The field of pulmonary medicine is now benefitting from a breathtaking revolution in the scientific understanding of signaling, metabolism, and cell and molecular biology. We are now collectively deciphering long-standing mysteries of medicine, and identifying more personalized targets for disease-modifying interventions. This progress is highlighted in the field of pulmonary vascular biology, a field that has seen remarkable success from molecular understanding of pathogenesis to the relatively rapid development and U.S. Food and Drug Administration (FDA) approval of more than 14 disease-modifying therapies. The early successes in this field highlight the importance of understanding basic biology and rapidly leveraging these discoveries into multicenter, randomized, placebo-controlled clinical trials, with evaluation of patient-centered outcomes. Partnerships between scientists, industry, academic clinical investigators, and the FDA, supported by rare disease regulatory incentives, have fueled this remarkable progress. The pulmonary arterial hypertension (PAH) field has clearly benefited from rapid translation of fundamental science to the bedside.
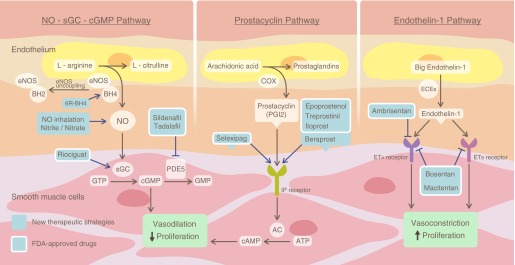
It is notable that the first-in-man therapies for PAH all targeted endothelial and smooth muscle vasoregulatory pathways, modulating vasoconstriction, redox (nitric oxide/reactive oxygen species) balance, and, to a much lesser extent, cellular proliferation, inflammation, thrombosis, metabolism, and more complex signaling hubs (1). The early drugs directly evolved from Nobel Prize–winning discoveries in the prostacyclin and nitric oxide (NO)–signaling cascades, highlighted by the development of intravenous, subcutaneous, oral, and inhaled prostacyclin analogs, as well as a newly approved small-molecule selexipeg, which target I-prostanoid receptors to increase intracellular cAMP levels (2). The NO-soluble guanylate cyclase–cyclic guanosine monophosphate pathway has similarly been targeted with the development of phosphodiesterase 5 inhibitors and small-molecule stimulators of the soluble guanylate cyclase enzyme, both of which increase smooth muscle cyclic guanosine monophosphate levels (1). The inhibition of endothelin-1 receptors A and B directly blocks vasoconstriction and indirectly promotes NO synthesis (2) (Figure 1).
Figure 1.
Current therapies for pulmonary arterial hypertension target prostacyclin, nitric oxide and endothelin-1 signaling pathways. U.S. Food and Drug Administration (FDA)-approved drugs and others in development are shown in the context of the canonical NO, prostacyclin, and endothelin-1 signaling pathways. Reproduced and modified with permission from Reference 2. 6R-BH4 = sapropterin dihydrochloride, tetrahydrobiopterin; AC = adenylyl cyclase; BH2 = dihydrobiopterin; BH4 = tetrahydrobiopterin; cGMP = cyclic guanosine monophosphate; COX = cyclooxygenase; ECEs = endothelin-converting enzymes; eNOS = endothelial NO synthase; ETA = endothelin receptor type A; ETB = endothelin receptor type B; GTP = guanosine-5′-triphosphate; IP = prostaglandin I2 (prostacyclin); PDE5 = phosphodiesterase 5; PGI2 = prostaglandin I2; sGC = soluble guanylyl cyclase.
Although these therapies have catalyzed efforts to diagnose and treat patients and improve patient quality of life, and, to a lesser extent, mortality, they do not cure the disease, and appear to have limited effects on the primary pathobiology, which we now appreciate involves major changes in endothelial and smooth muscle cell behavior, with the evolution of a glycolytic, apoptosis-resistant, and proliferative cellular phenotype, enhanced by a complex interplay of inflammation, fibrosis, and local hemostasis (3). Additional interplay of larger, stiffening, conducting vessels and right heart failure advance disease (4).
These challenges present opportunities for recent advances in fundamental science, particularly in the fields of cancer biology and metabolism, cell signaling networks, transcriptional regulation, systems biology, population genetics, and precision medicine. The fruits of these advances are already changing the practice of oncology and cardiology, in particular the speed with which a pathway is identified to FDA approval of a biological inhibitor, for example, a humanized antibody. Highlights include the immune checkpoint inhibitors that prime our immune system to attack cancer cells, and antibody inhibitors of proprotein convertase subtilisin/kexin type 9, a protein that controls cholesterol low-density lipoprotein levels that was discovered using population genetics to identify rare genomic variants that strongly modulate phenotype (5, 6).
In this review series, we introduce the readers to a number of the most exciting advances in science, explained clearly for both the novice and expert, and how these advances are impacting the patient with PAH. The areas covered will be relevant to most human lung diseases, and should be considered required knowledge for the physician and scientist. The reviews will cover four important areas:
-
1.
Past and present of molecular medicine of PAH, from population genetics to whole-genome sequencing and gene editing. Drs. Austin, West, Loyd, and Hemnes summarize how classical population genetic pedigree and association studies were used to identify the most common forms of hereditable PAH, and how new methods of whole-genome sequencing are accelerating the discovery of new, rare variants that cause PAH (7). The pathways identified, including the bone morphogenic protein (BMP) receptor type 2, endoglin, and activin A receptor type II–like 1, have highlighted the important role of transforming growth factor-β–BMP signaling in disease, as well as sex and other modifiers of receptor expression. The recent identification of mutations in the caveolin-1 and potassium channel two-pore domain subfamily K member 3 genes highlights the role of endothelial and smooth muscle vasoregualtory pathways. Identification of a new mutation leading to veno-occlusive disease, in the eukaryotic translation initiation factor 2 α kinase 4 gene, presents an opportunity to understand this rare disease. The identification of variants that can identify patients more likely to respond to specific drugs, like the endothelin-1 receptor antagonists, represents an example of precision medicine applied to the PAH field. Finally, the discovery of gene editing using the CRISPR-Cas9 and TALEN technologies is reviewed and how this might be used to target BMP receptor type 2 mutations in bone marrow followed by autologous stem cell transplantation.
-
2.
Focusing on developmental origins and disease inception for the prevention of pulmonary hypertension. Drs. Maron and Abman review how lung vascular development informs our understanding of disease pathogenesis and the identification of opportunities to screen for and diagnose “predisease” (8). New insights into primary and secondary prevention are considered, as well as new screening diagnostic technologies and approaches, particularly using exercise stress, and are being explored in patients.
-
3.
From cancer biology to new PAH therapeutics. Drs. Pullamsetti, Savai, Seeger, and Goncharova summarize new developments in our understanding of how the antiapoptotic, glycolytic, and proproliferative cellular phenotype evolves, and how critical signaling networks drive this process (9). Pathways central to metabolism and cancer are explored and now targeted as therapy for PAH. For example, hubs involving transcription factors mTORC, Akt, PI3K, FoxO, NFAT, and NF-kB are highlighted, in addition to dysregulated metabolic and mitochondrial signaling networks. Targeting these pathways has the potential to reverse more established disease, and many approved drugs used for cancer or immunosuppression are readily available for human use.
-
4.
Translating microRNA biology in pulmonary hypertension: it will take more than “miR” words. Drs. Chun, Bonnet, and Chan review how small noncoding microRNAs in the pulmonary vasculature and right ventricle can regulate gene transcription and control important signaling hubs that modulate disease progression (10). These signaling microRNAs are upstream of many of the classically dysregulated pathways in PAH. The authors will review how these microRNAs regulate gene expression, how they can be measured in circulating blood as biomarkers reflecting disease severity, and the principles of therapeutic targeting of microRNAs with antagomirs. The fundamental biology, signaling networks and current stratus of clinical development will all be discussed.
In conclusion, the field of pulmonary vascular biology continues to capitalize on profound advances in science and medicine. We anticipate that the scientific information shared with our readers in this series will rapidly translate into common clinical practice. This science will inform the basic language of medicine, analogous to how the PCR evolved from a simple, yet seemingly complicated, methodology for amplifying, identifying, and quantifying specific host and pathogen genes, to Nobel Prize stardom, and now the most common of diagnostic tools in our clinical arsenal.
Footnotes
Supported by National Institutes of Health grants 2R01HL098032, 1R01HL125886-01, P01HL103455, T32 HL110849, and T32 HL007563, and by the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 2.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res. 2014;115:115–130. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goncharova EA, Gladwin MT, Kawut SM. Update in pulmonary vascular diseases 2014. Am J Respir Crit Care Med. 2015;192:544–550. doi: 10.1164/rccm.201504-0829UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron BA, Gladwin MT, Simon MA. Update in pulmonary vascular disease 2015. Am J Respir Crit Care Med. 2016;193:1337–1344. doi: 10.1164/rccm.201601-0143UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 6.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 7.Austin ED, West J, Loyd JE, Hemnes AR. Molecular medicine of pulmonary arterial hypertension: from population genetics to precision medicine and gene editing. Am J Respir Crit Care Med. 2017;195:23–31. doi: 10.1164/rccm.201605-0905PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maron BA, Abman SH.Focusing on developmental origins and disease inception for the prevention of pulmonary hypertension Am J Respir Crit Care Med[online ahead of print] 17 Nov 2016DOI: 10.1164/rccm.201604-0882PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pullamsetti SS, Savai R, Seeger W, Goncharova EA.From cancer biology to new PAH therapeutics: targeting cell growth and proliferation signaling hubs Am J Respir Crit Care Med[online ahead of print] 14 Sept 2016DOI: 10.1164/rccm.201606-1226PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun HJ, Bonnet S, Chan SY.Translating microRNA biology in pulmonary hypertension: it will take more than “miR” words Am J Respir Crit Care Med[online ahead of print] 20 Sept 2016DOI: 10.1164/rccm.201604-0886PP [DOI] [PMC free article] [PubMed] [Google Scholar]