Abstract
Rationale: In the absence of a surgical lung biopsy, patients diagnosed with idiopathic pulmonary fibrosis (IPF) in clinical practice could participate in the INPULSIS trials of nintedanib if they had honeycombing and/or traction bronchiectasis plus reticulation, without atypical features of usual interstitial pneumonia (UIP), on high-resolution computed tomography (HRCT). Thus, the patients in these trials represented patients with definite UIP and a large subgroup of patients with possible UIP.
Objectives: To investigate the potential impact of diagnostic subgroups on the progression of IPF and the effect of nintedanib.
Methods: We conducted a post hoc subgroup analysis of patients with honeycombing on HRCT and/or confirmation of UIP by biopsy versus patients without either, using pooled data from the INPULSIS trials.
Measurements and Main Results: Seven hundred twenty-three (68.1%) patients had honeycombing and/or biopsy, and 338 (31.9%) patients had no honeycombing or biopsy. In these subgroups, respectively, the adjusted annual rate of decline in FVC in patients treated with placebo was −225.7 and −221.0 ml/yr, and the nintedanib versus placebo difference in the adjusted annual rate of decline in FVC was 117.0 ml/yr (95% confidence interval, 76.3–157.8) and 98.9 ml/yr (95% confidence interval, 36.4–161.5). There was no significant treatment-by-subgroup interaction (P = 0.8139). Adverse events were similar between the subgroups.
Conclusions: Patients with IPF diagnosed in clinical practice who had possible UIP with traction bronchiectasis on HRCT and had not undergone surgical lung biopsy had disease that progressed in a similar way, and responded similarly to nintedanib, to that of patients with honeycombing on HRCT and/or confirmation of UIP by biopsy.
Keywords: high-resolution computed tomography, HRCT, diagnosis, honeycombing, traction bronchiectasis
At a Glance Commentary
Scientific Knowledge on This Subject
Idiopathic pulmonary fibrosis (IPF) is a progressive disease characterized by decline in FVC. IPF is characterized by a high-resolution computed tomography (HRCT) image and/or histopathology features of usual interstitial pneumonia (UIP); the diagnosis of definite UIP on HRCT requires the presence of honeycombing.
What This Study Adds to the Field
These are the first data to show that the progression of disease is the same in placebo-treated patients with IPF with features of possible UIP with traction bronchiectasis on HRCT as in patients with honeycombing on HRCT and/or confirmation of UIP by surgical lung biopsy. Nintedanib has a consistent treatment effect in patients diagnosed with IPF who have features of possible UIP with traction bronchiectasis on HRCT as in patients who have honeycombing on HRCT and/or confirmation of UIP by surgical lung biopsy.
Idiopathic pulmonary fibrosis (IPF) is a specific form of interstitial pneumonia characterized by worsening dyspnea and progressive loss of lung function (1). According to the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association diagnostic guidelines published in 2011, diagnosis of IPF requires the exclusion of other known causes of interstitial lung disease and the presence of the usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT) in patients not subjected to a surgical lung biopsy (1). The UIP pattern includes subpleural basal predominance, reticular abnormality, honeycombing, and the absence of features inconsistent with UIP. For patients with a possible UIP pattern (i.e., reticulation with subpleural and basal predominance and absence of features inconsistent with UIP, but no honeycombing) or features inconsistent with UIP on HRCT, the guidelines state that a surgical lung biopsy is required to make a definitive diagnosis (1). The accuracy of a diagnosis of IPF increases with multidisciplinary discussion among clinicians, radiologists, and pathologists, particularly in cases in which the radiologic and histopathologic patterns are discordant (2, 3). However, because obtaining a surgical lung biopsy is not without risk for patients with severe physiologic impairment or substantial comorbidity, the risks of a surgical lung biopsy may outweigh the benefits of establishing a secure diagnosis of IPF (1, 4, 5).
Because the clinical course and response to treatment of patients who do not meet the current diagnostic criteria for IPF are unknown, investigating the behavior of disease across diagnostic subgroups is of great relevance (6). Furthermore, differences in diagnostic criteria required for participation in clinical trials in IPF may lead to different patient populations being assessed in different trials (7).
Nintedanib is an intracellular inhibitor of tyrosine kinases (8) that has been approved for the treatment of IPF in several countries and regions, including the United States (9), Europe (10), and Japan, and it has received a conditional recommendation for use in the latest international clinical practice guideline for the treatment of IPF (11). The two replicate, randomized, placebo-controlled phase III INPULSIS trials investigated the efficacy and safety of nintedanib 150 mg twice daily in patients with IPF (12). To enter the INPULSIS trials, patients had to have a diagnosis of IPF established ≤5 years before randomization and an HRCT scan performed within 12 months of randomization. In the absence of a surgical lung biopsy, patients had to have honeycombing and/or a combination of traction bronchiectasis and reticulation in the absence of atypical features of UIP on HRCT to be eligible to participate (12).
In both INPULSIS trials, nintedanib reduced the annual rate of decline in FVC by approximately 50% compared with placebo (primary endpoint) (12). For the two key secondary endpoints, time to first investigator-reported acute exacerbation and change from baseline in St. George’s Respiratory Questionnaire (SGRQ) total score over 52 weeks, a significant benefit of nintedanib versus placebo was observed in INPULSIS-2 but not in INPULSIS-1 (12). Adverse events were manageable for most patients; diarrhea was the most frequent adverse event in patients treated with nintedanib (12).
In this analysis, we investigated the potential impact of diagnostic subgroups on the effect of nintedanib in patients with IPF using pooled data from the INPULSIS trials. Some of these results have been reported in an abstract (13).
Methods
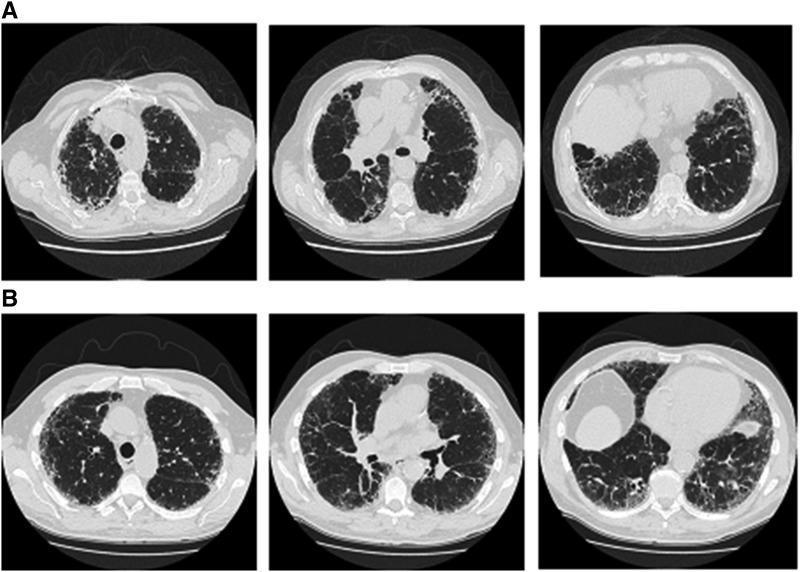
To qualify to enter the INPULSIS trials in the absence of a surgical lung biopsy, HRCT criteria A and B and C, or A and C, or B and C had to be met, where criterion A was definite honeycomb lung destruction with basal and peripheral predominance, criterion B was the presence of reticular abnormality and traction bronchiectasis consistent with fibrosis with basal and peripheral predominance, and criterion C was the absence of atypical features, specifically nodules and consolidation, with ground glass opacity, if present, being less extensive than a reticular opacity pattern. HRCT scans exemplifying patterns A and B and C, and B and C are shown in Figures 1A and 1B. All HRCT scans were assessed by one expert radiologist (D.M.H.). Surgical lung biopsies, if available, were reviewed by one expert pathologist (A.G.N.) and used to confirm eligibility. In cases in which there was disagreement between the radiologist and pathologist about whether a patient should be included in the trial, they discussed the case, and a consensus was reached.
Figure 1.
Eligibility criteria based on high-resolution computed tomography (upper, middle, and lower zones). (A) Criteria A, B, and C met. (B) Criteria B and C met.
Post hoc subgroup analyses of patients with honeycombing on HRCT and/or confirmation of UIP by surgical lung biopsy versus patients with features of possible UIP and traction bronchiectasis on HRCT (criteria B and C) and no surgical lung biopsy were conducted using pooled data from the two INPULSIS trials. Baseline characteristics were summarized by subgroup to determine whether there were any confounding factors. Analyses were conducted on the primary and key secondary endpoints by repeating the primary analysis of each endpoint within each subgroup. The annual rate of decline in FVC was analyzed based on random coefficient regression with fixed effects for trial, treatment, sex, age, height, and random effects for patient-specific intercept and time. Time to first investigator-reported acute exacerbation was analyzed based on a Cox’s regression model with terms for trial, treatment, sex, age, and height. The change from baseline in SGRQ over 52 weeks was analyzed based on a mixed model for repeated measures, with fixed effects for trial, treatment, visit, treatment-by-visit, baseline SGRQ total score, baseline SGRQ total score-by-visit, and random effect for the patient. To test if there was a different effect of nintedanib between the subgroups, an interaction P value was calculated. For the primary endpoint, the terms subgroup and an interaction term treatment-by-time-by-subgroup were included in the model. For the key secondary endpoints, the terms subgroup and interaction term treatment-by-subgroup were included in the model.
To check the robustness of the subgroup analyses, we also assessed the absolute change from baseline in FVC percent predicted over 52 weeks and the time to absolute decline in FVC ≥5% or ≥10% predicted, or death, over 52 weeks in each subgroup using the same approach as for the other endpoints to calculate the interaction P values. The absolute change from baseline in FVC percent predicted over 52 weeks was analyzed using a mixed model for repeated measures, with fixed effects for trial, treatment, visit, sex, age, height, treatment-by-visit, baseline FVC percent predicted, baseline FVC percent predicted-by-visit, and a random effect for the patient. The time to absolute decline in FVC ≥5% or 10% predicted, or death over 52 weeks was analyzed using a Cox’s regression model, with terms for trial, treatment, sex, age, and height. Analyses were based on data collected up to 372 days after randomization (52 wk plus 7 d margin). SAS version 9.2 or later (SAS Institute, Cary, NC) was used to perform the analyses.
Safety was assessed via clinical and laboratory evaluation, and the recording of adverse events with onset after the first dose and up to 28 days after the last dose of the study drug in patients who received ≥1 dose of the study drug. Safety analyses were repeated by subgroup and were descriptive.
Results
Patients
All the patients in the INPULSIS trials had a diagnosis of IPF established in clinical practice ≤5 years before randomization. Central review of HRCT scans of the 1061 patients treated in the trials showed that 567 (53.4%) of the patients had definite honeycomb lung destruction with basal and peripheral predominance (criteria A, B, and C, or criteria A and C), whereas in 468 (44.1%) of the patients, honeycombing was absent on HRCT but criteria B and C were met (Table 1). Radiological inclusion criteria were not fulfilled in 26 (2.5%) patients. Surgical lung biopsies were available for 229 (21.6%) patients. Of these 229 surgical lung biopsies, 130 showed definite UIP, 93 probable UIP, and 6 possible UIP.
Table 1.
Radiological Assessment and Availability of Surgical Lung Biopsy in Patients Treated in the INPULSIS Trials
| Nintedanib (n = 638) | Placebo (n = 423) | Total (n = 1061) | |
|---|---|---|---|
| Surgical lung biopsy available, n (%) | 144 (22.6)* | 85 (20.1)* | 229 (21.6)* |
| Radiological assessment, n (%) | |||
| Criteria A, B, and C | 264 (41.4)* | 199 (47.0)* | 463 (43.6)* |
| No surgical lung biopsy | 224 (35.1)* | 175 (41.4)* | 399 (37.6)* |
| Criteria A and C | 62 (9.7)* | 42 (9.9)* | 104 (9.8)* |
| No surgical lung biopsy | 57 (8.9)* | 38 (9.0)* | 95 (9.0)* |
| Criteria B and C | 296 (46.4) | 172 (40.7) | 468 (44.1) |
| No surgical lung biopsy | 213 (33.4)† | 125 (29.6)† | 338 (31.9)† |
| Did not fulfill radiological inclusion criteria (diagnosed based on surgical lung biopsy) | 16 (2.5)* | 10 (2.4)* | 26 (2.5)* |
Criterion A: definite honeycomb lung destruction with basal and peripheral predominance; criterion B: presence of reticular abnormality and traction bronchiectasis consistent with fibrosis with basal and peripheral predominance; criterion C: atypical features absent, specifically nodules and consolidation; ground glass opacity, if present, is less extensive than reticular opacity pattern.
Patients with honeycombing and/or biopsy (229 with biopsy, 399 [criteria A, B, and C] + 95 [criteria A and C] with honeycombing but not biopsy).
Patients without honeycombing or biopsy.
For the purposes of this subgroup analysis, 723 (68.1%) patients had honeycombing on HRCT and/or confirmation of UIP by surgical lung biopsy [i.e., a diagnosis of IPF according to current international guidelines (1)], and 338 (31.9%) patients had features of possible UIP and traction bronchiectasis on HRCT and no surgical lung biopsy (Table 1). There were no major differences in demographic and disease characteristics between the subgroups, and baseline characteristics were similar between the nintedanib and placebo groups within each subgroup, indicating that assessment of the effect of nintedanib across subgroups would not be affected by confounding factors (Table 2). In the subgroup of patients with honeycombing and/or biopsy, there were higher proportions of Asian patients (34.6 vs. 21.3%), patients with centrilobular emphysema (44.8 vs. 28.4%), and former or current smokers (75.8 vs. 64.2%) than in the subgroup of patients without honeycombing or biopsy.
Table 2.
Baseline Characteristics by Subgroup
| Honeycombing on HRCT and/or Confirmation of UIP Pattern by Surgical Lung Biopsy |
No Honeycombing or Surgical Lung Biopsy |
|||
|---|---|---|---|---|
| Nintedanib (n = 425) | Placebo (n = 298) | Nintedanib (n = 213) | Placebo (n = 125) | |
| Age, yr, mean (SD) | 66.3 (8.1) | 66.9 (7.9) | 67.2 (8.1) | 67.2 (7.9) |
| Male, n (%) | 341 (80.2) | 238 (79.9) | 166 (77.9) | 96 (76.8) |
| Race, n (%) | ||||
| White | 228 (53.6) | 165 (55.4) | 132 (62.0) | 83 (66.4) |
| Asian | 145 (34.1) | 105 (35.2) | 49 (23.0) | 23 (18.4) |
| Black | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing* | 50 (11.8) | 28 (9.4) | 32 (15.0) | 19 (15.2) |
| Former or current smoker, n (%) | 327 (76.9) | 221 (74.2) | 137 (64.3) | 80 (64.0) |
| Time since diagnosis, yr, mean (SD) | 1.7 (1.4) | 1.5 (1.3) | 1.5 (1.3) | 1.7 (1.4) |
| Presence of centrilobular emphysema, n (%)† | 192 (45.2) | 132 (44.3) | 62 (29.1) | 34 (27.2) |
| FVC, ml, mean (SD) | 2,690 (715) | 2,738 (809) | 2,760 (835) | 2,702 (815) |
| FVC, % predicted, mean (SD) | 79.1 (17.4) | 79.6 (17.9) | 81.1 (18.0) | 78.6 (19.1) |
| FEV1/FVC ratio, %, mean (SD) | 81.7 (6.0) | 81.7 (6.2) | 81.5 (5.6) | 81.7 (5.5) |
| DlCO, % predicted, mean (SD) | 46.4 (13.6) | 46.0 (12.7) | 49.4 (13.2) | 49.2 (14.8) |
| SGRQ total score, mean (SD)‡ | 39.4 (19.4) | 40.1 (18.5) | 39.7 (18.6) | 38.3 (18.7) |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; HRCT = high-resolution computed tomography; SGRQ = St. George’s Respiratory Questionnaire; UIP = usual interstitial pneumonia.
In France, regulations did not permit the collection of data on race.
Based on qualitative assessment of HRCT scans.
n = 415 for nintedanib and n = 295 for placebo in confirmation of UIP by surgical lung biopsy subgroup; n = 209 for nintedanib and n = 124 for placebo in features of possible UIP and traction bronchiectasis on HRCT and no biopsy.
Annual Rate of Decline in FVC
The adjusted annual rate of decline in FVC was consistent between placebo-treated patients with honeycombing and/or biopsy and without honeycombing or biopsy (−225.7 and −221.0 ml/yr, respectively).
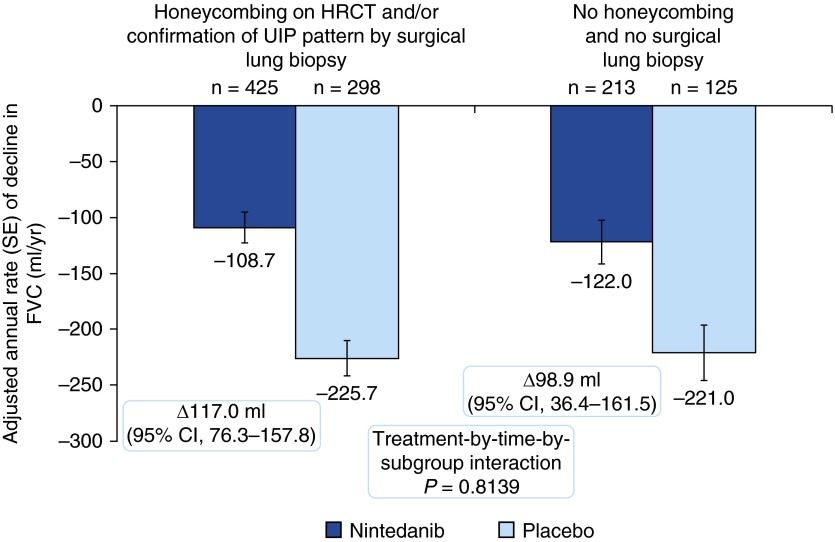
In the overall pooled population, nintedanib significantly reduced the annual rate of decline in FVC compared with placebo (difference of 109.9 ml/yr; 95% confidence interval [CI], 75.9–144.0; P < 0.0001) (12). In patients with honeycombing and/or biopsy, the adjusted annual rate of decline in FVC was −108.7 ml/yr in the nintedanib group (difference vs. placebo of 117.0 ml/yr; 95% CI, 76.3–157.8) (Figure 2). In patients without honeycombing or biopsy, the adjusted annual rate of decline in FVC was −122.0 ml/yr in the nintedanib group (difference vs. placebo of 98.9 ml/yr; 95% CI, 36.4–161.5) (Figure 2). The treatment-by-subgroup interaction P value was not significant (P = 0.8139), indicating that the treatment effect of nintedanib was not different between the subgroups. The treatment effect in both subgroups was also consistent with the treatment effect in the overall pooled population.
Figure 2.
Annual rate of decline in FVC (ml/yr) by subgroup. CI = confidence interval; HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia.
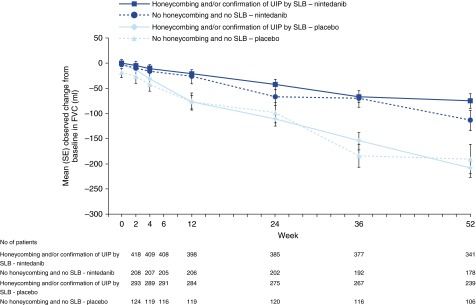
The primary endpoint results were supported by the observed changes from baseline in FVC over time in each subgroup (Figure 3). Importantly, the curves for change from baseline in FVC over time in the placebo groups were similar between the subgroups, indicating a similar rate of decline in FVC, irrespective of the diagnostic criteria. The results for the additional lung function outcomes were consistent across the subgroups and are presented in Table 3.
Figure 3.
Change from baseline in FVC over time by subgroup. SLB = surgical lung biopsy; UIP = usual interstitial pneumonia.
Table 3.
Lung Function Outcomes by Subgroup
| Honeycombing on HRCT and/or Confirmation of UIP Pattern by Surgical Lung Biopsy |
No Honeycombing or Surgical Lung Biopsy |
|||
|---|---|---|---|---|
| Nintedanib (n = 425) | Placebo (n = 298) | Nintedanib (n = 213) | Placebo (n = 125) | |
| Absolute change from baseline in FVC % predicted, adjusted mean (SE) | −2.66 (0.37) | −6.14 (0.42) | −3.43 (0.51) | −6.06 (0.62) |
| Nintedanib vs. placebo difference, adjusted mean (95% CI) | 3.48 (2.49–4.46) |
2.63 (1.18–4.08) |
||
| Treatment-by-subgroup interaction |
P = 0.8369 |
|||
| Time to absolute decline in FVC ≥5% predicted or death | |
|||
| Hazard ratio (95% CI) | 0.59 (0.49–0.72) |
0.65 (0.49–0.85) |
||
| Treatment-by-subgroup interaction |
P = 0.6745 |
|||
| Time to absolute decline in FVC ≥10% predicted or death | |
|||
| Hazard ratio (95% CI) | 0.59 (0.46–0.76) |
0.63 (0.43–0.94) |
||
| Treatment-by-subgroup interaction | P = 0.7735 | |||
Definition of abbreviations: CI = confidence interval; HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia.
Investigator-reported Acute Exacerbations
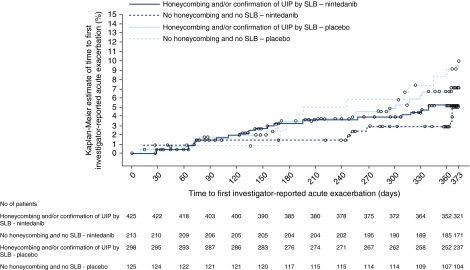
The proportion of patients with ≥1 acute exacerbation was low in placebo-treated patients in both subgroups, but was consistent between patients without honeycombing or biopsy and patients with honeycombing and/or biopsy (9.6% [12 patients] vs. 6.7% [20 patients], respectively) (Figure 4). The proportion of nintedanib-treated patients with ≥1 acute exacerbation was 4.7% (10 patients) and 4.9% (21 patients) in these subgroups, respectively (Figure 4).
Figure 4.
Time to first acute exacerbation over 52 weeks by subgroup. SLB = surgical lung biopsy; UIP = usual interstitial pneumonia.
In the overall pooled population, there was a numerical but not statistically significant reduction in the risk of having a first acute exacerbation in favor of nintedanib (hazard ratio [HR], 0.64; 95% CI, 0.39–1.05; P = 0.0823) (12). In patients with honeycombing and/or biopsy, the HR for time to first acute exacerbation was 0.75 (95% CI, 0.40–1.38) in favor of nintedanib (Figure 4). In patients without honeycombing or biopsy, the HR for time to first acute exacerbation was 0.50 (95% CI, 0.22–1.17) in favor of nintedanib (Figure 4). The treatment-by-subgroup interaction P value was not significant (P = 0.3747), indicating that the treatment effect of nintedanib was not different between the diagnostic subgroups. The treatment effect in both subgroups was also consistent with the treatment effect in the overall pooled population.
SGRQ Total Score
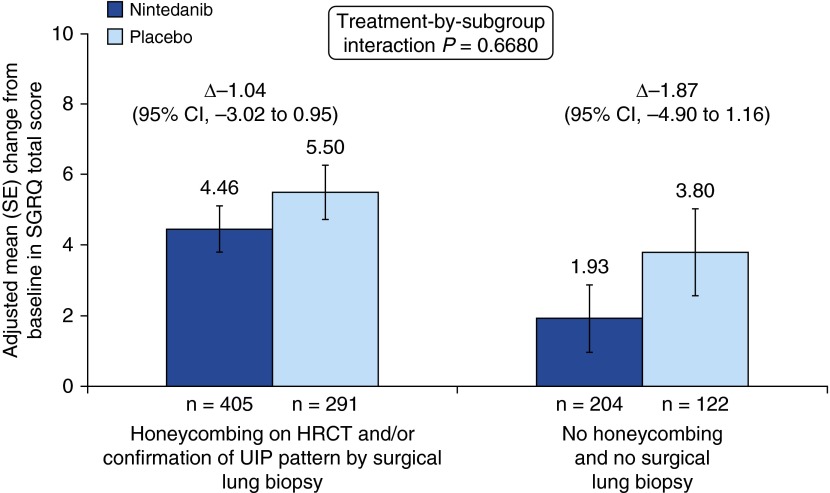
Among placebo-treated patients, the adjusted mean change from baseline in SGRQ total score at week 52 was similar in patients with honeycombing and/or biopsy than in patients without honeycombing or biopsy (5.50 vs. 3.80) (Figure 5). The adjusted mean change from baseline in SGRQ total score at week 52 was 4.46 and 1.93 for nintedanib-treated patients in these subgroups, respectively (Figure 5).
Figure 5.
Change from baseline in St. George’s Respiratory Questionnaire (SGRQ) total score over 52 weeks by subgroup. CI = confidence interval; HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia.
In the overall pooled population, there was no significant difference between nintedanib and placebo in the change from baseline in SGRQ total score at week 52 (between-group difference of −1.43 points; 95% CI, −3.09 to 0.23; P = 0.0923) (12). In patients with honeycombing and/or biopsy, the nintedanib versus placebo difference in the adjusted mean change from baseline in SGRQ total score at week 52 was −1.04 (95% CI, −3.02 to 0.95) (Figure 5). In patients without honeycombing or biopsy, the nintedanib versus placebo difference in the adjusted mean change from baseline in SGRQ total score at week 52 was −1.87 (95% CI, −4.90 to 1.16) (Figure 5). The treatment-by-subgroup interaction P value was not significant (P = 0.6680), which indicated that the treatment effect of nintedanib was not different between the diagnostic subgroups. The treatment effect in both subgroups was also consistent with the treatment effect in the overall pooled population.
Adverse Events
A summary of adverse events in each subgroup is presented in Table 4. There were no major differences in the safety profile of nintedanib between the subgroups by diagnostic criteria. The proportions of patients who had ≥1 serious adverse event were comparable between the nintedanib and placebo groups within each subgroup and were consistent with the overall pooled population (12). Also consistent with the results in the overall population, diarrhea was the most frequent adverse event in nintedanib-treated patients in both subgroups, reported in 64.0% of patients with honeycombing and/or biopsy and 59.2% of patients without honeycombing or biopsy, compared with 18.8 and 17.6% of placebo-treated patients in these subgroups, respectively. However, as observed for the overall population, only a small proportion of nintedanib-treated patients (4.5 and 4.2% in these subgroups, respectively) permanently discontinued the study medication due to diarrhea.
Table 4.
Exposure and Adverse Events by Subgroup
| Honeycombing on HRCT and/or Confirmation of UIP by Surgical Lung Biopsy |
No Honeycombing or Surgical Lung Biopsy |
|||
|---|---|---|---|---|
| Nintedanib (n = 425) | Placebo (n = 298) | Nintedanib (n = 213) | Placebo (n = 125) | |
| Exposure, mean (SD) | 10.1 (3.5) | 10.7 (2.9) | 10.7 (3.0) | 11.1 (2.6) |
| Any adverse event(s) | 407 (95.8) | 268 (89.9) | 202 (94.8) | 111 (88.8) |
| Most frequent adverse event(s)* | ||||
| Diarrhea | 272 (64.0) | 56 (18.8) | 126 (59.2) | 22 (17.6) |
| Nausea | 107 (25.2) | 23 (7.7) | 49 (23.0) | 5 (4.0) |
| Nasopharyngitis | 63 (14.8) | 51 (17.1) | 24 (11.3) | 17 (13.6) |
| Cough | 58 (13.6) | 41 (13.8) | 27 (12.7) | 16 (12.8) |
| Vomiting | 53 (12.5) | 7 (2.3) | 21 (9.9) | 4 (3.2) |
| Decreased appetite | 49 (11.5) | 21 (7.0) | 19 (8.9) | 3 (2.4) |
| Bronchitis | 39 (9.2) | 28 (9.4) | 28 (13.1) | 17 (13.6) |
| Progression of IPF† | 44 (10.4) | 44 (14.8) | 20 (9.4) | 17 (13.6) |
| Weight decreased | 39 (9.2) | 11 (3.7) | 23 (10.8) | 4 (3.2) |
| Upper respiratory tract infection | 36 (8.5) | 30 (10.1) | 22 (10.3) | 12 (9.6) |
| Abdominal pain | 34 (8.0) | 9 (3.0) | 22 (10.3) | 1 (0.8) |
| Dyspnea | 31 (7.3) | 30 (10.1) | 18 (8.5) | 18 (14.4) |
| Severe adverse event(s) | 115 (27.1) | 72 (24.2) | 59 (27.7) | 27 (21.6) |
| Serious adverse event(s) | 131 (30.8) | 94 (31.5) | 63 (29.6) | 33 (26.4) |
| Fatal adverse event(s) | 28 (6.6) | 23 (7.7) | 9 (4.2) | 8 (6.4) |
| Adverse event(s) leading to treatment discontinuation‡ | 90 (21.2) | 41 (13.8) | 33 (15.5) | 14 (11.2) |
| Diarrhea | 19 (4.5) | 1 (0.3) | 9 (4.2) | 0 (0.0) |
| Progression of IPF† | 10 (2.4) | 15 (5.0) | 3 (1.4) | 6 (4.8) |
| Nausea | 12 (2.8) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
Definition of abbreviations: HRCT = high-resolution computed tomography; IPF = idiopathic pulmonary fibrosis; UIP = usual interstitial pneumonia.
Data are n (%) for exposure.
Adverse events reported by >10% of patients in any treatment group.
Corresponds to the Medical Dictionary for Regulatory Activities term “IPF,” which included disease worsening and acute exacerbations of IPF.
Adverse events leading to treatment discontinuation in >2% of patients in any treatment group.
Discussion
All the patients who participated in the INPULSIS trials had been diagnosed with IPF in accordance with clinical practice, based on clinical assessment and reasoning, including consideration of HRCT data. We believe that a considerable number of patients who have possible UIP according to current diagnostic guidelines also have traction bronchiectasis, and that the subgroup of patients in our study who had a combination of traction bronchiectasis and reticulation on HRCT represent an important subgroup of the patients with IPF seen in clinical practice. In this post hoc analysis of data from 1061 patients treated in the INPULSIS trials, we have shown, for the first time, that the rate of decline in FVC in patients with possible UIP with traction bronchiectasis and no surgical lung biopsy is the same as in patients with a diagnosis of IPF according to current guidelines (i.e., with honeycombing on HRCT and/or confirmation of UIP on surgical biopsy) (1). The proportion of patients who had acute exacerbations was also consistent between these subgroups. Furthermore, we have demonstrated that there was no difference in the treatment effect of nintedanib on the rate of decline in FVC, acute exacerbations, or change in SGRQ score between these diagnostic subgroups. These are the first data to show the efficacy of a treatment for IPF in reducing FVC decline in patients with possible UIP with traction bronchiectasis. The adverse event profile of nintedanib in both subgroups was similar and as expected based on the adverse events reported in the overall patient population (12).
The presence of honeycombing on HRCT has been correlated with a lower FVC percent predicted and lower diffusing capacity of the lung for carbon monoxide (DlCO) percent predicted in some but not all studies (1, 14, 15). In our study, FVC percent predicted and DlCO percent predicted at baseline were similar in patients with honeycombing as in patients without honeycombing or biopsy.
Challenges exist to basing the diagnosis of IPF on the presence of honeycombing on HRCT or a surgical lung biopsy. Atypical HRCT patterns are common in patients with biopsy-proven UIP (16). Not all patients with IPF have honeycombing on HRCT (17). Data from the INSIGHTS-IPF (Investigating Significant Health Trends in Idiopathic Pulmonary Fibrosis) registry of patients with IPF in clinical practice in Germany found that of 447 patients with an HRCT scan, 23.7% had possible UIP (18). In the INPULSIS trials, fewer than half of the patients had honeycombing on HRCT at baseline. Interobserver agreement for the identification and differentiation of honeycombing (e.g., vs. traction bronchiectasis) on HRCT is low and is complicated by the presence of emphysema, which may mimic honeycombing (7, 16, 17, 19‒21). Furthermore, in patients with IPF who have comorbidities, which are common in patients with IPF (22), or in patients with severe physiologic impairment, surgical lung biopsy may pose a significant risk (4).
In conclusion, our findings show that patients diagnosed with IPF in accordance with clinical practice who have possible UIP with traction bronchiectasis on HRCT (confirmed by central review) have disease that progresses in a similar way and responds similarly to nintedanib, as do patients who have honeycombing on HRCT and/or confirmation of UIP by biopsy in accordance with current diagnostic guidelines. The striking similarity observed in the natural history of disease and the effect of nintedanib treatment between these subgroups supports the use of a working diagnosis of IPF in clinical practice for the large group of patients who have possible UIP with traction bronchiectasis, including patients who may be unable or unwilling to undergo surgical lung biopsy. These findings have implications for clinical trial design and for the criteria used to diagnose IPF in clinical practice.
Acknowledgments
Acknowledgment
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Julie Fleming and Wendy Morris of Fleishman-Hillard Group Ltd., London, United Kingdom, during the preparation of this article. The authors were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development and have approved the final version.
Footnotes
Supported by the National Institute of Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College (A.G.N. and D.M.H). The INPULSIS trials were funded by Boehringer Ingelheim.
Author Contributions: All the authors were involved in study design, analysis, and interpretation of data, as well as revising the manuscript for important intellectual content.
Originally Published in Press as DOI: 10.1164/rccm.201602-0402OC on June 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaherty KR, Andrei AC, King TE, Jr, Raghu G, Colby TV, Wells A, Bassily N, Brown K, du Bois R, Flint A, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175:1054–1060. doi: 10.1164/rccm.200606-833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KR, King TE, Jr, Raghu G, Lynch JP, III, Colby TV, Travis WD, Gross BH, Kazerooni EA, Toews GB, Long Q, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170:904–910. doi: 10.1164/rccm.200402-147OC. [DOI] [PubMed] [Google Scholar]
- 4.Kreider ME, Hansen-Flaschen J, Ahmad NN, Rossman MD, Kaiser LR, Kucharczuk JC, Shrager JB. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1140–1144. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Ryerson CJ, Urbania TH, Richeldi L, Mooney JJ, Lee JS, Jones KD, Elicker BM, Koth LL, King TE, Jr, Wolters PJ, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42:750–757. doi: 10.1183/09031936.00131912. [DOI] [PubMed] [Google Scholar]
- 6.Huie TJ, Brown KK. Definitions of disease: should possible and probable idiopathic pulmonary fibrosis be enrolled in treatment trials? Respir Investig. 2015;53:88–92. doi: 10.1016/j.resinv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Hansell DM, Goldin JG, King TE, Jr, Lynch DA, Richeldi L, Wells AU. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: a position paper from the Fleischner Society. Lancet Respir Med. 2015;3:483–496. doi: 10.1016/S2213-2600(15)00096-X. [DOI] [PubMed] [Google Scholar]
- 8.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehringer Ingelheim Pharmaceuticals, Inc. OFEV (nintedanib) prescribing information. 2016 [accessed 2016 Apr 15] Available from http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Ofev/ofev.pdf.
- 10.Boehringer Ingelheim. OFEV (nintedanib). Summary of product characteristics. 2016 [accessed 2016 Apr 18] Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003821/WC500182474.pdf.
- 11.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 12.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Wells A, Nicholson AG, Richeldi L, Flaherty KR, Le Maulf F, Stowasser S, Schlenker-Herceg R, Hansell DM. Consistent effect of nintedanib on decline in FVC in patients across subgroups based on HRCT diagnostic criteria: results from the INPULSIS trials in IPF [abstract] Am J Respir Crit Care Med. 2015;191:A1022. [Google Scholar]
- 14.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, Raghu G, King TE, Jr, Bradford WZ, Schwartz DA, et al. Idiopathic Pulmonary Fibrosis Study Group. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Shehu E, Gjonbrataj J, Bahn YE, Rho BH, Lee MY, Choi WI. Clinical findings and outcomes in patients with possible usual interstitial pneumonia. Respir Med. 2015;109:510–516. doi: 10.1016/j.rmed.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Sverzellati N, Lynch DA, Hansell DM, Johkoh T, King TE, Jr, Travis WD. American Thoracic Society-European Respiratory Society classification of the idiopathic interstitial pneumonias: advances in knowledge since 2002. Radiographics. 2015;35:1849–1871. doi: 10.1148/rg.2015140334. [DOI] [PubMed] [Google Scholar]
- 17.Jacob J, Hansell DM. HRCT of fibrosing lung disease. Respirology. 2015;20:859–872. doi: 10.1111/resp.12531. [DOI] [PubMed] [Google Scholar]
- 18.Behr J, Kreuter M, Hoeper MM, Wirtz H, Klotsche J, Koschel D, Andreas S, Claussen M, Grohé C, Wilkens H, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015;46:186–196. doi: 10.1183/09031936.00217614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh SL, Sverzellati N, Devaraj A, Keir GJ, Wells AU, Hansell DM. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69:216–222. doi: 10.1136/thoraxjnl-2013-203843. [DOI] [PubMed] [Google Scholar]
- 20.Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, Bankier AA, Lee KS, Müller NL, Song JW, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 21.Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM UIP Observer Consort. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax. 2016;71:45–51. doi: 10.1136/thoraxjnl-2015-207252. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46:1113–1130. doi: 10.1183/13993003.02316-2014. [DOI] [PubMed] [Google Scholar]