Abstract
Rationale: Biomarkers for survival in amyotrophic lateral sclerosis (ALS) would facilitate the development of novel drugs. Although respiratory muscle weakness is a known predictor of poor prognosis, a comprehensive comparison of different tests is lacking.
Objectives: To compare the predictive power of invasive and noninvasive respiratory muscle strength assessments for survival or ventilator-free survival, up to 3 years.
Methods: From a previously published report respiratory muscle strength measurements were available for 78 patients with ALS. Time to death and/or ventilation were ascertained. Receiver operating characteristic analysis was used to determine the cutoff point of each parameter.
Measurements and Main Results: Each respiratory muscle strength assessment individually achieved statistical significance for prediction of survival or ventilator-free survival. In multivariate analysis sniff trans-diaphragmatic and esophageal pressure, twitch trans-diaphragmatic pressure (Tw Pdi), age, and maximal static expiratory mouth pressure were significant predictors of ventilation-free survival and Tw Pdi and maximal static expiratory mouth pressure for absolute survival. Although all measures had good specificity, there were differing sensitivities. All cutoff points for the VC were greater than 80% of normal, except for prediction of 3-month outcomes. Sequential data showed a linear decline for direct measures of respiratory muscle strength, whereas VC showed little to no decline until 12 months before death/ventilation.
Conclusions: The most powerful biomarker for mortality stratification was Tw Pdi, but the predictive power of sniff nasal inspiratory pressure was also excellent. A VC within normal range suggested a good prognosis at 3 months but was of little other value.
Keywords: amyotrophic lateral sclerosis, survival, diaphragm, maximal inspiratory pressure, sniff nasal inspiratory pressure
At a Glance Commentary
Scientific Knowledge on the Subject
Although respiratory muscle weakness is known to be associated with a poor prognosis in amyotrophic lateral sclerosis (ALS), there are no comprehensive data that compare the prognostic power of different tests of inspiratory and expiratory muscle function at different time intervals. However, such data would facilitate enrichment of phase IIa studies of novel agents aiming to reduce mortality in ALS.
What This Study Adds to the Field
In a cohort of patients with ALS followed until 100% mortality occurred, invasive and noninvasive tests of respiratory muscle strength were performed, allowing the predictive power for death or noninvasive ventilation of each test at time intervals up to 3 years to be assessed and for relevant cutoff points to be obtained. The best performing tests were sniff and twitch trans-diaphragmatic pressure, but maximal sniff nasal inspiratory pressure also had excellent predictive power; sniff nasal inspiratory pressure values of 52, 71, 85, and 88 cm H2O displayed greater than or equal to 95% sensitivity for predicting death or noninvasive ventilation use at 6, 12, 24, and 36 months, respectively. Although vital capacity also had good predictive power, the cutoff point indicating a poor prognosis was within the normal range (i.e., >80% predicted) for all time intervals beyond 3 months, making this a less useful stratification variable for most studies.
Amyotrophic lateral sclerosis (ALS) is a progressive and devastating neurologic condition characterized by progressive muscle weakness. Although riluzole is licensed for ALS treatment (1), the benefits in clinical practice have proved modest and new drugs are urgently required. ALS-related respiratory muscle weakness is relatively uncommon at presentation, but hypercapnic respiratory failure caused by muscle weakness is common in established ALS. Although noninvasive ventilation (NIV) is an effective therapy, especially for patients with nonbulbar disease (2, 3), it comes with a poorer prognosis and the inconvenience of wearing a ventilator apparatus; therefore, it may be considered a “hard” adverse endpoint.
For most conditions, including ALS, trials powered to detect improvements in survival are expensive. This may delay the evaluation and introduction of new drugs or technologies (e.g., diaphragm pacing). Therefore biomarkers, either as a surrogate outcome measure or as a stratification tool, would be useful to enrich the study population for those at high risk of meeting the endpoint. In ALS, mortality or the need for ventilator support are objective endpoints that additionally have obvious validity to patients, regulators, and payers.
Several studies have identified respiratory muscle weakness, measured directly or as VC, as a predictor of daytime respiratory failure (4) or death (5–7). Respiratory muscle strength could therefore be a valuable biomarker. However, there are some gaps in the literature diminishing the value of current data. First, the literature disproportionately depends on VC as a measure of respiratory muscle strength. Although VC has some advantages (e.g., widespread availability), the relationship of directly measured respiratory muscle strength with VC is, as expected from the pulmonary pressure volume curve (8), not linear (9). Therefore it is unknown whether VC is responsive to disease progression throughout the entire disease course. Second, no large-scale study has compared the prognostic power of direct measurement of diaphragm function as trans-diaphragmatic pressure with noninvasive measures. Third, the prognostic value of nonvolitional measures of respiratory muscle function have not been evaluated, with the exception of Pinto and coworkers (10), who undertook phrenic nerve stimulation. However, they used an electrical technique, which can be unreliable even in experienced hands (11), and used the compound muscle action potential as outcome measure rather than a measure of force. Lastly, most studies are limited by the lack of sequential data and a short follow-up, leaving a proportion of the participants alive, thus diminishing statistical power.
Between 1996 and 2000, we studied (4) a large group of patients with ALS (n = 81) using invasive and noninvasive measures of respiratory muscle function (12); however, our prior publication presented only cross-sectional data and explored the relationship between respiratory muscle strength and daytime and nocturnal hypercapnia. In the current analysis, conducted between 2014 and 2015, the value of these measures for the prediction of death or initiation of NIV was assessed.
Methods
Participants
The present study used respiratory muscle function data from a previously published ALS patient cohort, containing 81 patients (16 females) studied between 1996 and 2000 (4). Entry into the study was not dependent on the presence or absence of respiratory symptoms. The date of death was ascertained in 2014, using hospital and central NHS records. Treatment with NIV was obtained from the clinical records, where available.
Participants were recruited from the King's MND Care and Research Centre, where a diagnosis of ALS was confirmed by a consultant with a special interest in ALS and classified as El Escorial “possible, probable, or definite” (13). The study was approved by the Kings College Hospital ethics committee and all patients provided written informed consent.
Measurements
At the time of assessment, none of the patients had received ventilatory support, although several were subsequently treated with noninvasive positive-pressure ventilation. The respiratory muscle strength measurement protocol has been described previously (4). The following parameters were included: VC, maximal static inspiratory and expiratory mouth pressures (MIP and MEP, respectively), maximal sniff nasal inspiratory pressure (SNIP), maximal sniff esophageal (Sn Pes) and trans-diaphragmatic pressures (Sn Pdi), unpotentiated twitch trans-diaphragmatic pressure (Tw Pdi) using cervical magnetic stimulation (14, 15), maximal cough gastric pressure (Cough Pga) (16), and the unpotentiated twitch abdominal pressure elicited by magnetic stimulation of the 10th thoracic intervertebral space (Tw T10) (17).
Statistical Analyses
A stepwise selection method using Cox regression was performed to determine the relationship between each variable and both survival and ventilation-free survival. The following variables were included in the stepwise regression analysis: Tw Pdi (cm H2O), Sn Pdi (cm H2O), Sn Pes (cm H2O), SNIP (cm H2O), SNIP (percent-predicted), VC (percent-predicted), MIP (cm H2O), MIP (percent-predicted), MEP (cm H2O), MEP (percent-predicted), Cough Pga (cm H2O), age, and sex. In selection steps, the score tests for each variate not in the model are performed, and are used to determine which variate should be added to or removed from the model. The stepwise selection significance levels for entering and removing were 0.2 and 0.1, respectively. The selection process was repeated until no further effect could be added to the model or if the current model is identical to a previously visited model. Sensitivity and specificity analyses, for the prediction of survival and ventilation-free survival, were performed for each parameter at 3-month intervals until 18 months and thereafter at 6-month intervals until the third year. Furthermore, the relationship of the strongest predictors from the multivariate analysis model with noninvasive measures of respiratory muscle strength was investigated.
The values derived from the receiver operating characteristic (ROC) plot, offering the greatest sensitivity and sensitivity (i.e., ratio of sensitivity/[1-specificity], as a function of time), were plotted for each parameter. Kaplan-Meier survival analyses were performed, correlating death or the date of initiation of NIV for each parameter, with three groups based on normal values for each test: (1) greater than or equal to 80% predicted (i.e., within the normal range), (2) 45% to less than 80% predicted, and (3) less than 45% predicted.
For patients in whom multiple measures were available, graphical plots were used to determine the trajectory of each parameter before death.
Results
After review of the source (paper) data (4) and the date of death, 78 patients (17 females) were included. Only 21.8% of the sample were women; this was not the result of any deliberate recruitment bias and compares with an overall clinical population with a male/female ratio of approximately 3:2.
Riluzole was taken by the majority of patients. The long time-interval between respiratory function measurement (1996–2000) and survival analysis (2014–2015) unfortunately ensured a 100% mortality rate in our cohort. The patients survived a mean 744 days from the date of inclusion in the previously published cohort (4). NIV was offered to patients managed by our institution, but this was not universal practice in the United Kingdom at the time. Therefore, NIV was not offered to some participants. In addition, because of the wide geographic referral base of the King's MND Care and Research Centre, NIV data could not be obtained for some (n = 9) cases.
The mean (SD) age of the included patients was 61 (8.7) years and they had a mean (SD) ALS functional rating score of 28 (6), a mean (SD) Norris limb score of 41 (12.9) out of 63, and a mean Norris bulbar score of 34 (8.5) out of 39 (Table 1). As expected and as shown in Table 1, a range of respiratory muscle weakness was observed in the patient population; inherent to the condition not all patients had the stamina to complete all the tests and where so we opted to prioritize respiratory muscle tests. Numbers for the datasets available for each parameter are also given in Table 1.
Table 1.
Demographic Data of Participants
| Score Indicating Normal Function | n (of 78) | Mean | SD | Median | Q1, Q3 | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, yr | — | 78 | 61 | 8.7 | 61 | 55, 66 |
| Sex, male/female | — | 78 | 61/17 | — | ||
| ALS FRS | 40 | 55 | 28 | 5.6 | 29 | 23, 32 |
| Norris limb | 63 | 58 | 41 | 12.9 | 41.5 | 36, 49 |
| Norris bulbar | 39 | 58 | 34 | 8.5 | 35 | 30, 39 |
| MRC UL | 60 | 48 | 53 | 7.5 | 55 | 46.5, 59 |
| MRC LL | 40 | 48 | 33 | 8.7 | 37 | 28.5, 40 |
| MRC neck | 10 | 48 | 9 | 1.2 | 10 | 8.5, 10 |
| Respiratory muscle strength | ||||||
| Tw Pdi, cm H2O | >18 | 77 | 15.0 | 12.7 | 9.9 | 5.7, 23.9 |
| Sn Pdi, cm H2O | >70 | 77 | 57.6 | 43.3 | 47 | 19.7, 88.9 |
| Sn Pes, cm H2O | >60 | 77 | 54.0 | 32.3 | 45 | 31.9, 71 |
| MIP, cm H2O | — | 75 | 36.2 | 26.0 | 28 | 18, 47 |
| MIP, % predicted | — | 75 | 43.6 | 29.2 | 35 | 21.2, 63 |
| MEP, cm H2O | — | 75 | 48.6 | 30.1 | 39 | 28, 67 |
| MEP, % predicted | — | 75 | 40.7 | 22.2 | 41.35 | 22.2, 58 |
| VC, % predicted | — | 74 | 71.5 | 27.6 | 68.5 | 50, 97.8 |
| SNIP, cm H2O | >60 | 62 | 51.0 | 31.7 | 44 | 27.7, 66 |
| SNIP, % predicted | — | 62 | 52.8 | 30.8 | 44.85 | 27.9, 76.75 |
| Cough Pga, cm H2O | — | 75 | 91.8 | 61.0 | 83 | 42.25, 121.8 |
| Tw T10, cm H2O | — | 70 | 15.9 | 14.0 | 12 | 5.7, 22 |
Definition of abbreviations: ALS FRS = Amyotrophic Lateral Sclerosis Functional Rating Scale; Cough Pga = cough gastric pressure; LL = lower limb; MEP = maximal static expiratory mouth pressure; MIP = maximal static inspiratory mouth pressure; MRC = Medical Research Council; Q1 = quartile 1; Q3 = quartile 3; SNIP = sniff nasal inspiratory pressure; Sn Pdi = sniff trans-diaphragmatic pressure; Sn Pes = sniff esophageal pressure; Tw Pdi = twitch trans-diaphragmatic pressure; Tw T10 = twitch abdominal pressure elicited by magnetic stimulation of the 10th thoracic intervertebral space; UL = upper limb.
Three individuals scored zero for some of the respiratory muscle strength assessments because of an inability to perform the maneuver.
Predictive Value of Respiratory Muscle Strength Tests for Death and NIV
A stepwise regression analysis was performed in all patients who had complete data (n = 57) for all tested variables (Tw Pdi, Sn Pdi, Sn Poes, SNIP, VC, MIP, MEP, Cough Pga, age, and sex).
In the stepwise regression analysis for ventilation-free survival (Table 2), all respiratory muscle strength assessments achieved statistical significance (P < 0.05) in their individual test scores, whereas no significance was observed for sex (P = 0.83) or age (P = 0.81). Sn Pdi and Tw Pdi had the highest values in the individual test scores, whereas the lowest values were registered for age and sex. In the final multivariate analysis model for ventilation-free survival, five variables retained significance: (1) age (P = 0.0027), (2) Tw Pdi (P < 0.0001), (3) Sn Pdi (P = 0.0025), (4) Sn Poes (P = 0.0275), and (5) MEP % predicted (P = 0.0544) (Table 3).
Table 2.
Stepwise Regression for Ventilation-Free and Absolute Survival: Individual Score Test Results
| Ventilation-Free Survival |
Absolute Survival |
|||
|---|---|---|---|---|
| Chi-Square | P Value | Chi-Square | P Value | |
| Age, yr | 0.0556 | 0.8136 | 2.5544 | 0.1100 |
| Sex | 0.0458 | 0.8305 | 0.1746 | 0.6761 |
| Tw Pdi, cm H2O | 47.9799 | <0.0001 | 22.3576 | <0.0001 |
| Sn Pdi, cm H2O | 54.3358 | <0.0001 | 21.3279 | <0.0001 |
| Sn Pes, cm H2O | 29.6091 | <0.0001 | 20.0359 | <0.0001 |
| SNIP, cm H2O | 30.1857 | <0.0001 | 19.7347 | <0.0001 |
| SNIP, % predicted | 34.5232 | <0.0001 | 20.1526 | <0.0001 |
| VC, % predicted | 32.6306 | <0.0001 | 16.8896 | <0.0001 |
| MIP, cm H2O | 21.9363 | <0.0001 | 15.1205 | 0.0001 |
| MIP, % predicted | 24.0194 | <0.0001 | 14.3513 | 0.0002 |
| Cough Pga, cm H2O | 15.4459 | <0.0001 | 16.6363 | <0.0001 |
| MEP, cm H2O | 22.6263 | <0.0001 | 19.5375 | <0.0001 |
| MEP, % predicted | 30.7708 | <0.0001 | 19.0626 | <0.0001 |
Definition of abbreviations: Cough Pga = cough gastric pressure; MEP = maximal static expiratory mouth pressure; MIP = maximal static inspiratory mouth pressure; SNIP = sniff nasal inspiratory pressure; Sn Pdi = sniff trans-diaphragmatic pressure; Sn Pes = sniff esophageal pressure; Tw Pdi = twitch trans-diaphragmatic pressure.
Table 3.
Stepwise Regression for Ventilation-Free and Absolute Survival: Final Multivariate Analysis Model of Maximum Likelihood Estimates
| Parameter | Parameter Estimate | Standard Error | Chi-Square | P Value | Hazard Ratio | 95% CI of Hazard Ratio |
|---|---|---|---|---|---|---|
| Ventilation-free survival |
||||||
| Age, yr | −0.06332 | 0.02112 | 8.9861 | 0.0027 | 0.939 | 0.901–0.978 |
| Tw Pdi, cm H2O | −0.13279 | 0.03104 | 18.3039 | <0.0001 | 0.876 | 0.824–0.931 |
| Sn Pdi, cm H2O | −0.03014 | 0.00996 | 9.1576 | 0.0025 | 0.970 | 0.952–0.989 |
| Sn Pes, cm H2O | 0.02091 | 0.00949 | 4.8578 | 0.0275 | 1.021 | 1.002–1.040 |
| MEP, % predicted | −0.02138 | 0.01111 | 3.7003 | 0.0544 | 0.979 | 0.958–1.000 |
| Absolute survival |
||||||
| Tw Pdi, cm H2O | −0.03754 | 0.01201 | 9.7724 | 0.0018 | 0.963 | 0.941–0.986 |
| MEP, cm H2O | −0.01529 | 0.00570 | 7.1953 | 0.0073 | 0.985 | 0.974–0.996 |
Definition of abbreviations: CI = confidence interval; MEP = maximal static expiratory mouth pressure; Sn Pdi = sniff trans-diaphragmatic pressure; Sn Pes = sniff esophageal pressure; Tw Pdi = twitch trans-diaphragmatic pressure.
In the stepwise regression analysis for absolute survival (Table 2), each respiratory muscle strength assessment achieved statistical significance (P < 0.05) in their individual score tests, whereas no significance was observed for sex (P = 0.68) or age (P = 0.11). The highest values in the individual score tests were observed for Sn Pdi and Tw Pdi, whereas the lowest values were registered for sex and age. In the final analysis model for absolute survival, two variables retained significance: Tw Pdi (P = 0.0018) and MEP (P = 0.0073) (Table 3).
The sensitivity and specificity of the key noninvasive (VC, SNIP, MIP, and MEP) and invasive (Tw Pdi and Sn Pdi) respiratory muscle strength measures to predict death or the use of NIV as a function of time before that event and at time points up to 3 years are shown in Tables 4 and 5, and Table E2 in the online supplement for the MEP. The cutoff values and area under the curve, identified from the ROC analysis, as a function of time before that event and at time points up to 3 years are shown in Figures 1 and E1, respectively. ROC analyses showed area under the curve greater than 0.8 at most time points for all tests, but the numerical range of cutoff points varied. It should be noted that although all measures had good to excellent specificity, there were widely differing sensitivities for the prediction of death or ventilator use. The VC value that gave the best cutoff point was higher than 80% predicted at all time points, except for outcome prediction at 3 months.
Table 4.
Receiver Operating Characteristic Analysis for Key Respiratory Muscle Strength Parameters up to 3 Years of Follow-up for Ventilation-Free Survival: Noninvasive Respiratory Muscle Strength Parameters
| Projected Time (mo) | Vital Capacity (% Predicted) |
Sniff Nasal Inspiratory Pressure (% Predicted) |
Maximal Static Inspiratory Mouth Pressure (% Predicted) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff | AUC | Sensitivity | Specificity | Cutoff | AUC | Sensitivity | Specificity | Cutoff | AUC | Sensitivity | Specificity | |
| 3 | 78 | 0.837 | 0.62 | 0.96 | 49 | 0.877 | 0.61 | 0.95 | 51 | 0.731 | 0.46 | 0.96 |
| 6 | 81 | 0.894 | 0.61 | 0.97 | 52 | 0.859 | 0.55 | 0.95 | 54 | 0.796 | 0.49 | 0.97 |
| 9 | 85 | 0.906 | 0.63 | 0.97 | 71 | 0.865 | 0.50 | 0.96 | 64 | 0.813 | 0.44 | 0.97 |
| 12 | 88 | 0.892 | 0.61 | 0.97 | 71 | 0.847 | 0.53 | 0.96 | 64 | 0.822 | 0.46 | 0.97 |
| 15 | 105 | 0.848 | 0.37 | 0.98 | 82 | 0.846 | 0.48 | 0.97 | 83 | 0.807 | 0.27 | 0.98 |
| 18 | 105 | 0.868 | 0.37 | 0.96 | 82 | 0.845 | 0.52 | 0.97 | 83 | 0.844 | 0.30 | 0.98 |
| 24 | 106 | 0.854 | 0.41 | 0.98 | 85 | 0.865 | 0.55 | 0.98 | 84 | 0.849 | 0.32 | 0.98 |
| 36 | 111 | 0.883 | 0.46 | 0.98 | 88 | 0.981 | 0.83 | 0.98 | 93 | 0.909 | 0.31 | 0.98 |
Definition of abbreviation: AUC = area under the curve.
Data for the maximal static expiratory mouth pressure are shown in Table E2.
Table 5.
Receiver Operating Characteristic Analysis for Key Respiratory Muscle Strength Parameters up to 3 Years of Follow-up for Ventilation-Free Survival: Invasive Respiratory Muscle Strength Parameters
| Twitch Trans-diaphragmatic Pressure (cm H2O) |
Sniff Trans-diaphragmatic Pressure (cm H2O) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Projected Time (mo) | Cutoff | AUC | Sensitivity | Specificity | Cutoff | AUC | Sensitivity | Specificity |
| 3 | 9.8 | 0.913 | 0.78 | 0.96 | 47.1 | 0.926 | 0.74 | 0.96 |
| 6 | 17.0 | 0.919 | 0.60 | 0.97 | 47.1 | 0.951 | 0.82 | 0.97 |
| 9 | 17.0 | 0.962 | 0.69 | 0.97 | 55.0 | 0.976 | 0.85 | 0.97 |
| 12 | 18.6 | 0.943 | 0.70 | 0.98 | 77.2 | 0.963 | 0.65 | 0.98 |
| 15 | 26.1 | 0.943 | 0.47 | 0.98 | 89.0 | 0.950 | 0.60 | 0.98 |
| 18 | 26.1 | 0.938 | 0.52 | 0.98 | 89.0 | 0.952 | 0.67 | 0.98 |
| 24 | 28.4 | 0.922 | 0.45 | 0.98 | 103.0 | 0.933 | 0.59 | 0.98 |
| 36 | 28.4 | 0.958 | 0.77 | 0.98 | 108.5 | 0.978 | 0.85 | 0.98 |
Definition of abbreviation: AUC = area under the curve.
Data for the maximal static expiratory mouth pressure are shown in Table E2.
Figure 1.
Cutoff values of the respiratory muscle strength parameters with greatest sensitivity for prediction of time to death or noninvasive ventilation. Graphical analysis showing the cutoff point identified from the receiver operating characteristic analysis for the prediction of death or noninvasive ventilation for the key respiratory muscle strength parameters as a function of time before that event. MEP = maximal static expiratory mouth pressure; MIP = maximal static inspiratory mouth pressure; SNIP = sniff nasal inspiratory pressure; Sn Pdi = sniff trans-diaphragmatic pressure; Tw Pdi = twitch trans-diaphragmatic pressure.
Figure 2 shows the Kaplan-Meier survival curves for the three principle techniques (VC, SNIP, and MIP) used for noninvasive assessment of inspiratory muscle strength. For each parameter, the participants were categorized into the following subgroups: greater than or equal to 80% predicted (i.e., within the normal range), 45% to less than 80% predicted, and less than 45% predicted. Although, a result within the normal range was associated with the longest survival for all three noninvasive inspiratory muscle strength parameters, VC discriminated less between moderate and severe weakness compared with SNIP and MEP.
Figure 2.
Kaplan-Meier analysis (for the composite endpoint of death or noninvasive ventilation use) for the (A) VC, (B) sniff nasal inspiratory pressure, and (C) maximal static inspiratory mouth pressure. The participants were categorized into three subgroups: greater than or equal to 80% predicted (i.e., within the normal range), 45 to less than 80% predicted, and less than 45% predicted. The P values of log-rank tests of equality over subgroups are all less than or equal to 0.0001.
Sequential measures of respiratory muscle strength were obtained for only 25 participants on 2–11 occasions either because it was impractical for most of the visits or the patient declined to participate. Figure 3 shows the serial behavior of respiratory muscle strength parameters in those 25 patients in whom serial measures were available. The VC declined slowly until 12 months before death, followed by a rapid decline until death. In contrast, a more linear pattern was observed for direct measures of respiratory muscle strength, including SNIP (% predicted), Sniff Pdi (cm H2O), Twi pdi (cm H2O), MIP (% predicted), and MEP (% predicted).
Figure 3.
Longitudinal respiratory muscle strength data in a subset of patients for whom sequential measures were available (n = 25) for the (A) VC, (B) sniff nasal inspiratory pressure (SNIP), (C) twitch trans-diaphragmatic pressure (Twi Pdi), (D) sniff trans-diaphragmatic pressure (Sniff Pdi), (E) maximal static inspiratory mouth pressure (MIP), (F) maximal static expiratory mouth pressure (MEP), and (G) gastric pressure during maximal cough (Cough Pga). Each series of dots represents the trajectory of this parameter for an individual patient. NIV = noninvasive ventilation.
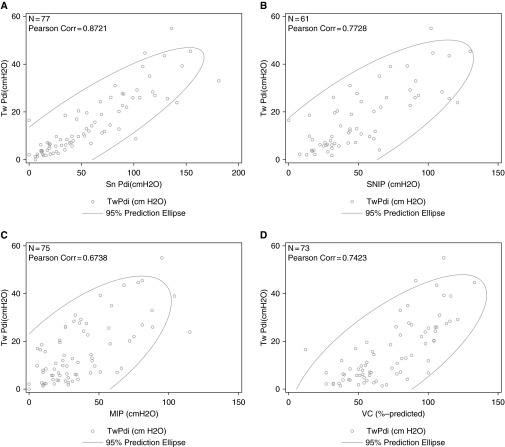
A correlation of Tw Pdi with Sn Pdi and with noninvasive measures of respiratory muscle strength, including MIP, SNIP, and VC, was observed (Figure 4).
Figure 4.
The correlation of twitch trans-diaphragmatic pressure (Twi Pdi) with (A) sniff trans-diaphragmatic pressure (Sn Pdi), (B) sniff nasal inspiratory pressure (SNIP), (C) maximal static inspiratory mouth pressure (MIP), and (D) VC.
Discussion
For most conditions, trials powered to detect improvements in survival are expensive. This may delay the evaluation and introduction of novel drugs or technologies aiming to reduce mortality. Therefore biomarkers, either as a surrogate outcome or as a stratification tool, would be useful to enrich trials for those at high risk of meeting the study endpoint.
Critique of the Method
Each respiratory muscle strength assessment individually achieved statistical significance for prediction of death or ventilator-free survival. These findings are in line with previous studies, reporting that respiratory muscle strength measures are strong predictors of death or the need to use NIV. Our study did not evaluate all tests of respiratory muscle function or questionnaires, such as the SINQ-5 (18); in particular supine VC has been identified as a sensitive marker of isolated diaphragm dysfunction in ALS (19). This test is attractive in the sense that spirometry is widely available but we caution that many patients with ALS cannot easily transfer onto a testing couch especially as the condition progresses, and its prognostic value remains unknown. Other techniques of reported value for assessment of respiratory muscle function in ALS include measurement of compound muscle action potential elicited by phrenic nerve stimulation (10, 20), but because this was not measured in the current study we are unable to comment on its value in comparison with measurement of force as Pdi.
We also acknowledge that our analysis is strictly confined to indices of respiratory muscle function. Thus, we have deliberately not sought to confirm whether a bulbar presentation is associated with a poor prognosis, which has been explored in larger studies. Similarly nocturnal hypoventilation, which is known to be associated with a poor prognosis, was not explored in this analysis, at least in part because most authorities now consider this to be an indication for NIV. Finally, we are aware that sequential measures, particularly when used in conjunction with sophisticated modeling techniques as described by Carreiro and coworkers (21), may offer an alternative predictive strategy. However, particularly for the purposes of recruiting to clinical trials, or counseling patients, it is often preferable to base a decision on a single measurement.
The current cohort is not sufficiently powerful to dissect factors predictive of death once NIV has been established. Even if it had been, it could not have accommodated other aspects of ventilator support that are culturally influenced (22), such as the use of 24-hour tracheostomy ventilation (T-IPPV). It is anecdotally accepted that patients with ALS receiving T-IPPV and enteral feeding live longer and may die for reasons other than ALS (23); unsurprisingly, the overall burden of disease involvement may be predictive in T-IPPV users (24).
Although the cohort is, to our knowledge, the largest ALS cohort to have had invasive measures of respiratory muscle strength, only limited serial noninvasive respiratory muscle strength data were available.
The choice of death or NIV use as endpoint requires defense, especially because the patients were studied when NIV was being introduced as a therapeutic option for ALS in the United Kingdom. Although all patients at the King’s Centre were considered for NIV as appropriate, this may not have applied to other referring centers. This issue was confirmed by a pooled analysis of 2,477 patients with ALS participating in three (negative) therapeutic trials reported between 2004 and 2006 (25), a similar period to the time at which the respiratory muscle strength measurements were made in the current study. Unsurprisingly, variation in ventilation practice was reported between countries and at different institutions within the same country, with ventilator interventions ranging from 0 to 23.1%. Because of this variation, a larger sample size was required for the study of composite endpoints compared with death alone. However, the practical arguments in favor of the composite endpoint of death or ventilation from a drug development perspective are first that ventilation itself represents a considerable healthcare burden and second that, because ventilation itself increases longevity (2), the use of NIV might obscure a true treatment effect of a novel therapeutic agent.
Significance of the Findings
Although respiratory muscle weakness is known as a poor prognostic feature in ALS (4–7) and other neuromuscular disorders, such as the muscular dystrophies or Pompe disease, there are no comprehensive data that compare the prognostic power of different tests of inspiratory and expiratory muscle function at different time intervals in any condition. Therefore, the current study assessed the value of noninvasive and invasive respiratory muscle strength assessments, at different time points up to 3 years, for the prediction of death or the initiation of NIV in a large cohort of patients with ALS (n = 78) (10, 19–21).
Sn Pdi and Twi Pdi were the best performing tests for prediction of ventilator-free survival, but SNIP also had excellent predictive power. Although VC also had good predictive power for ventilation-free survival, the cutoff value indicating a poor prognosis was within the normal range (i.e., >80% predicted) for all time intervals beyond 3 months, making this a less useful stratification variable for most studies. In addition, in a longitudinal subset of our cohort the inferiority of VC for enrichment against an endpoint of ventilator-free survival was observed. This is in line with findings from a pooled analysis (in 2,477 patients with ALS) reporting that relatively high VC values may be observed close to a clinical meaningful event (e.g., tracheostomy) (25). In that study, VC measurements were available for 50 patients with ALS requiring tracheostomy within 30 days of the need for tracheostomy, and were 50% or more of the predicted value in 11 patients, 60% or more of the predicted value in seven patients, and 70% or more of the predicted value in five patients. Combined, these data suggest that VC is a poor tool for assessing change until the period immediately before death, in contrast to Sn Pdi, Twi Pdi, and SNIP, for which linear declines were observed in the longitudinal cohort.
In the multivariate analysis for absolute survival, Tw Pdi and MEP reached statistical significance. This confirms for the first time a modest, but measurable impact of expiratory muscle function on survival in ALS. The predictive effect of MEP was substantially weaker than the predictive value of inspiratory muscle strength assessments, but was retained in multivariate analysis, and is therefore not likely to be an epiphenomenon. It has been previously reported that a profound expiratory muscle weakness is associated with the inability to generate transient supramaximal flow during a cough (26), which has been hypothesized to reduce defense against respiratory tract infection and aspiration. Impaired survival has been reported after NIV in those with mucous accumulation (27); this perhaps points to the inability to satisfactorily expectorate mucous. The attenuation of the effect, considering ventilator free-survival, was likely caused by the benefits of NIV in reducing the impact of chest infection both by improving blood gases but also by preventing basal atelectasis. This observation strengthens the case for further study of devices that facilitate expectoration of sputum (28, 29).
The Tw Pdi may be considered a gold standard, because it is independent of patient motivation or tester aptitude (14). Our data confirm the expected relationship between volitional and noninvasive tests of inspiratory muscle function and the Tw Pdi. Although Tw Pdi and Sn Pdi may be considered direct measures of diaphragm strength, Sn Poes and SNIP reflect overall inspiratory muscle strength; while the diaphragm does constitute the majority of inspiratory muscle function in normal humans it is clear that Sn Poes and Sn Pdi are not therefore measuring exactly the same quantity. In that context Table 3 is of interest because it is noted that although much the strongest association with ventilation-free survival was achieved by Tw Pdi, the associations with Sn Pdi and Sn Poes were of opposite polarity so that whereas in univariate analysis a reduced Sn Poes was associated with reduced survival, in multivariate analysis an increased Sn Poes was statistically associated with a slightly increased risk of death or ventilation. We suspect this paradox occurs because disease impact on the nondiaphragmatic muscles (which is what Sn Poes captures once Pdi has been stratified for during the process of multivariate analysis) is a marker of wider regional involvement.
In conclusion, the value of respiratory muscle strength as a biomarker is confirmed by the current analysis. By providing detailed sensitivity and specificity predictions, the present data can be used to permit smaller sample sizes in future ALS trials and thus to permit a more rapid sorting of treatments into those with benefit and those without. Although VC has a good sensitivity, the cutoff points identified by ROC analysis were largely within the normal range and our longitudinal data confirmed that change in VC was of small amplitude until close to death or the need for NIV. Our data suggest that direct measurement of respiratory muscle strength may be a more useful biomarker.
Acknowledgments
Acknowledgment
The authors thank Ismar Healthcare (Lier, Belgium) for their support with editing of the manuscript, which was funded by BioMarin Pharmaceutical Inc.
Footnotes
Supported by a grant from the Muscular Dystrophy Association of America (for original data collection, R.A.L.), BioMarin Pharmaceutical Inc. (for current survival analysis), and the National Institute for Health Research Respiratory Biomedical Research Unit at the Royal Brompton and Harefield National Health Service (NHS) Foundation Trust and Imperial College, London (M.I.P.). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Author Contributions: All authors contributed to the concept of the study, critically reviewed the manuscript, and approved this submission for publication. R.A.L. made the original measurements and reviewed the source data to create the material for the present analysis. K.Y. and E.J. undertook statistical analysis of the survival data to provide the analysis results for this paper. M.I.P. reviewed the source data with R.A.L. to create the material for the present analysis and prepared the first and subsequent draft of the manuscript. P.N.L. and J.M. were supervisors for the neurologic and respiratory muscle aspects, respectively, of the original data collection.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201604-0848OC on August 5, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 2.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 3.Lyall RA, Donaldson N, Fleming T, Wood C, Newsom-Davis I, Polkey MI, Leigh PN, Moxham J. A prospective study of quality of life in ALS patients treated with noninvasive ventilation. Neurology. 2001;57:153–156. doi: 10.1212/wnl.57.1.153. [DOI] [PubMed] [Google Scholar]
- 4.Lyall RA, Donaldson N, Polkey MI, Leigh PN, Moxham J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain. 2001;124:2000–2013. doi: 10.1093/brain/124.10.2000. [DOI] [PubMed] [Google Scholar]
- 5.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–719. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- 6.Ringel SP, Murphy JR, Alderson MK, Bryan W, England JD, Miller RG, Petajan JH, Smith SA, Roelofs RI, Ziter F, et al. The natural history of amyotrophic lateral sclerosis. Neurology. 1993;43:1316–1322. doi: 10.1212/wnl.43.7.1316. [DOI] [PubMed] [Google Scholar]
- 7.Stambler N, Charatan M, Cedarbaum JM ALS CNTF Treatment Study Group. Prognostic indicators of survival in ALS. Neurology. 1998;50:66–72. doi: 10.1212/wnl.50.1.66. [DOI] [PubMed] [Google Scholar]
- 8.Rahn H, Otis AB, Chadwick LE, Fenn WO. The pressure-volume diagram of the thorax and lung. Am J Physiol. 1946;146:161–178. doi: 10.1152/ajplegacy.1946.146.2.161. [DOI] [PubMed] [Google Scholar]
- 9.De Troyer A, Borenstein S, Cordier R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax. 1980;35:603–610. doi: 10.1136/thx.35.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto S, Pinto A, de Carvalho M. Phrenic nerve studies predict survival in amyotrophic lateral sclerosis. Clin Neurophysiol. 2012;123:2454–2459. doi: 10.1016/j.clinph.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Mier A, Brophy C, Moxham J, Green M. Twitch pressures in the assessment of diaphragm weakness. Thorax. 1989;44:990–996. doi: 10.1136/thx.44.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Man WD, Moxham J, Polkey MI. Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur Respir J. 2004;24:846–860. doi: 10.1183/09031936.04.00029004. [DOI] [PubMed] [Google Scholar]
- 13.Brooks BR. El Escorial World Federation of Neurology Criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci. 1994;124:96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 14.Hamnegård CH, Wragg SD, Mills GH, Kyroussis D, Polkey MI, Bake B, Moxham J, Green M. Clinical assessment of diaphragm strength by cervical magnetic stimulation of the phrenic nerves. Thorax. 1996;51:1239–1242. doi: 10.1136/thx.51.12.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Similowski T, Fleury B, Launois S, Cathala HP, Bouche P, Derenne JP. Cervical magnetic stimulation: a new painless method for bilateral phrenic nerve stimulation in conscious humans. J Appl Physiol (1985) 1989;67:1311–1318. doi: 10.1152/jappl.1989.67.4.1311. [DOI] [PubMed] [Google Scholar]
- 16.Man WD, Kyroussis D, Fleming TA, Chetta A, Harraf F, Mustfa N, Rafferty GF, Polkey MI, Moxham J. Cough gastric pressure and maximum expiratory mouth pressure in humans. Am J Respir Crit Care Med. 2003;168:714–717. doi: 10.1164/rccm.200303-334BC. [DOI] [PubMed] [Google Scholar]
- 17.Kyroussis D, Mills GH, Polkey MI, Hamnegard C-H, Koulouris N, Green M, Moxham J. Abdominal muscle fatigue after maximal ventilation in humans. J Appl Physiol (1985) 1996;81:1477–1483. doi: 10.1152/jappl.1996.81.4.1477. [DOI] [PubMed] [Google Scholar]
- 18.Steier J, Jolley CJ, Seymour J, Teschler H, Luo YM, Polkey MI, Moxham J.Screening for sleep-disordered breathing in neuromuscular disease using a questionnaire for symptoms associated with diaphragm paralysis Eur Respir J 201137400–405 [DOI] [PubMed] [Google Scholar]
- 19.Lechtzin N, Wiener CM, Shade DM, Clawson L, Diette GB. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest. 2002;121:436–442. doi: 10.1378/chest.121.2.436. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins JA, Sakamuri S, Katz JS, Forshew DA, Guion L, Moore D, Miller RG. Phrenic nerve conduction studies as a biomarker of respiratory insufficiency in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:213–220. doi: 10.3109/21678421.2015.1112406. [DOI] [PubMed] [Google Scholar]
- 21.Carreiro AV, Amaral PM, Pinto S, Tomás P, de Carvalho M, Madeira SC. Prognostic models based on patient snapshots and time windows: predicting disease progression to assisted ventilation in amyotrophic lateral sclerosis. J Biomed Inform. 2015;58:133–144. doi: 10.1016/j.jbi.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Tagami M, Kimura F, Nakajima H, Ishida S, Fujiwara S, Doi Y, Hosokawa T, Yamane K, Unoda K, Hirose T, et al. Tracheostomy and invasive ventilation in Japanese ALS patients: decision-making and survival analysis: 1990-2010. J Neurol Sci. 2014;344:158–164. doi: 10.1016/j.jns.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi H, Oppenheimer EA. ALS patients on TPPV: totally locked-in state, neurologic findings and ethical implications. Neurology. 2003;61:135–137. doi: 10.1212/01.wnl.0000069925.02052.1f. [DOI] [PubMed] [Google Scholar]
- 24.Lo Coco D, Marchese S, La Bella V, Piccoli T, Lo Coco A. The amyotrophic lateral sclerosis functional rating scale predicts survival time in amyotrophic lateral sclerosis patients on invasive mechanical ventilation. Chest. 2007;132:64–69. doi: 10.1378/chest.06-2712. [DOI] [PubMed] [Google Scholar]
- 25.Gordon PH, Corcia P, Lacomblez L, Pochigaeva K, Abitbol JL, Cudkowicz M, Leigh PN, Meininger V. Defining survival as an outcome measure in amyotrophic lateral sclerosis. Arch Neurol. 2009;66:758–761. doi: 10.1001/archneurol.2009.1. [DOI] [PubMed] [Google Scholar]
- 26.Polkey MI, Lyall RA, Green M, Nigel Leigh P, Moxham J. Expiratory muscle function in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 1998;158:734–741. doi: 10.1164/ajrccm.158.3.9710072. [DOI] [PubMed] [Google Scholar]
- 27.Peysson S, Vandenberghe N, Philit F, Vial C, Petitjean T, Bouhour F, Bayle JY, Broussolle E. Factors predicting survival following noninvasive ventilation in amyotrophic lateral sclerosis. Eur Neurol. 2008;59:164–171. doi: 10.1159/000114037. [DOI] [PubMed] [Google Scholar]
- 28.Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. Eur Respir J. 2003;21:502–508. doi: 10.1183/09031936.03.00048102. [DOI] [PubMed] [Google Scholar]
- 29.Mustfa N, Aiello M, Lyall RA, Nikoletou D, Olivieri D, Leigh PN, Davidson AC, Polkey MI, Moxham J. Cough augmentation in amyotrophic lateral sclerosis. Neurology. 2003;61:1285–1287. doi: 10.1212/01.wnl.0000092018.56823.02. [DOI] [PubMed] [Google Scholar]