of vessel-associated diseases.
Several key environmental cues that modulate VSMC plasticity have been reported. For example, the contractile phenotype of VSMC is markedly converted into the synthetic phenotype by the simulation with platelet-derived growth factor (PDGF) [21]. On the other hand, insulin, insulin-like growth factor-1 (IGF-1), and laminin-mediated integrin signaling pathways stimulate the phenotypic switch, from the synthetic type to the contractile type [20,23,24]. In the disease status, it has been reported that the inflammatory response is closely related with the phenotypic conversion of VSMC. For instance, the loss of TNFα significantly suppresses VSMC proliferation, reducing the wall thickness and lesion size during the developmental stages of atherosclerosis [18,19]. Likewise, our results show that the stimulation of contractile type of VSMCs with TNFα and PDGF downregulated the marker gene expression, such as SMA, calponin, and SM22α (Fig. 1A). Therefore, inflammatory cytokines may provide key environmental cues for the phenotypic change of VSMC during the pathogenesis of cardiovascular diseases.
The differentiation or dedifferentiation of VSMC is controlled by a coordinated regulation of transcriptional factors [3]. In the present study, we provide evidence that transcriptional activity of myocardin, KLF4, KLF8, and KLF5 is cross-regulated to orchestrate the control mechanism of the VSMC phenotype. KLF8 has been identified as a transcriptional repressor that suppresses target gene expression in association with c-terminal binding protein (CtBP), which is also known as transcriptional repressor [25]. Although the function of KLF4 and KLF5 in the physiology of VSMC has been well established [26,27,28,29,30], the role of KLF8 in VSMC is still ambiguous. In the present study, we have provided evidence that KLF8 stimulates the differentiation of VSMC. For example, the expression level of KLF8 was markedly reduced during the TNFα-induced dedifferentiation of VSMC (Figs. 1B and 1C). In addition, the expression of KLF8 was highly elevated in the contractile phenotype of VSMC compared with the synthetic phenotype, and forced expression of KLF8 in the synthetic phenotype of VSMC significantly enhanced the expression of contractile marker proteins (Fig. 3). Finally, the ectopic expression of KLF8 significantly repressed the promoter activity of KLF5, which plays an important role in TNFα-induced dedifferentiation of VSMC [22]. Therefore, we can suggest that KLF8 plays an essential role in the differentiation of VSMC.
Furthermore, we raised an important issue regarding the determination of VSMC phenotype. Although each transcriptional regulator seems to act independently during the differentiation or dedifferentiation process, we observed that each transcriptional regulator cross-regulates the transcriptional activity of one another. Previously, it has been reported that myocardin is associated with serum response factor (SRF) transcription factor to directly regulate the expression of smooth muscle marker genes [31,32]. In addition to this direct effect on SMC marker gene promoter, our results show that myocardin activated the promoter activity of KLF8 (Fig. 2C). The activation of KLF8 promoter activity by myocardin seems not to be mediated by direct action on KLF8 promoter region, but rather enhanced the promoter activity of KLF4 (Fig. 2D), which in turn up-regulated the promoter activity of KLF8 (Fig. 2E). It is noteworthy that the promoter region of KLF8 possesses the binding sites of KLF4 (Fig. 2A). In addition, it has been reported that KLF4 stimulates the differentiation of VSMC [33]. The promoter region of KLF8 also contains the NFκB binding site (Fig. 2A). It has previously been reported that the transcriptional activity of NFκB is regulated by the PI3k/Akt signaling pathways [34,35,36]. Accordingly, our results show that the modulation of Akt1 expression significantly affected the promoter activity of KLF8 (Figs. 2F and 2G). It also has been reported that FAK-dependent gene expression of KLF8 is mediated by the PI3K/Akt signaling pathway in human ovarian epithelial cancer cells [37]. Furthermore, it has been reported that Akt1 regulates the differentiation of VSMC [20]. As such, the expression of KLF8 is regulated by multiple transcriptional regulators, such as myocardin, KLF4, and NFκB. Therefore, it is reasonable to suggest that various signals merge with the expression of KLF8, and its expression plays an essential role in the regulation of VSMC phenotype.
Currently, the mechanism underlying KLF8-induced VSMC differentiation is still ambiguous. One possible mechanism may be the KLF8-dependent suppression of KLF5. Our results show that TNFα downregulated the expression of KLF8, whereas the expression of KLF5 was significantly enhanced by TNFα (Figs. 1B, 1C, 4A). Moreover, the ectopic expression of KLF8 suppressed the promoter activity of KLF5 (Fig. 4B). The forced expression of KLF5 suppressed the expression of contractile marker genes, such as SMA and SM22α (Figs. 4D and 4E), leading to the loss of AngII-induced contraction of VSMC (Fig. 4F). Congruently, previous reports have shown that the expression of KLF5 is significantly elevated in proliferating VSMC [29,38]. It also has been reported that the expression of KLF5 is suppressed in contractile phenotype of VSMC but is reactivated by various environmental cues, such as atherosclerosis, vein graft, and vascular injury [39,40,41]. In these regards, KLF8-dependent regulation of KLF5 expression may very well play an essential role in the phenotypic conversion of VSMC.
In conclusion, the expression of KLF8 is mediated by myocardin, KLF4, and NFκB signaling pathways, thereby stimulating the differentiation of VSMC. KLF8 blocks the expression of KLF5 which plays an essential role in the dedifferentiation of contractile VSMC. In the presence of TNFα, which is an important inflammatory cytokine, the expression of KLF8 is downregulated, thereby relieving the repression activity of KLF5, which leads to the expression of KLF5. Consequently, KLF5 induces the phenotypic conversion of VSMCs and stimulates the loss of contractile function of smooth muscles. Therefore, KLF8 could be the potential therapeutic target for vascular diseases.
ACKNOWLEDGEMENTS
This work was supported for two years by Pusan National University Research Grant (2015).
Footnotes
Author contributions: J.M.H. and S.J.Y. performed experiments. S.Y.J. and H.S.L. assisted data analysis. S.J.K. and H.K.S. provided critical comments on the manuscript. S.S.B. generated main idea and wrote manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 2.Wynne BM, Chiao CW, Webb RC. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin ii and endothelin-1. J Am Soc Hypertens. 2009;3:84–95. doi: 10.1016/j.jash.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 4.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haldar SM, Ibrahim OA, Jain MK. Kruppel-like factors (KLFs) in muscle biology. J Mol Cell Cardiol. 2007;43:1–10. doi: 10.1016/j.yjmcc.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans PM, Zhang W, Chen X, Yang J, Bhakat KK, Liu C. Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J Biol Chem. 2007;282:33994–34002. doi: 10.1074/jbc.M701847200. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto S, Suzuki T, Muto S, Aizawa K, Kimura A, Mizuno Y, Nagino T, Imai Y, Adachi N, Horikoshi M, Nagai R. Positive and negative regulation of the cardiovascular transcription factor KLF5 by p300 and the oncogenic regulator SET through interaction and acetylation on the DNA-binding domain. Mol Cell Biol. 2003;23:8528–8541. doi: 10.1128/MCB.23.23.8528-8541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura T, Suzuki T, Aizawa K, Munemasa Y, Muto S, Horikoshi M, Nagai R. The deacetylase HDAC1 negatively regulates the cardiovascular transcription factor Krüppel-like factor 5 through direct interaction. J Biol Chem. 2005;280:12123–12129. doi: 10.1074/jbc.M410578200. [DOI] [PubMed] [Google Scholar]
- 9.Meng F, Han M, Zheng B, Wang C, Zhang R, Zhang XH, Wen JK. All-trans retinoic acid increases KLF4 acetylation by inducing HDAC2 phosphorylation and its dissociation from KLF4 in vascular smooth muscle cells. Biochem Biophys Res Commun. 2009;387:13–18. doi: 10.1016/j.bbrc.2009.05.112. [DOI] [PubMed] [Google Scholar]
- 10.He M, Han M, Zheng B, Shu YN, Wen JK. Angiotensin II stimulates KLF5 phosphorylation and its interaction with c-Jun leading to suppression of p21 expression in vascular smooth muscle cells. J Biochem. 2009;146:683–691. doi: 10.1093/jb/mvp115. [DOI] [PubMed] [Google Scholar]
- 11.Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, Maemura K, Kubota N, Kadowaki T, Nagai R. SUMOylation of Krüppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med. 2008;14:656–666. doi: 10.1038/nm1756. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZY, Wang X, Zhou Y, Offner G, Tseng CC. Destabilization of Krüppel-like factor 4 protein in response to serum stimulation involves the ubiquitin-proteasome pathway. Cancer Res. 2005;65:10394–10400. doi: 10.1158/0008-5472.CAN-05-2059. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Sun X, Ran Q, Wilkinson KD, Murphy TJ, Simons JW, Dong JT. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene. 2005;24:3319–3327. doi: 10.1038/sj.onc.1208497. [DOI] [PubMed] [Google Scholar]
- 14.Du JX, Yun CC, Bialkowska A, Yang VW. Protein inhibitor of activated STAT1 interacts with and up-regulates activities of the pro-proliferative transcription factor Krüppel-like factor 5. J Biol Chem. 2007;282:4782–4793. doi: 10.1074/jbc.M603413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai-Kowase K, Kumar MS, Hoofnagle MH, Yoshida T, Owens GK. PIAS1 activates the expression of smooth muscle cell differentiation marker genes by interacting with serum response factor and class I basic helix-loop-helix proteins. Mol Cell Biol. 2005;25:8009–8023. doi: 10.1128/MCB.25.18.8009-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du JX, Bialkowska AB, McConnell BB, Yang VW. SUMOylation regulates nuclear localization of Krüppel-like factor 5. J Biol Chem. 2008;283:31991–32002. doi: 10.1074/jbc.M803612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kober F, Canault M, Peiretti F, Mueller C, Kopp F, Alessi MC, Cozzone PJ, Nalbone G, Bernard M. MRI follow-up of TNF-dependent differential progression of atherosclerotic wall-thickening in mouse aortic arch from early to advanced stages. Atherosclerosis. 2007;195:e93–e99. doi: 10.1016/j.atherosclerosis.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Yun SJ, Ha JM, Kim EK, Kim YW, Jin SY, Lee DH, Song SH, Kim CD, Shin HK, Bae SS. Akt1 isoform modulates phenotypic conversion of vascular smooth muscle cells. Biochim Biophys Acta. 2014;1842:2184–2192. doi: 10.1016/j.bbadis.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Ha JM, Yun SJ, Kim YW, Jin SY, Lee HS, Song SH, Shin HK, Bae SS. Platelet-derived growth factor regulates vascular smooth muscle phenotype via mammalian target of rapamycin complex 1. Biochem Biophys Res Commun. 2015;464:57–62. doi: 10.1016/j.bbrc.2015.05.097. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Yun SJ, Kim YH, Ha JM, Jin SY, Lee HS, Kim SJ, Shin HK, Chung SW, Bae SS. Essential role of krüppel-like factor 5 during tumor necrosis factor α-induced phenotypic conversion of vascular smooth muscle cells. Biochem Biophys Res Commun. 2015;463:1323–1327. doi: 10.1016/j.bbrc.2015.06.123. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi K, Shibata K, Morita T, Iwasaki K, Watanabe M, Sobue K. Insulin receptor substrate-1/SHP-2 interaction, a phenotype-dependent switching machinery of insulin-like growth factor-I signaling in vascular smooth muscle cells. J Biol Chem. 2004;279:40807–40818. doi: 10.1074/jbc.M405100200. [DOI] [PubMed] [Google Scholar]
- 24.Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52:2562–25629. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- 25.van Vliet J, Turner J, Crossley M. Human Krüppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000;28:1955–1962. doi: 10.1093/nar/28.9.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao D, Niu X, Ning N, Hao G. Regulation of angiotensin II-Induced Krüppel-like factor 5 expression in vascular smooth muscle cells. Biol Pharm Bull. 2006;29:2004–2008. doi: 10.1248/bpb.29.2004. [DOI] [PubMed] [Google Scholar]
- 27.Garvey SM, Sinden DS, Schoppee Bortz PD, Wamhoff BR. Cyclosporine up-regulates Krüppel-like factor-4 (KLF4) in vascular smooth muscle cells and drives phenotypic modulation in vivo. J Pharmacol Exp Ther. 2010;333:34–42. doi: 10.1124/jpet.109.163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 29.Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I. Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost. 2005;3:1569–1576. doi: 10.1111/j.1538-7836.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Sinha S, Dandré F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 33.Li HX, Han M, Bernier M, Zheng B, Sun SG, Su M, Zhang R, Fu JR, Wen JK. Krüppel-like factor 4 promotes differentiation by transforming growth factor-beta receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J Biol Chem. 2010;285:17846–17856. doi: 10.1074/jbc.M109.076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- 35.Mansell A, Khelef N, Cossart P, O'Neill LA. Internalin B activates nuclear factor-kappa B via Ras, phosphoinositide 3-kinase, and Akt. J Biol Chem. 2001;276:43597–43603. doi: 10.1074/jbc.M105202200. [DOI] [PubMed] [Google Scholar]
- 36.Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM. Interferon alpha /beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001;276:13756–13761. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008;283:13934–13942. doi: 10.1074/jbc.M709300200. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Sawaki D, Aizawa K, Munemasa Y, Matsumura T, Ishida J, Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J Biol Chem. 2009;284:9549–9557. doi: 10.1074/jbc.M806230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bafford R, Sui XX, Wang G, Conte M. Angiotensin II and tumor necrosis factor-alpha upregulate survivin and Kruppel-like factor 5 in smooth muscle cells: Potential relevance to vein graft hyperplasia. Surgery. 2006;140:289–296. doi: 10.1016/j.surg.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Hoshino Y, Kurabayashi M, Kanda T, Hasegawa A, Sakamoto H, Okamoto E, Kowase K, Watanabe N, Manabe I, Suzuki T, Nakano A, Takase S, Wilcox JN, Nagai R. Regulated expression of the BTEB2 transcription factor in vascular smooth muscle cells: analysis of developmental and pathological expression profiles shows implications as a predictive factor for restenosis. Circulation. 2000;102:2528–2554. doi: 10.1161/01.cir.102.20.2528. [DOI] [PubMed] [Google Scholar]
- 41.Ogata T, Kurabayashi M, Hoshino Y, Ishikawa S, Takeyoshi I, Morishita Y, Nagai R. Inducible expression of BTEB2, a member of the zinc-finger family of transcription factors, in cardiac allograft arteriosclerosis. Transplant Proc. 2000;32:2032–2033. doi: 10.1016/s0041-1345(00)01544-x. [DOI] [PubMed] [Google Scholar]


