To the Editor:
Muscle wasting during critical illness has been suggested to contribute to survivor functional disability (1). Two B-mode ultrasound measures have been reported that quantify wasting (2, 3): (1) combined thickness of the rectus femoris (RF) and vastus intermedius muscles (“muscle layer thickness,” henceforth referred to as “thickness”) (4, 5); and (2) RF cross-sectional area (RFCSA), which correlates with lower-limb strength in other clinical circumstances (6). The degree to which either of these ultrasound measures reflect muscle weakness in the critically ill is unclear (7).
First, we hypothesized that, like change in RFCSA (ΔRFCSA), change in thickness (Δthickness) would underestimate loss of muscle size, as measured by the histological gold standard (myofiber thickness) and the biochemical gold standard of protein:DNA ratio measured in skeletal muscle biopsies. Second, we hypothesized that ΔRFCSA and Δthickness would both be related to muscle weakness.
Subjects were patients of the Musculoskeletal Ultrasound in Critical Illness: Longitudinal Evaluation study (NCT01106300) (8), the original study having been approved by University College London (London, UK) Ethics Committee A. All patients were recruited within 24 hours of admission to a university hospital and a community hospital (August 2009–April 2011) and were expected to survive intensive care unit (ICU) admission after being invasively ventilated for over 48 hours and in the ICU longer than 7 days. Excluded were those with pregnancy, lower-limb amputation, primary neuromuscular pathology, or disseminated cancer. Next-of-kin assent and retrospective patient consent were obtained.
Images were acquired on ICU Days 1, 7, and 10. ICU RFCSA assessment and reliability have been previously described (8). Thickness was measured at the midpoint of RF between the two fascial lines. Images were excluded if the femur was not visible.
ΔThickness and ΔRFCSA were compared with change in myofiber cross-sectional area (ΔfiberCSA) and protein:DNA in sequential vastus lateralis muscle biopsies acquired on Days 1 and 7, as described previously (8).
Manual muscle testing was performed (9) on Day 10 if patients could follow three or more of De Jonghe’s five command criteria, and a knee extension component score of 4/5 or less was used to define lower-limb weakness (10).
Bland-Altman comparisons were used to establish: (1) interrater reliability of thickness measurements; and (2) longitudinal bias between Δthickness and ΔRFCSA over the study period. Normality was assessed using D’Agostino and Pearson omnibus normality tests, and data were analyzed using two-tailed Student’s t test or Mann-Whitney U test, as appropriate. Differential longitudinal change in muscle size (Δthickness vs. ΔRFCSA) was compared using two-way repeated measures analysis of variance. A bivariable logistical regression was performed, with knee extensor weakness as the dependent variable and ultrasound measurements as the independent variable.
Of the initial cohort of 62 patients with serial muscle ultrasounds, 8 had incomplete or missing electronic scan records. Of the remaining 54, 11 had one scan or more in which the femur was not visualized. Two assessors analyzed images at 21 time points to establish interrater reliability. Thickness measurements were highly correlated between observers (A.S.M. and Z.A.P.: Pearson’s r = 0.98) with an intraclass coefficient of 0.986 (95% confidence interval [CI], 0.965–0.994). A Bland-Altman plot demonstrated minimal bias of −0.07 (±0.2) cm (95% CI, −0.46 to 0.32 cm).
Nineteen patients had thickness, RFCSA, fiberCSA and protein:DNA ratio measured on Day 1 and Day 7. ΔThickness significantly underestimated ΔfiberCSA (−4.6% [95% CI, −14.19 to 4.95] vs. −16.4% [95% CI, −32.0 to −0.74]; P = 0.025) and change in protein:DNA ratio (−4.6% [95% CI, −14.19 to 4.95] vs. −30.9% [95% CI, −51.2 to −10.6]; P = 0.019). We have previously shown ΔRFCSA to underestimate change in protein:DNA ratio (−10.3% [95% CI, −6.1 to −14.5] vs. −29.5% [95% CI, −13.4 to −45.6%]; P = 0.03), but not ΔfiberCSA (−10.3% [95% CI, −6.1 to −14.5] vs. −17.5% [95% CI, −5.8 to −29.3]; P = 0.31) (8).
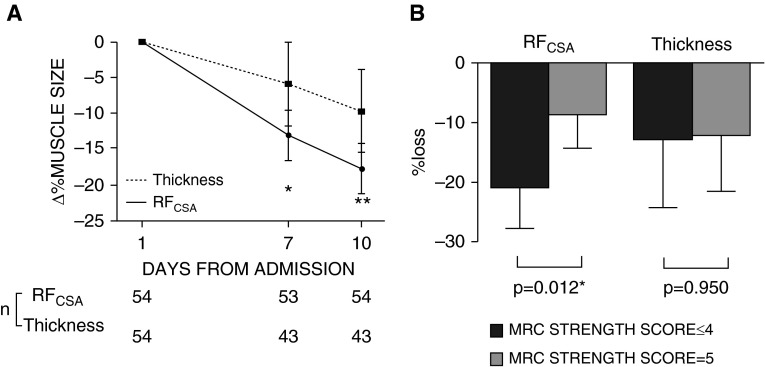
ΔThickness and ΔRFCSA correlated (r2 = 0.22, P = 0.049), but a Bland-Altman comparison between Δthickness and ΔRFCSA over 10 days revealed a bias of −8.3 (±19.7)% (95% CI, −46.7 to 30.7) for thickness, resulting in significant underestimation of muscle wasting at Days 7 and 10 (Figure 1A and Table 1).
Figure 1.
(A) Change in rectus femoris cross-sectional area (RFCSA) and muscle layer thickness over 10 days of critical illness. *P < 0.05 and **P < 0.01 using two-way repeated measures analysis of variance. (B) Knee Extensor Medical Research Council (MRC) Strength Score and loss of muscle size as measured by RFCSA and thickness (n = 27). *P < 0.05 using two-tailed unpaired Student’s t test. Data are presented as mean (95% confidence interval) (in B, the whiskers represent half of a symmetrical 95% confidence interval around the mean).
Table 1.
Comparison of Change in Muscle Limb Thickness and Rectus Femoris Cross-Sectional Area at Days 7 and 10 of Critical Illness
| ΔThickness (%) | ΔRFCSA (%) | P Value | |
|---|---|---|---|
| Day 7 | −5.88 (−11.69 to −0.06) | −13.0 (−16.52 to −9.48) | 0.031* |
| Day 10 | −9.36 (−15.43 to −3.84) | −17.72 (−21.15 to −14.29) | 0.004* |
Definition of abbreviations: ΔRFCSA = change in rectus femoris cross-sectional area; ΔThickness = change in muscle limb thickness.
P < 0.05 using two-way repeated measures analysis of variance.
Of the 63 patients, 40 were able to obey commands and underwent volitional strength testing on Day 10, among whom thickness was available in 27.
ΔRFCSA was greater in those with knee extensor weakness than those without (20.7% [95% CI, 13.7–27.7] vs. 8.4% [95% CI, 2.5–14.3], respectively; P = 0.012). ΔThickness did not differ between these groups (12.6% [95% CI, 0.94–24.2] vs. 12.1 [95% CI, 2.7–21.5], respectively; P = 0.95; Figure 1B). In a bivariable logistical regression, ΔRFCSA was associated with knee extensor weakness (odds ratio, 1.101 [95% CI, 1.011–1.199]; P = 0.027), but Δthickness was not (odds ratio, 1.001 [95% CI, 0.960–1.044]; P = 0.947).
All other things being equal, muscle strength and size are proportional—the latter acting as a proxy for the former in ICU, where nonvolitional objective measures of strength are logistically challenging. Our results suggest that ΔRFCSA reflects knee extensor weakness and muscle loss better than Δthickness. ΔThickness also underestimated ΔRFCSA (a −8% bias on Bland-Altman plot being relevant, given that a 10% change in RFCSA is considered sufficient to affect function [11])—in part, perhaps, because it is a unidimensional measure when compared with (two-dimensional) muscle area or (three-dimensional) volume. The specific relationship of tissue edema to ultrasound measures remains unclear (3, 8), although edema may also affect fiberCSA (12).
Although these data are derived from the largest cohort available for longitudinal radiopathological correlation, our study is limited by its size. The cohort size was further limited by one-third of patients not being able to perform volitional strength testing, albeit this was in keeping with published rates (13). Finally, measurement of thickness was not an original primary goal of image analysis, a fact that might account for the lack of femoral image availability in one-third of patients. Although considered unlikely to have impacted the observations made, nonrandom bias cannot be excluded.
We have previously shown RFCSA studies to indicate muscle quality (3) and not to underestimate muscle fiberCSA. We now show that thickness measurements significantly underestimate ICU muscle wasting compared with RFCSA. In addition, RFCSA is a more reliable proxy for muscle strength in a setting where volitional and nonvolitional muscle strength measurements are challenging. We suggest measurement of ΔRFCSA as a biomarker for proximal lower-limb muscle loss and knee extensor weakness during early critical illness.
Footnotes
Supported by the U.K. National Institute of Health Research (NIHR) (Z.A.P.), the Batchworth Charitable Trust, the Moulton Charitable Foundation, and the NIHR University College London Hospitals Biomedical Research Centre (UCLH BRC) (A.S.M.), the Research Councils UK (S.D.H.), the NIHR Clinical Research Facility and BRC at Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust (GSST) and King’s College London (N.H. and B.C.), and by University College London and UCLH BRC (H.E.M., Z.A.P., and A.S.M.). The NIHR doctorate fellowship (2010–2014) underpinned the core patient population study on which this follow-up was built, as did funding from the Batchworth Charitable Trust, Moulton Foundation, and European Society of intensive Care Medicine. Additional funding was received from the Whittington Hospital NHS Trust and the European Society of Intensive Care Medicine.
Author Contributions: Concept and design—Z.A.P., P.S.S., J.M., S.D.H., N.H., and H.E.M.; data collection—Z.A.P., A.S.M., J.R., B.C., and A.R.; analysis and interpretation—Z.A.P., A.S.M., B.C., P.S.S., A.R., J.M., S.D.H., N.H., and H.E.M.; manuscript drafting and revision—Z.A.P., A.S.M., B.C., P.S.S., A.R., J.M., S.D.H., N.H., and H.E.M.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 2.Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, Annoni R, Puthucheary Z, Gordon IR, Morris PE, Denehy L. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30:1151.e9–14. doi: 10.1016/j.jcrc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, Moxham J, Harridge S, Hart N, Montgomery HE. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43:1603–1611. doi: 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 4.Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23:273–280. doi: 10.1016/S0261-5614(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 5.Gruther W, Benesch T, Zorn C, Paternostro-Sluga T, Quittan M, Fialka-Moser V, Spiss C, Kainberger F, Crevenna R. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med. 2008;40:185–189. doi: 10.2340/16501977-0139. [DOI] [PubMed] [Google Scholar]
- 6.Seymour JM, Ward K, Sidhu PS, Puthucheary Z, Steier J, Jolley CJ, Rafferty G, Polkey MI, Moxham J. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 7.Connolly B, MacBean V, Crowley C, Lunt A, Moxham J, Rafferty GF, Hart N. Ultrasound for the assessment of peripheral skeletal muscle architecture in critical illness: a systematic review. Crit Care Med. 2015;43:897–905. doi: 10.1097/CCM.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 8.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 9.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 10.Connolly BA, Jones GD, Curtis AA, Murphy PB, Douiri A, Hopkinson NS, Polkey MI, Moxham J, Hart N. Clinical predictive value of manual muscle strength testing during critical illness: an observational cohort study. Crit Care. 2013;17:R229. doi: 10.1186/cc13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, Gosker HR, Schols AM, Moxham J, Polkey MI, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauptmann S, Klosterhalfen B, Weis J, Mittermayer C, Kirkpatrick CJ. Skeletal muscle oedema and muscle fibre necrosis during septic shock: observations with a porcine septic shock model. Virchows Arch. 1994;424:653–659. doi: 10.1007/BF00195781. [DOI] [PubMed] [Google Scholar]
- 13.Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15:R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]