Summary
In Drosophila, the Apaf-1 related killer (Dark) forms an apoptosome that activates procaspases. To investigate function, we have determined a near atomic structure of Dark double rings using cryo-electron microscopy. We then built a nearly complete model of the apoptosome that includes 7- and 8-blade β-propellers. We find that the preference for dATP during Dark assembly may be governed by Ser325, which is in close proximity to the 2′ carbon of the deoxyribose ring. Interestingly, β-propellers in V-shaped domains of the Dark apoptosome are more widely separated, relative to these features in the Apaf-1 apoptosome. This wider spacing may be responsible for the lack of cytochrome c binding to β-propellers in the Dark apoptosome. Our structure also highlights the roles of two loss of function mutations that may block Dark assembly. Finally, the improved model provides a framework to understand apical procaspase activation in the intrinsic cell death pathway.
In Brief> ETOC
Cheng et al., present a structure of the Dark apoptosome at 4.4 Å resolution. A nearly complete atomic model clarifies dATP selectivity during nucleotide exchange, suggests a role for the β-propellers in assembly and provides a framework to understand apical procaspase activation

Introduction
An Apaf-1 related killer in Drosophila, known as Dark, binds dATP and assembles into an apoptosome, which activates procaspases in the intrinsic cell death pathway (Rodriguez et al., 1999; Zhou et al., 1999). Dark is essential for many cell deaths during development and for stress-induced apoptosis (Kanuka et al., 1999; Zhou et al., 1999; Rodriguez et al., 1999; Daish et al., (2004).; Srivastava et al., 2007; Mills et al., 2006). Apaf-1 and Dark are members of the signal transduction ATPases with numerous domains (STAND; Danot et al., 2009), a subgroup of the AAA+ ATPase super-family. However, these molecules do not couple nucleotide hydrolysis to mechanical work. Instead, family members undergo conformational changes during nucleotide exchange to form apoptosomes that activate procaspases (Yuan and Akey, 2013). Dark assembly in vitro creates a double ring comprised of two octameric platforms that pack face-to-face with D8 symmetry (Yu et al., 2006).
An N-terminal Caspase Recruitment Domain (CARD) in Dark is a member of the α-helical, Death Domain super-family. This Dark domain interacts with the N-terminal CARD of Dronc and recruits the apical procaspase to the apoptosome (Dorstyn et al., 1999; Pang et al., 2015). Dronc is activated and then cleaves DrICE, which functions as an executioner to proteolyze downstream targets (Chew et al., (2004).; Dorstyn & Kumar, 2008; Kumar, 2007). Dark molecules also contain a nucleotide binding domain (NBD), helical domain 1 (HD1) and winged helix domain (WHD) that are building blocks of the central hub (Pang et al., 2015; Yuan et al., 2011a). In addition, the NBD and HD1 form a nucleotide binding pocket that binds dATP, but not ATP, during nucleotide dependent assembly (Yu et al., 2006). Helical domain 2 (HD2) follows the WHD and extends from the hub to support a V-shaped domain comprised of tandem 7- and 8-blade β-propellers (Yu et al., 2006; Yuan et al., 2011a; Pang et al., 2015). In Apaf-1, these WD40 domains act as a sensor to bind cytochrome c (Yuan et al, 2010, 2013), which is released from mitochondria in response to cell death signals (Tait and Green 2010). However, bovine cytochrome c does not bind to the Dark apoptosome to mediate assembly in vitro (Yu et al., 2006), even though there is strong conservation with the insect homolog of the heme protein. In addition, cytochrome c is not required for Dronc activation (Dorstyn et al., 2002, 2004; Dorstyn and Kumar, 2008), but may play an as yet undefined role in some tissues (Arama et al., 2006; Mendes et al., 2006; Kornbluth and White, 2005).
In this paper, we report the structure of a Dark apoptosome determined at near atomic resolution with cryo-electron microscopy. However, a gradient of resolution is present in the 3D density map. Side chains are resolved in the central hub while peripheral β-propellers are imaged at lower resolution. Thus, we used a focused 3D classification in RELION (Scheres, 2012; Zhou et al., 2015) to improve the electron density map for the β-propellers. We then built a model of these domains with Drosophila sequences, based on a crystal structure of mouse Apaf-1 (Reubold et al. 2011). Beta-propellers in Dark have an altered geometry with a larger gap between them, relative to their conformation in the human apoptosome (Cheng et al., 2016; Zhou et al., 2015). This altered conformation may explain why the Dark apoptosome is unable to bind cytochrome c. Our analysis also suggests how relative affinities for dATP and ATP are established in Dark and Apaf-1 apoptosomes (Cheng et al., 2016). The Dark structure also reveals a novel interaction between the 7-blade β-propeller and the central hub, while providing a rationale for two loss of function mutations that may block assembly. Finally, a comparison of Drosophila and human apoptosomes suggests a general model for the activation of apical procaspases.
Results
Structure determination
In previous work, we resolved α-helices in a 3D structure of the Dark double ring, built homology models for the CARD, hub domains and HD2 arms, and proposed that the V-shaped domain is comprised of tandem 7- and 8-blade β-propellers (Yuan et al., 2011a). To provide further insights, we have determined a near atomic structure of the Dark apoptosome in the ground state. A sharpened map with a global resolution of ~4.4Å was obtained from ~17000 particles with movie frame alignment (Li et al., 2013), gold standard refinement with D8 symmetry, and corrections for residual motions and radiation damage with RELION (Scheres, 2012, 2014). An estimate of the local resolution (Kucukelbir et al., 2014) indicates that side chain resolution (3.5–4.0Å) is present in the hub, while β-propellers in the V-shaped domain have a lower resolution (7–10 Å; Figures S1A, S1B). This was confirmed by direct inspection of the map. We then used a focused 3D classification in RELION (Scheres 2012; Zhou et al., 2015) to improve our map of the V-shaped domain. This approach provided a local map of the β-propellers with a gold standard resolution of ~7.3 Å (Figure S1A).
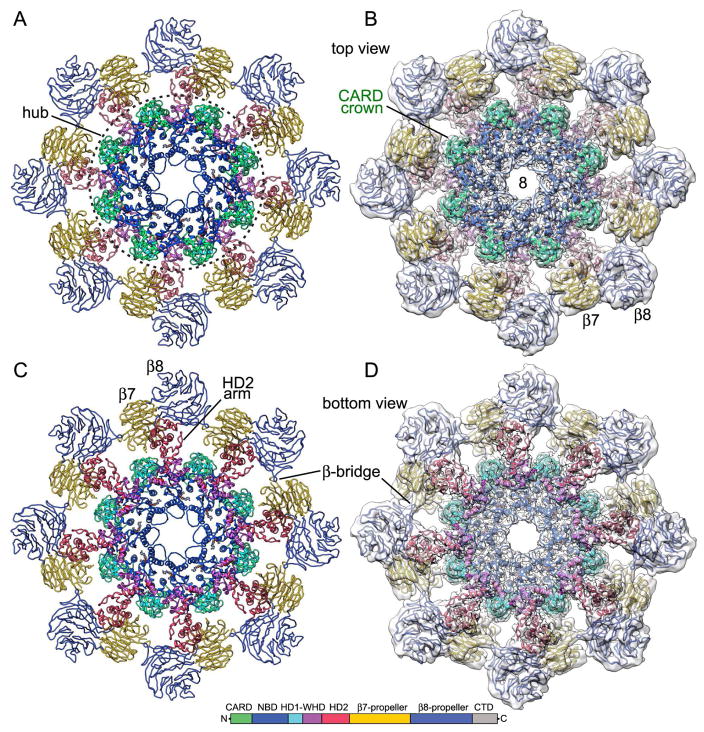
A previous model of the CARD, hub domains and HD2 (PDB 4V4L; Yuan et al., 2011a) was docked into the global 3D map with Chimera (Pettersen et al., (2004) and refined with molecular dynamics flexible fitting (MDFF; Trabuco et al., 2008), local rebuilding in Coot (Emsley et al., 2010), and real space refinement with PHENIX (Adams et al., 2010). To complete the analysis, tandem 7- and 8-blade β-propellers were modeled in the local map with similar domains from an Apaf-1 crystal structure (Reubold et al., 2011; see Table S1 for data collection and refinement statistics). A composite density map was constructed in Chimera by zoning around the CARDs, hub, and HD2, which were docked into the global map; the same approach was applied to β-propeller models docked into the local map of the V-shaped domain. The two density maps were then combined to create a final composite map. This map was rendered as an iso-surface to show top and oblique views of the double ring (Figurea 1A, 1B). In addition, top and bottom views of a single octagonal ring are shown (Figures 1C, 1D) and a Dark subunit is highlighted in all four panels of this figure (in grey). Top and bottom views of the final apoptosome model are shown as ribbons with color coded subunit domains, both without (Figures 2A, 2C) and with the density map (Figures 2B, 2D). In the model, NBD, HD1 and WHD interact with adjacent subunits to form the central hub, while N-terminal CARDs in each subunit sit on top of their respective NBDs to form a crown. The HD2 domains from each subunit extend from the central hub to support β-propellers in the V-shaped domain.
Figure 1. Overview of double and single Dark rings.
(A) A top view of the combined 3D density map is shown with the top ring in blue, the bottom ring in yellow and a single Dark subunit in grey. V-shaped domains are from a map obtained with focused 3D classification at ~7.3 Å resolution. (B) An oblique view is shown of the composite 3D density map. (C, D) Top and bottom views of a single octagonal ring are shown. A V-shaped domain with β-propellers (β7, β8) is indicated.
Figure 2. Ribbon model of a single Dark ring.
(A, B) A top view is shown of a single ring, without and with and the composite 3D density map. Dark domains are color-coded (see color key). The central hub is located within a dashed circle and the CARD crown is indicated. (C, D) Bottom views of the final model are shown and distinctive features are labeled.
The overall fit for the CARDs, central hub domains and HD2 arms was quantitated with a model vs map FSC curve. This gave an FSC0.5 value of 4.3 Å that is similar to the gold standard FSC0.143 value for this map region (4.2 Å) (Figures S1B, S1A; green curves). We also constructed a sequence alignment in Chimera (Pettersen et al., 2004) for Dark, Apaf-1 and CED-4 apoptosomes with annotations for secondary structure and pertinent features (Figure S2). Interactions of a Dark subunit within a single ring are shown in Figure S3, along with the extended monomer. We aligned our model with a recent Dark structure determined at near atomic resolution (Pang et al., 2015; PDB 3J9L), and this gave an r.m.s.d. of 2.3 Å between Cα’s after excluding the β-propellers. The r.m.s.d. of domains ranged from 1.14 to 2.81 with the largest difference in the HD2 arms, which display a bit of flexibility. A similar comparison with an earlier model (Yuan et al., 2011a; PDB 4V4L) gave an r.m.s.d. of 3.4 Å, which provides a benchmark for the accuracy of homology modeling with a density map at α-helical resolution (~7 Å).
Subunit structure and interactions
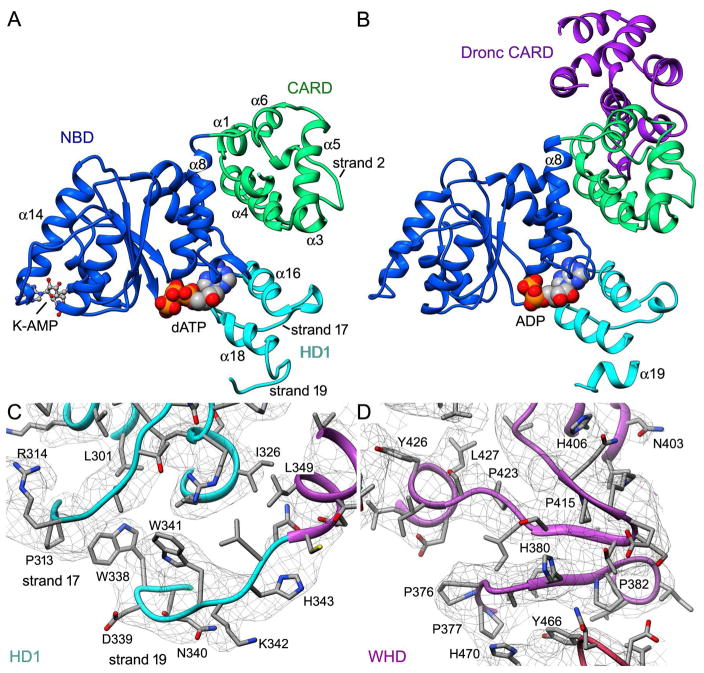
Our structure reveals a number of domain modifications that deviate from previous models (Yuan et al., 2011a, 2010; Qi et al., 2010; Riedl et al., 2005; Reubold et al., 2011). In particular, the fold of the Dark CARD has 5 α-helices rather than 6, with the canonical second helix (denoted α2) replaced with a strand (Val24-Met28) (Figure 3A; Figure S2). However, this alteration does not prevent binding by the Dronc CARD to form a post-activation complex (Figure 3B; Pang et al., 2015). Moreover, Dark CARD-NBD interactions remain intact in this complex. The Dark CARD is linked to helix α8 in the NBD with a short, 4 residue loop and the interaction between these domains is stabilized by hydrogen bonds (Thr94-Gln98, Ser17-Try106) and a salt bridge (Asp14-Arg110). An interaction network is also present between residues in the CARD (His47, Arg60) and HD1 (Asp295 and Glu296) to maintain the relative position of these domains.
Figure 3. Novel domain features in the Dark apoptosome.
(A,B) The CARD-NBD-HD1 module within the central hub is shown in the absence (panel A; this work) and presence of bound Dronc CARD (panel B; PDB 39JK; Pang et al., 2015). Three predicted α-helices have been replaced with extended strands as indicated in our model (panel A). For the structure in panel B, Dark was forced to assemble with a bound nucleotide diphosphate (modeled as ADP) by the addition of a Dronc CARD. (C) Strands 17 and 19 in HD1 have replaced α-helices that are present in other family members and are shown in the density map. (D) The signature β-hairpin in the WHD is shown (Ser409-Q414) as part of a small β-sheet that is dominated by 5 prolines (Pro376, 377, 382, 415, and 423).
A second structural change has occurred in HD1 which packs against the NBD to form the nucleotide binding pocket. The HD1 in other structures contains four α-helices (α16-α19) (Riedl et al., 2005; Qi et al. 2010; Cheng et al. 2016; Zhou et al., 2015). However, in Dark two helices in HD1 (α16, α18) pack against the NBD in close proximity to dATP in the Dark apoptosome, while two predicted helices (α17, α19) form strands located on the outside of the domain (Figures 3A, 3C). Strand 17 is delimited by Pro309, Pro313 and Pro321, while strand 19 contains Trp338 and Trp341, which stabilize a turn. The ensuing WHD interacts with both HD1 and NBD, mediated in part by helix α20 which follows strand 19. The winged helix domain gets its name from a β-hairpin (residues 411–423) that forms part of a small 3-stranded β-sheet (Figure 3D).
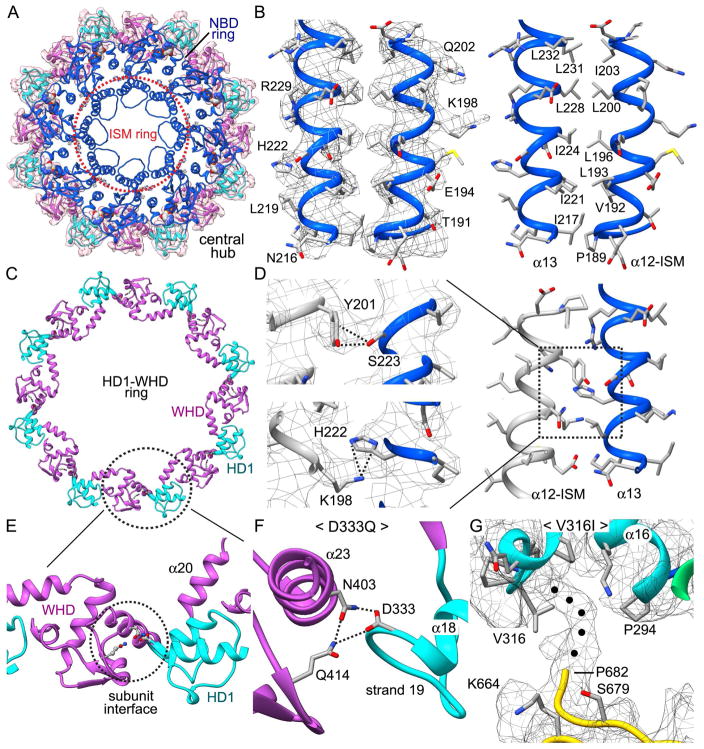
Correct assembly of the Dark apoptosome is an important step in Dronc activation. Our structure allows us to describe interactions that stabilize the association of Dark subunits within a single ring. In the hub, lateral interactions between adjacent NBDs form an inner ring, while inter-monomer contacts between WHD and HD1 create an outer ring (Figures 4A, 4C). An insert in the NBD, known as the initiator specific motif (ISM, Danot et al., 2009) is part of a conserved helix-loop-helix structural motif (Yuan et al., 2010; 2011a). In particular, the α12 and α13 helices interact through an extensive hydrophobic zipper (Figure 4B), which is conserved in the human apoptosome (Cheng et al., 2016). These helices also form lateral interactions with symmetry mates to form the ISM ring, which lines the central pore of the NBD ring (Figures 4A, 4D). Interestingly, helix α13 makes three contacts to helix α12 in the adjoining subunit with a hydrogen bond between Asn216-Glu194 (not shown), a putative π-cation interaction (His222-K198; Figure 4D, bottom left panel) and a novel hydrogen-bond between Tyr201-Ser223 (Figure 4D, top left panel). In the latter, the side chain OH of Ser223 points directly towards the aromatic ring of Tyr201 while the OH on the tyrosine ring is positioned to donate a proton back to the side chain oxygen of Ser223. In positioning the NBD within the central ring, a three-way interaction is present between Asp14 in the CARD and the NBD of the neighboring subunit (Asp111, Arg142).
Figure 4. Interactions within the central hub.
(A) A top view is shown of the central hub. The ISM ring is indicated by a dashed orange circle, while the HD1-WHD ring is enclosed within a zoned map. (B) A close-up is shown of the α-12/α-13 helical pair from the ISM ring. A hydrophobic zipper is present between the two α-helices. Side chain density in the map is shown in the left panel, while residues in the zipper are labeled in the right hand panel. (C) A ribbon model of the HD1-WHD ring is shown and one interface between adjacent subunits is indicated with a dashed oval. (D) Interactions within the boxed area on the right are highlighted. (E, F) A D333Q point mutation may knock out a three-way interaction at the WHD-HD1 subunit interface. (G) A V316I point mutation would block interactions of HD1 with an un-modeled main chain density (marked with black dots) that originates from a loop in the 7-blade β-propeller.
To further stabilize the central hub, HD1 interacts with the WHD in the adjacent subunit with hydrogen bonds between Asp333, Asn403, and Gln414, along with interactions between His343 and Ser396 to form the HD1-WHD ring (Figure 4C). A loss of function point mutation (D333Q) (Srinvastava et al., 2007) would weaken or eliminate a three-way contact at the subunit interface between adjacent domains in the HD1-WHD ring in which Asp333 is the central player (Figures 4E, 4F).
Nucleotide binding pocket
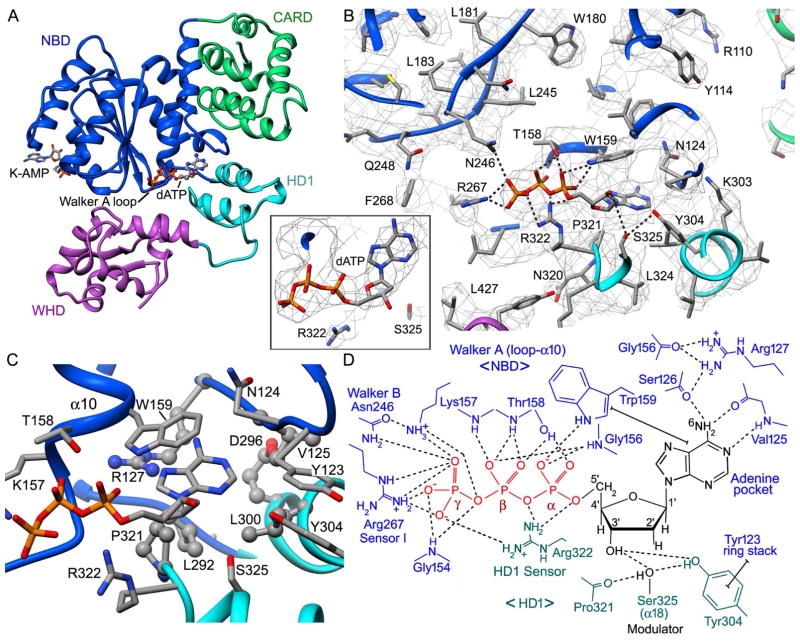
The nucleotide binding site in Dark is located at the interface of the NBD and HD1 (Figure 5A). An overview of this section of the map is shown (Figure 5B) along with an inset of dATP at a higher threshold (Figure 5B inset). The dATP binding site contains residues from Walker A and B boxes with regions that mediate binding to the adenine base, deoxyribose sugar and triphosphate moiety. The adenine binding pocket is formed by Asn124, Phe120, Arg127 and Trp159 in the NBD, along with Pro321, Leu300, and Leu292 in HD1. In addition, the adenine is ring-stacked with Trp159. An arginine (Arg127) is strictly conserved and makes hydrogen bonds to Gly156 and Ser126 to form part of the pocket wall, while the adenine base is positioned by hydrogen bonds to Ser126 and Val125 (Figure 5D).
Figure 5. dATP binding in the Dark apoptosome.
(A) The dATP binding pocket is located at the interface of the NBD and HD1. Bound dATP is shown in ball and stick representation. (B) The dATP binding pocket is shown within the density map. Some hydrogen bonds and salt bridges are indicated by dashed lines. Arginine 322 from HD1 may act as a sensor for dATP. middle inset: The fit of dATP in the density map is shown at a higher threshold with the adenine base in an anti configuration. (C) The binding pocket for the adenine ring is formed primarily by hydrophobic residues, whose side chains are shown in ball and stick representation. (D) Interactions of dATP are summarized in a molecular schematic. Hydrogen bonds and salt bridges are shown by dashed lines and ring stacking interactions are indicated with capped solid lines.
The triphosphate makes numerous hydrogen bonds to Walker A residues in the P-loop and at the end of helix α10 (Figure 5D). These interactions involve main chain amides (Gly154, Gly156, Thr158, Lys157), and side chains. In particular, Trp159 forms a hydrogen bond with the α-phosphate, the side chain of Thr158 interacts with the α- and β-phosphates, and a conserved lysine (Lys157) interacts with the γ-phosphate. The γ-phosphate also interacts with Sensor I (NBD: Arg267) and an “HD1 sensor” (Arg322), while the latter also interacts with the α- and β-phosphates. The HD1 sensor in Dark is located on helix α18, whereas a tyrosine residue in the HD1-WHD loop takes on this role in CED-4 and Apaf-1 apoptosomes (Cheng et al., 2016; Qi et al., 2010; Figure S2). The repositioning of this sensor results from the loss of the HD1-WHD loop in Dark, coupled with local rearrangements of the NBD-HD1 interface, which brings helices α16 and α18 into close contact with bound dATP. Changes in this region alter the interaction profile of the NBD-HD1-WHD triplet so that Dark can form a ring with 8 subunits.
We also compared the conformation of dATP in apoptosome and Apaf-1 structures (Table S2). The energetically favorable anti conformer of the adenine base is present in most cases. However, a recent structure for the ground state Dark apoptosome has a syn conformation for dATP (Pang et al., 2015). To clarify this point, we carried out real space refinement with PHENIX using the published Dark apoptosome model and EM density map (PDB 3J9L; EMD-2871) and started the computation with dATP in the favored anti configuration. The nucleotide binding pocket of PDB-3J9L aligns well with our model after refinement (r.m.s.d. of 0.68 Å; Figure S4).
The geometry of the dATP binding site indicates that the Dark apoptosome may have a negligible dATPase activity. This is based on the overall similarity of nucleotide binding sites in Dark and Apaf-1 (this work, see T. Cheng et al. 2016), coupled with the observation that the human apoptosome has no measurable activity (Reubold et al., 2009; Kim et al., 2005). In support of this idea, a comparison with a crystal structure of the CED-4 apoptosome (Qi et al., 2010) shows a Mg+2 ion adjacent to ATP, which may activate a water molecule during catalysis. This cation in CED-4 is liganded by side chains of Ser166, Asp250 and Lys191. In Dark, these residues are replaced by Thr158, Leu245 and Asn182, and the position of Thr158 would likely preclude it from acting as a ligand if a Mg+2 ion were bound in an equivalent position. In addition, no obvious density is present for a bound Mg+2 ion at this resolution. We also note that a critical glutamate/aspartate in the Walker B motif is replaced with an asparagine (Asn246) that interacts with the γ-phosphate. Finally, no arginine finger is provided by an adjacent subunit in the Dark apoptosome to accelerate catalysis. It remains to be determined whether the Dark monomer has dATPase activity, but residue changes mentioned above would argue for an activity that is comparable to or even lower than that measured for Apaf-1 (4–5 ATP molecules hydrolyzed/monomer/hr; Reubold et al., 2009).
In Dark, dATP is strictly required over ATP for nucleotide-dependent assembly of the ground state apoptosome (Yu et al., 2006). The basis for nucleotide selectivity by Dark is not known but ATP and dATP differ only at the 2′ position in the sugar ring, where an OH is replaced by a hydrogen atom. Thus, discrimination between ATP and dATP may involve a steric read out at the 2′ position of the sugar. We suggest that the side chain of Ser325 may discriminate between ribose and deoxyribose rings. The serine side chain interacts with the carbonyl of Pro321 and with the OH of Tyr304. In addition, Ser325 and Tyr304 each make hydrogen bonds to the 3′ OH of the deoxyribose ring (Figure 5D). This positions the side chain of Ser325 to within ~3.3–3.7 Å of the 2′ carbon (see Figure S4). This close approach may preclude ATP from binding to Dark due to a steric clash with the 2′ OH. In addition, Ser325 is conserved in Apaf-1 where the side chain is positioned ~4.3 Å from the 2′ carbon, and in this case both ATP and dATP can bind and drive assembly. However, the proximity of Ser325 to the 2′ position in Apaf-1 may be responsible for the 4–10x lower affinity exhibited for ATP relative to dATP, when cytochrome c is present (Jiang and Wang, 2000; Reubold et al. 2009).
HD2 and β-propellers
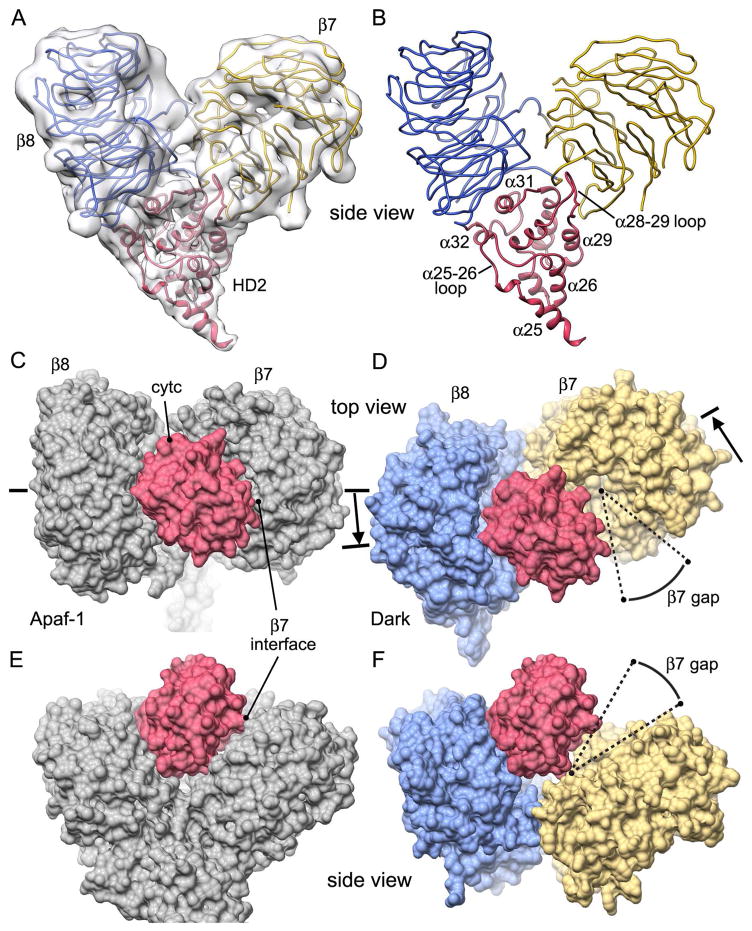
The HD2 arm extends from the WHD in the central hub and is comprised of 8 α-helices with 3 loops. This arm supports the 7- and 8-blade β-propellers through interactions at the base of the V-shaped domain (Figures 6A, 6B). Thus, the β-propellers rest on helices α30, α31 and α32 and two large loops stabilize the interface. Loop α28-α29 penetrates into a cleft near the a-strand of blade 1 in the 7-blade β-propeller, while loop α30-α31 (Lys548-Leu553) interacts with both β-propellers at the base of the V-domain.
Figure 6. HD2 and β-propeller interactions.
(A) Domains within the V-shaped region are shown as ribbons within the improved density map. (B) Helices in HD2 form an apex upon which the two β-propellers rest and stabilize the V-shaped domain. Individual α-helices and loops in HD2 are labeled. (C, D) Calculated molecular surfaces are shown for V-shaped domains in human (panel C) and Dark apoptosomes (panel D). Cytochrome c (in red) is tightly bound to the 8-blade β-propeller of Apaf-1. A similar tight interaction with Dark would leave a large gap relative to the 7-blade β-propeller, which has rotated outwards (see arrow). (E, F) Side views are shown of the models in panels C and D. Note that human HD2 is not shown in panels D and F.
Currently, there is no high resolution structure available for the Dark β-propellers. Hence, these domains were modeled using β-propellers in a crystal structure of mouse Apaf-1 (Experimental Procedures; Reubold et al., 2011). Additional restraints were provided by 15 predicted WD40 repeats, a C-terminal caspase cleavage site at the junction between the 8-blade β-propeller and a putative CTD (Akdemir et al., 2006) and an improved density map of the V-shaped domain at ~7.3 Å resolution. In the final model, WD-motifs (and possible replacement residues) are located at appropriate positions on the c-strand in each blade of the β-propellers. In addition, “fit-in-map” values from Chimera were 0.908 and 0.925, respectively for Dark 7- and 8-blade β-propellers. In the model, these β-propellers have an unusual topology that is shared with Apaf-1. In brief, a long linker extends from helix α32 in HD2 (Phe584-Arg600) to form a displaced outer strand on blade 8 in the 8-blade β-propeller, before forming a long linker to the innermost a-strand (Gln601-Gly610) of blade 1 in the 7-blade β-propeller. The rest of this β-propeller then ensues with the outermost d-strand of blade 7 forming a linker with a short helix that connects to the first blade of the 8-blade β-propeller. Finally, the c-strand of the final blade of the 8-blade β-propeller exits at Arg1264 near the HD2 arm.
Interestingly, blade 6 in the 8-blade β-propeller has a large loop between the c- and d- strands (Phe1170-Cys1181) that extends laterally to contact the final blade of the 7-blade β-propeller in a neighboring subunit (β-bridge, Figures 1, 2 and Figure S2). Interactions between Tyr1177 and Tyr915, with contributions from Glu1178 and Lys916, may contribute to the formation of this inter-subunit bridge. In addition, an un-modeled loop is present between the c- and d-strands of blade 2 in the 7-blade β-propeller, which makes a direct contact with strand 17 of HD1 (Arg314-Val316). A loss of function mutation mutation (V316I) inserts a bulkier side chain at this position that may clash with un-modeled main chain density at the base of this loop (Figure 4G; Figure S3D marked with an asterisk). This mutation highlights an interaction between the 7-blade β-propeller and HD1, which may stabilize an extended conformation of Dark during assembly.
In the human apoptosome, cytochrome c is bound in a cleft between the two β-propellers using shape complimentarity (Yuan et al., 2013; Cheng et al., 2016, Zhou et al., 2015; Figures 5C, 5E), whereas Dark does not bind the heme protein (Yu et al., 2005). To investigate this issue, we aligned Dark and Apaf-1 V-shaped domains with their HD2 arms. Interestingly, the Dark β-propellers are rotated relative to their human counterparts with the 8-blade β-propeller having moved inwards towards the central hub (~15 Å), while the 7-blade β-propeller has moved outwards (~20 Å; see arrows, Figures 6C, 6D). These movements accommodate an additional subunit in the Dark apoptosome relative to the Apaf-1 complex. In addition, the 7-blade β-propeller has rotated by 30–40° away from the 8-blade β-propeller, which creates a wider cleft in the Dark V-shaped domain. To demonstrate this, we docked the human 8-blade β-propeller onto its counterpart in the Dark subunit with Chimera. We then used the transformation matrix to position cytochrome c within the V-shaped domain of Dark (Figure 6D), in the same orientation relative to the 8-blade β-propeller. Previous work has shown that cytochrome c makes a tight interaction with the 8-blade β-propeller of Apaf-1, while maintaining a narrow gap between itself and the 7-blade β-propeller. This gap can be crossed by long side chains to make hydrogen bonds and salt bridges in the Apaf-1 complex (Cheng et al., 2016). However, the modeled gap between cytochrome c and the 7-blade β-propeller in Dark is significantly larger and wedge-shaped, due to the wider cleft that is present between β-propellers (Figures 6D, 6F). This altered geometry of the V-shaped domain may contribute to the inability of the Dark apoptosome to bind cytochrome c (Yu et al., 2006), as one interaction surface for the heme protein has been nearly eliminated.
The exit point of the final β-strand in the 8-blade β-propeller corresponds to the C-terminus of strand c (Arg1264). We surmise that a flexible linker is present between Arg1264 and Asp1289 with the latter being the first residue in a known caspase cleavage site (D1289AVD; Akdemir et al., 2006). Residues that follow the cleavage site may form a novel CTD (Ala1293-Ser1440) that is disordered in our map. Based on our model this caspase cleavage site should be accessible. To test this, we showed that caspase-3 is able to cleave Dark monomers and double rings and generates a slightly shorter form as visualized by SDS PAGE (Figure S5A). In addition, caspase-3 cleavage left the double ring particles intact, based on their mobility on a glycerol gradient (not shown) and their morphology by cryo-EM (Figure S5B).
NBD adenylylation and caged micelles
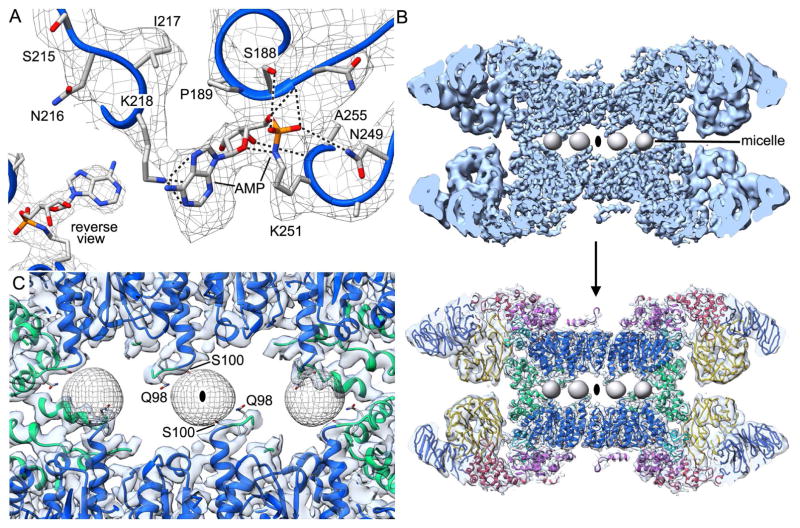
A novel density is present in the NBD and was visible in a previous map at 7Å resolution (Yuan et al., 2011a). This density can be modeled as an adenylylation of Lys251 based on known post-translational modifications (Figure 7A and inset; Itzen et al., 2011). In this case, the α-phosphate of ATP undergoes nucleophilic attack by the ε-NH2 group of lysine to form a phosphoramidate bond with AMP. The AMP group is held in place by multiple interactions between the phosphate group and backbone amides of Ser188 and Ala252, along with weaker interactions to side chains of Ser188, Asn187 and Asn249. In addition, a hydrogen bond is present between the 3′-OH of the ribose ring and the amide of Ala252, while the adenine base makes a π-cation interaction with Lys218. Adenylylation of Dark may reflect a stress response in baculovirus infected insect cells during over-expxression. Alternatively, adenylylation of Lys251 may help stabilize the NBD ring during assembly and could be lost under some purification conditions, as it is not present in two other recent maps (Pang et al. 2015). Since this modification occurred in sf9 insect cells a similar process may occur in Drosophila.
Figure 7. Adenylylation of the NBD and caged micelles.
(A) A novel adenylylation of Lys251 is present in Dark. Hydrogen bonds and π-cation interactions to Lys218 are indicated with dashed lines. At this threshold the Lys218-adenine ring interaction is visible. Inset bottom left: The Lys251 modification is shown in a reverse view at a higher density threshold. (B) DHPG/NP-40 mixed micelles are caged between opposing rings in the Dark apoptosome. Micelle densities were low pass filtered to ~15 Å and four of these are viewed along a two-fold axis in this cut-away view. Top: density map; Bottom: map with docked model. (C) A view along an alternate 2-fold axis reveals micelle interactions with the CARD-NBD loop (Gln98) and helix α8 (Ser100) from opposing NBDs.
Dark has a low isoelectric point. With this in mind, we used diheptanoylphosphatidylglycerol (DHPG) as an additive during freezing on holey carbon grids. We reasoned that this short chain detergent would bind to the carbon film creating a negatively-charged surface that would repel Dark double rings, to improve particle loading within holes, and this proved to be correct. DHPG may also bind to the air-water interface to prevent preferential interactions at both of these surfaces. A minimal amount of the non-ionic detergent (NP-40) was also used to solubilize DHPG aggregates and act as a surfactant during freezing. Unexpectedly, we found 8 egg-shaped densities of ~20 Å diameter sandwiched between the two apoptosome rings, with each density positioned on a 2-fold axis between pairs of opposing NBDs (Figures 7B, 7C). This density was not observed in a map calculated without DHPG (Yuan et al., 2011a), so identification of this feature as a small mixed micelle is reasonable, given the short chain length of this detergent-like phospholipid.
Discussion
Dark structure and assembly
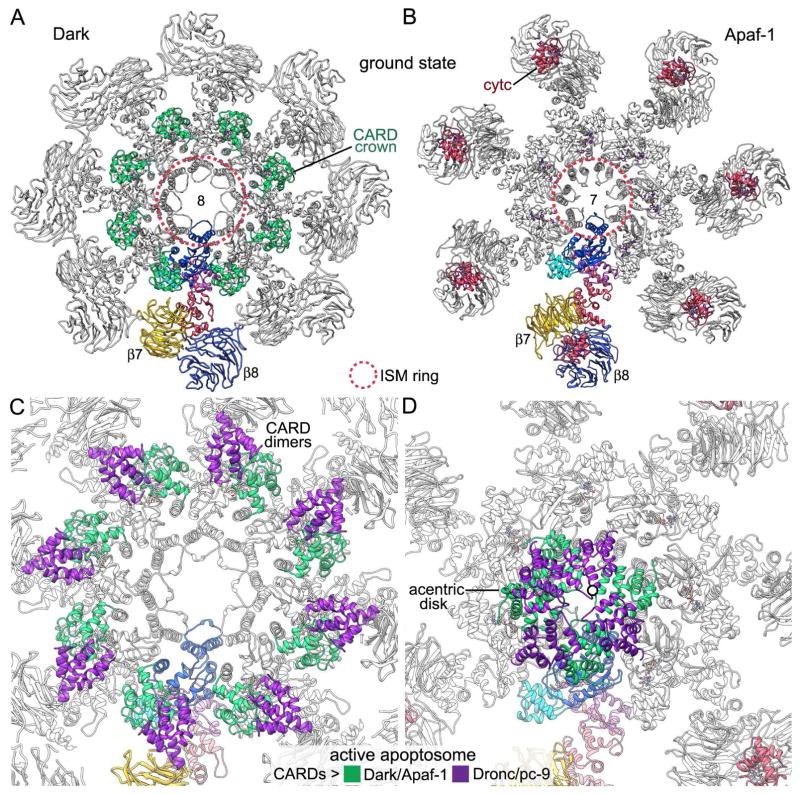
Dark single rings and Apaf-1 apoptosomes share a similar organization for the central hub, HD2 arms and V-shaped domains. A major exception however, concerns the presence of an additional subunit in the Dark ring, yet the particle diameters are similar (Yuan et al., 2011a). To achieve this altered packing, the NBD-HD1-WHD triplet within each Dark monomer has undergone local rearrangements. This includes changes in the side-to-side packing of the helix-loop-helix motif within the ISM ring, which has moved to a slightly larger radius (~5 Å) in Dark (Figures 8A, 8B). In addition, a more acute angle is present between HD1 and WHD domains in the Dark subunit, when viewed from along the 8-fold symmetry axis. This allows a central hub with a similar diameter to be formed by 8 rather than 7 subunits. Finally, the HD2 arm is more strongly bent towards the top surface of the Dark ring, which moves the β-propellers upwards (Yuan et al., 2011a). This movement is coupled with a rotation of the V-shaped domain and formation of a bridge between β-propellers in adjacent subunits. An interaction between the 7-blade β-propeller and HD1 may stabilize the extended conformation of Dark that is required for assembly. This interaction may be abrogated by a V316I point mutation (Srinvastava et al., 2007).
Figure 8. CARDs in ground state and active apoptosomes.
(A, B) Top views are shown of Dark and Apaf-1 apoptosomes in the ground state (left: this work, PDB 5JUL; right: Zhou et al., 2015; PDB 3JBT). A single subunit is color coded and the CARD crown and cytochrome c molecules are labeled and color-coded in green and red, respectively. ISM rings in the apoptosomes are marked with a dashed circle (red). (C) A zoomed in view is shown of a post-activation Dark apoptosome (Pang et al., 2015; PDB 3J9K). CARD-CARD heterodimer formation by Dark and Dronc creates an expanded crown that does not perturb intrinsic Dark CARD-NBD interactions. (D) A similar view is shown of the active human apoptosome (Cheng et al., 2016, PDB 5JUY). The offset of an acentric 8-CARD disk is apparent. The spiral-shaped CARD disk differs dramatically in location and organization relative to the expanded CARD crown in the post-activation Dark apoptosome shown in panel C.
Nucleotide exchange is a required step in formation of an extended Dark monomer in the canonical model of assembly. Thus, a large number of interactions are formed between dATP, the NBD and HD1 to stabilize the NBD-HD1 pair in the correct orientation to interact with the WHD. The WHD-HD2 interface in Dark is extensive and may be similar in closed and extended conformations, as occurs in Apaf-1 (Cheng et al., 2016; Yuan et al. 2013, 2010; Riedl et al., 2005; Reubold et al., 2011). Changes induced by dATP binding would align the WHD within the hub to interact with adjacent monomers during ring formation, while positioning the HD2 arm. Thus, an extended conformation of Dark may promote the formation of a lateral dimer, thereby creating a seed for growth of the octameric ring. Further details of the assembly mechanism must await a structure of the Dark monomer and structures of possible assembly intermediates.
A recent near atomic structure provides further insights into Dark assembly, when interpreted in light of our model. In this work, the addition of Dronc CARDs to Dark allowed the formation of a double-ring apoptosome with bound nucleotide diphosphate, which was modeled as ADP (Pang et al., 2015). The structure of this novel double-ring particle may represent a post-activation state in which Dronc catalytic domains have been released by auto-cleavage (Pang et al., 2015). Remarkably, Dark assembly in this case did not require nucleotide exchange, which can be explained as follows. An intrinsic flexibility of Dark monomers may allow dATP to displace nucleotide diphosphate during sequential incubations at 37° C and room temperature in a standard assembly reaction (Yu et al., 2006). When Dronc CARDs are added to Dark monomers, they form Dronc CARD-Dark CARD heterodimers that do not disturb interactions between the Dark CARD and the NBD (Figures 3A, 3B). The Dronc CARD also interacts with the lateral surface of the 7-blade β-propeller, which has to move slightly to accommodate the CARD-CARD heterodimer (Pang et al., 2015). Hence, formation of the CARD-CARD heterodimer creates a bridge between the NBD and the V-shaped domain that may stabilize an extended conformation of the Dark subunit. This in turn, would favor ring assembly and bypass the requirement for nucleotide exchange.
Dronc activation
Dronc is an apical procaspase that contains an N-terminal CARD (residues 1–109), a long linker (residues 110–193), and the two catalytic domains (residues 194–450; Yan et al., 2004). A model of proximity-induced dimerization has been proposed for Dronc activation (Dorstyn & Kumar, 2008; Snipas et al, 2008; Boatright et al., 2003) in which a dimer is stabilized by an internal cleavage at Glu252 (Yan et al., 2006). In normal cells, Dronc activation is inhibited by the BIR2 domain of DIAP1 which forms a complex with a linker region (Chai et al., 2003). Reaper, Hid and Grim (RHG) proteins relieve Dronc inhibition by binding to the BIR2 domain (Wu et al., 2001; Hawkins et al., 2000), while inhibitor of apoptosis (IAP) motifs in the RHG proteins bind to the BIR1 domain of DIAP1 to release DrICE inhibition (Yan et al., 2004; Wang et al., 1999; Kaiser et al., 1998). Hence, activation of apical and executioner procaspases may require Dark assembly coupled with the release of DIAP1 from Dronc and DrICE. For Dronc, the unblocking step may occur in solution or possibly when the apical procaspase is bound to the Dark apoptosome.
Wild type Dronc molecules are further auto-proteolyzed when they associate with Dark and a large complex with Dronc CARDs can be separated from soluble and active Dronc dimers after ~4 hours (Pang et al., 2015). In these post-activation complexes, Dronc and Dark CARDs interact within a single Dark ring. However, double rings are formed from single rings in the absence of the catalytic domains, through bridging interactions between Dronc CARDs (Pang et al., 2015). In addition, the N- and C-termini of Dronc CARDs face inwards, towards the center of the double ring complex. This arrangement would make it hard to activate DrICE in a double-ring particle because the extended Dronc linker (~84 residues) must be threaded through very narrow portals between adjacent CARDs from opposed rings, if Dronc catalytic domains are to be located on the outside where they would have access to the executioner caspase. Hence, these double rings may represent post-activation complexes that have dimerized at high protein concentration.
Apical procaspases are recruited to apoptosomes through CARD-CARD interactions (reviewed in Bratton and Salvesen, 2010). However, the orientation of CARDs differs significantly in Dark and Apaf-1 apoptosomes and is dependent on the state of the complex. In the ground state, N-terminal CARDs on the Dark apoptosome form an octagonal crown with each CARD interacting with the NBD and HD1 in the same subunit (Figure 8A; this work, Yuan et al., 2011a; Pang et al., 2015). Conversely, in Apaf-1 the CARD has a much longer linker to its NBD and is disordered in the ground state apoptosome (Figure 8B; Yuan et al., 2010, 2013; Zhou et al., 2015). In the Dark apoptosome, Dronc binding creates CARD-CARD heterodimers which form a larger crown on the central hub that may be similar in active and post-activation states. However, formation of the active Dark complex does not alter the original CARD crown that is present in the ground state (compare Figures 3A, 3B, 8A, 8C; see Pang et al., 2015). In stark contrast, an acentric CARD disk is formed when procaspase-9 molecules are bound to the human apoptosome. The resulting disk contains four Apaf-1 CARDs along with 3 or 4 procaspase-9 CARDs, which form pairs that are packed in a tight spiral that is slightly offset from the particle axis (Cheng et al., 2016; Figure 8D). Thus, it appears unlikely that Dark and Dronc CARDs will form a similar disk in the active state, due to the very short linker between the Dark CARD and NBD, coupled with the similarity of CARD-NBD interactions in ground state and post-activation complexes (this work, Pang et al., 2015; Yuan et al., 2011a).
We have shown previously that active Dark-Dronc complexes exist primarily as single rings after co-assembly, although they tended to aggregate through non-specific interactions when concentrated for cryo-EM and a few double rings were present (Yuan et al., 2011a). Thus, Dark single rings with bound Dronc may represent the active apoptosome, in which interacting CARDs are located on the central hub as an expanded crown (Pang et al., 2015; Figure 8C). In this scenario, the long linker between the Dronc CARD and catalytic domains may play a role in activation that is analogous to the role of a similar linker in procaspase-9 (Yuan et al., 2011b; Cheng et al., 2016). However, this model of Dronc activation differs from the Apaf-1/procaspase-9 paradigm, in which platform formation enables the assembly of a quasi-helical CARD disk. The lack of a bona fide CARD disk in the Dark apoptosome would argue for a general model of apical procaspase activation. In this model, Dronc molecules form dimers that are flexibly-tethered to the Dark apoptosome during activation (Yan et al., 2006). After Dronc dimerization, competing proteolytic reactions may occur that use Dronc or DrICE as substrates for the Dark-Dronc complex, followed by a second stage in which released Dronc dimers (Pang et al., 2015) may cleave DrICE molecules in solution. Finally, our data suggest that the Apaf-1 apoptosome will have one or possibly two procaspase-9 dimers in the active complex (Cheng et al., 2016), while the Dark apoptosome with a full occupancy (Pang et al., 2015) will have four Dronc dimers anchored via CARD-CARD interactions.
Experimental Procedures
Protein expression, purification and assembly
His-tagged Dark was cloned previously into pFastBac for expression in baculovirus infected sf9 insect cells (Yu et al. 2006). Cells were harvested 48 hours after infection and homogenized in buffer T (20 mM Tris pH 7.5, 50 mM NaCl, 1 mM PMSF, 1 mM Benzamidine, 1 mM beta-mercaptoethanol). The lysate was centrifuged (100,000 g for 30 min) and the supernatant was loaded onto a 2 ml Ni-NTA column, and Dark was eluted in 250 mM Imidazole in buffer T. The eluate was dialyzed in 25 mM potassium phosphate, pH 7.5 and 50 mM NaCl. The sample was further purified by binding to 2 mls of hydroxyapatite (BioRad HTP) in batch, and then eluted with 250 mM potassium phosphate, pH 7.5 and 50 mM NaCl. Dark was dialysed into buffer A (10 mM Hepes pH 7.5, 20 mM KCl, 1 mM EDTA, 1 mM EGTA, 1.5 mM MgCl2) and frozen at ~0.5 mg/ml until use. To assemble the apoptosome, Dark was mixed with 10 mM dATP and 10 mM EDTA at 37 °C for 30 minutes, followed by room temperature incubation overnight (Yu et al. 2006; Yuan et al., 2011a).
Data acquisition and image processing
Dark apoptosomes were concentrated with a spin ultrafiltration unit (Amicon Ultra MWCO 10K) to ~2 mg/ml with the following additives present at the indicated final concentrations: NP-40 (0.025%), DHPG (diheptanoylphosphatidylglycerol; 0.05%), cytochrome c (0.5 mg/ml), DeoxybigChaps (0.01%), and lysine (0.03 mg/ml). A sample volume of 2.5 μl was applied onto a C-Flat 300 mesh R1.2/1.3 holey grid (Protochips) that had been freshly glow discharged and then was blotted for 2.5 seconds in 100% humidity at 20° C and plunge frozen in liquid ethane using a Vitrobot Mark 3 (FEI company). A Titan Krios electron microscope equipped with a K2 Summit direct electron detector (GATAN), a spherical aberration corrector and a Gatan Image Filter (GIF) with a slit width of ~20 eV were used for data acquisition at 300 kV. Data were collected in super resolution mode at nominal magnifications of 81,000 X and 105,000 X, corresponding to 0.675 and 0.52 Å per pixel, at a dose rate of 10.25 and 7.8 electrons per physical pixel per second, respectively. Each movie exposure was fractionated into 23 frames and lasted 6.9 s, which amounts to a total dose of 42 and 50 electrons per Å2 for the 81,000 X and 105,000 X movie data sets. Defocus ranged from −1.5 to −2.4 μm.
In all 1991 movies were collected and then corrected for gain and binned 2X in IMOD (Kremer et al., 1996), yielding pixel sizes of 1.35 and 1.04 Å. Beam induced motions were corrected with UCSF MotionCorr (Li et al., 2013) and frames 2–23 were used for further processing. Corrected micrographs from the 105,000 X data set were scaled to 1.35 Å per pixel to match the 81,000 X data and contrast transfer function parameters were estimated by CTFFIND3 for all micrographs in the data set (Mindell and Grigorieff, 2003). An initial set of 88485 particles was selected by e2boxer.py (Tang et al., 2007) and a subset of 24153 particles was obtained by manual inspection followed by 2D classification in RELION-1.3 (Scheres, 2012). The best particles were then subjected to 3D classification in which a published map (EMD-5235) was low pass filtered to 60 Å and used as a reference. A density map at 5.3 Å resolution (gold-standard FSC0.143) was obtained with D8 symmetry refinement. Per particle beam-induced motion correction and B-factor weighting for radiation damage were carried out in RELION-1.3 (Scheres, 2014) with frames 2–23, a running average of 7 frames, and a standard deviation of 1 pixel for translations. A final set of 17769 particles was obtained after further 3D classification on the polished particles. A final map with a global resolution of 4.4 Å was obtained after D8 refinement, which was sharpened using automatic B-factor estimation (Rosenthal and Henderson 2003) with a soft mask applied after correction for the modulation transfer function of the K2 Summit direct electron detector. Local resolution was estimated using Resmap (Kucukelbir et al, 2014). An improved local map for β-propellers in the V-shaped domain was obtained with focused 3D classification (Zhou et al., 2015). We subsequently used the zone and vop functions in Chimera (Pettersen et al., 2004) to create a composite 3D map with the higher resolution central hub and arms, which contained improved density for the β-propellers from the focused 3D classification. We also applied a local normalization to the map with EMAN2 (Tang et al., 2007), so that all domains could be visualized with a single threshold.
Model building
Domains from the CARD to HD2, residues 1–583, were built using a previously published model (PDB 1VT4; Yuan et al., 2011a), which was fit into the density as a rigid body with Chimera (Pettersen et al., 2004), and the overall fit was further improved with Molecular Dynamics Flexible Fitting (MDFF; Trabuco et al., 2008). Manual adjustments were made in Coot (Emsley et al., 2010), with alternating cycles of real space refinement in PHENIX (Adams et al., 2010) using secondary structure and geometry restraints. A model for an adenylylated lysine was generated in Jligand (Lebedev et al., 2012) and refinement restraints were generated in phenix.elbow (Adams et al., 2010). Beta-propellers are comprised of a roughly cylindrical array of blades, each of which is formed by four strands (denoted a, b, c, d) in an anti-parallel β-sheet, with the a-strand next to a central pore and the d-strand located on the outer edge. Dark β-propeller sequences (residues 584–1264) were aligned to comparable domains in a crystal structure of mouse Apaf-1 (PDB 3SFZ; Reubold et al., 2011) and a starting model for each β-propeller was constructed with MODELLER (Sali and Bundell, 1993). These models were flexibly fit into the density with MDFF (Trabuco et al., 2008), followed by cycles of manual adjustment in Coot (Emsley et al., 2010) and refinement in PHENIX (Adams et al., 2010). Neither of the β-propellers is circular in cross-section and this is more pronounced for the 7-blade β-propeller. This distortion required a separate docking of individual blades within the density map during modeling of the 7-blade β-propeller with the homology model (Reubold et al., 2011). The 8-blade β-propeller has an elliptical shape that MDFF was successful in fitting (Trabuco et al., 2008). We also omitted portions of three loops in the 7-blade β-propeller from the final model, including residues: 683–698, 726–729 and 855–858. Although the resolution of the V-shaped domain is only ~7.3Å, side chains were left on β-propellers to facilitate docking of β-sheets within the map. MolProbity was used to evaluate the overall fitness of the model (Davis et al., 2007). Final figures were made with Chimera (Pettersen et al., 2004) and Adobe Photoshop.
Supplementary Material
Highlights.
A nearly complete model of the Dark apoptosome is presented including β-propellers
Insights are provided for dATP selectivity during nucleotide exchange and assembly
A novel role is uncovered for Dark β-propellers in apoptosome assembly
A general model is proposed for activation of apical procaspases on the apoptosome
Acknowledgments
We thank Janelia Farm for data collection time on the Krios 1 microscope. The Ludtke and Akey laboratories are supported by NIH grants (S.J.L. R01 GM080139; C.W.A. R01 GM63834).
Footnotes
Accession numbers
Coordinates for the Dark apoptosome model (PDB: 5JUL) and density maps (EMD-8177) have been deposited.
Author contributions
T.C.C., I.V.A. and S.Y. prepared proteins for the experiments. T.C.C. and C.W.A. designed the experiments and prepared frozen grids, Z.Y., T.C.C. and C.W.A. collected the data. T.C.C, S.L. and C.W.A analyzed the data and the paper was written with contributions from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdemir F, Farkas R, Chen P, Juhasz G, Medved’ová L, Sass M, Wang L, Wang X, Chittaranjan S, Gorski SM, Rodriguez A, Abrams JM. Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development. 2006;133:1457–1465. doi: 10.1242/dev.02332. [DOI] [PubMed] [Google Scholar]
- Arama E, Bader M, Srivastava M, Bergmann A, Steller H. The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. The EMBO Journal. 2006;25:232–243. doi: 10.1038/sj.emboj.7600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Molecular Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Yan N, Huh JR, Wu JW, Li W, Hay BA, Shi Y. Molecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1-dependent Dronc ubiquitination. Nature Structural Biology. 2003;10:892–898. doi: 10.1038/nsb989. [DOI] [PubMed] [Google Scholar]
- Cheng TC, Hong C, Akey IV, Yuan S, Akey CW. A near atomic structure of the human apoptosome. Elife. 2016;5 doi: 10.7554/eLife.17755. pii: e17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SK, Akdemir F, Chen P, Lu WJ, Mills K, Daish T, Kumar S, Rodriguez A, Abrams JM. The apical caspase Dronc governs programmed and unprogrammed cell death in Drosophila. Developmental Cell. 2004;7:897–907. doi: 10.1016/j.devcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Daish TJ, Mills K, Kumar S. Drosophila caspase Dronc is required for specific developmental cell death pathways and stress-induced apoptosis. Developmental Cell. 2004;7:909–915. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, III, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucl Acids Res. 2007;35:375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danot O, Marquenet E, Vidal-Ingigliardi D, Richet E. Wheel of life, wheel of death: a mechanistic insights into signaling by STAND proteins. Structure. 2009;17:172–182. doi: 10.1016/j.str.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Kumar S. A biochemical analysis of the activation of the Drosophila caspase DRONC. Cell Death and Differentiation. 2008;15:461–470. doi: 10.1038/sj.cdd.4402288. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Mills K, Lazebnik Y, Kumar S. The two cytochrome c species, DC3 and DC4, are not required for caspase activation and apoptosis in Drosophila cells. The Journal of Cell Biology. 2004;167:405–410. doi: 10.1083/jcb.200408054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L, Read S, Cakouros D, Huh JR, Hay BA, Kumar S. The role of cytochrome c in caspase activation in Drosophila melanogaster cells. The Journal of Cell Biology. 2002;156:1089–1098. doi: 10.1083/jcb.200111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L, Colussi PA, Quinn LM, Richardson H, Kumar S. DRONC, an ecdysone-inducible Drosophila caspase. Proceedings of the National Academy of Sciences. 1999;96:4307–4312. doi: 10.1073/pnas.96.8.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallographica Section D Biological Crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrao R, Wu H. Helical assembly in the death domain (DD) superfamily. Current Opinion in Structural Biology. 2012;22:241–247. doi: 10.1016/j.sbi.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes & Development. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Hawkins CJ, Yoo SJ, Peterson EP, Wang SL, Vernooy SY, Hay BA. The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. Journal of Biological Chemistry. 2000;275:27084–27093. doi: 10.1074/jbc.M000869200. [DOI] [PubMed] [Google Scholar]
- Itzen A, Blankenfeldt W, Goody RS. Adenylylation: renaissance of a forgotten post-translational modification. Trends in Biochemical Sciences. 2011;36:221–228. doi: 10.1016/j.tibs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. Journal of Biological Chemistry. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Vucic D, Miller LK. The Drosophila inhibitor of apoptosis DIAP1 suppresses cell death induced by the caspase drICE. FEBS Letters. 1998;440:243–248. doi: 10.1016/s0014-5793(98)01465-3. [DOI] [PubMed] [Google Scholar]
- Kanuka H, Sawamoto K, Inohara N, Matsuno K, Okano H, Miura M. Control of the cell death pathway by Dapaf-1 a Drosophila Apaf-1/CED-4 related caspase activator. Molecular Cell. 1999;4:757–769. doi: 10.1016/s1097-2765(00)80386-x. [DOI] [PubMed] [Google Scholar]
- Kim HE, Du F, Fang M, Wang X. Formation of an apoptosome is initiated by cytochrome c induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proceedings of the National Academy of Sciences. 2005;102:17545–17550. doi: 10.1073/pnas.0507900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S, White K. Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm) Journal of Cell Science. 2005;118:1779–1787. doi: 10.1242/jcs.02377. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. Journal of Structural Biology. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nature Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death and Differentiation. 2007;13:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Lebedev AA, Young P, Isupov MN, Moroz OV, Vagin AA, Murshudov GN. JLigand: a graphical tool for the CCP4 template-restraint library. Acta Crystallogr D Biol Crystallogr. 2012;68:431–440. doi: 10.1107/S090744491200251X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mooney P, Zheng S, Booth C, Braunfeld MB, Gubbens S, Agard DA, Cheng Y. Electron counting and beam-induced motion correction enables near atomic resolution single particle cryoEM. Nature Methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. Journal of Structural Biology. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mendes CS, Arama E, Brown S, Scherr H, Srivastava M, Bergmann A, Steller H, Mollereau B. Cytochrome c-d regulates developmental apoptosis in the Drosophila retina. EMBO reports. 2006;7:933–939. doi: 10.1038/sj.embor.7400773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K, Daish T, Harvey KF, Pfleger CM, Hariharan IK, Kumar S. The Drosophila melanogaster Apaf-1 homologue ARK is required for most, but not all, programmed cell death. The Journal of Cell Biology. 2006;172:809–815. doi: 10.1083/jcb.200512126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142:334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Pang Y, Bai XC, Yan C, Hao Q, Chen Z, Wang JW, Scheres SH, Shi Y. Structure of the apoptosome: mechanistic insights into activation of an initiator caspase from Drosophila. Genes & Development. 2015;29:277–287. doi: 10.1101/gad.255877.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A Visualization System for Exploratory Research and Analysis. Journal of Computational Chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Qi S, Pang Y, Hu Q, Liu Q, Li H, Zhou Y, He T, Liang Q, Liu Y, Yuan X, Luo G, Li H, Wang J, Yan N, Shi Y. Crystal Structure of the Caenorhabditis elegans apoptosome reveals an octameric assembly of CED-4. Cell. 2010;141:446–457. doi: 10.1016/j.cell.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Reubold TF, Wohlgemuth S, Eschenburg S. Crystal structure of full-length apaf-1: how the death signal is relayed in the mitochondrial pathway of apoptosis. Structure. 2011;19:1074–1083. doi: 10.1016/j.str.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Reubold TF, Wohlgemuth S, Eschenburg S. A new model for the transition of APAF-1 from inactive monomer to caspase-activating apoptosome. Journal of Biological Chemistry. 2009;284:32717–32724. doi: 10.1074/jbc.M109.014027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Li W, Chao Y, Schwarzenbacher R, Shi Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature. 2005;434:926–933. doi: 10.1038/nature03465. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Oliver H, Zou H, Chen P, Wang X, Abrams JM. Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nature Cell Biology. 1999;1:272–279. doi: 10.1038/12984. [DOI] [PubMed] [Google Scholar]
- Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. Journal of Molecular Biology. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. Journal of Molecular Biology. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Scheres SH. Beam-induced motion correction for submegadalton cryo-EM particles. eLife. 2014;3:e03665. doi: 10.7554/eLife.03665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH. RELION: implementation of a Bayesian approach to cryo-EM structure determination. Journal of Structural Biology. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipas SJ, Drag M, Stennicke HR, Salvesen GS. Activation mechanism and substrate specificity of the Drosophila initiator caspase DRONC. Cell Death and Differentiation. 2008;15:938–945. doi: 10.1038/cdd.2008.23. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Scherr H, Lackey M, Xu D, Chen Z, Lu J, Bergmann A. ARK, the Apaf-1 related killer in Drosophila, requires diverse domains for its apoptotic activity. Cell Death and Differentiation. 2006;14:92–102. doi: 10.1038/sj.cdd.4401931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews Molecular Cell Biology. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. EMAN2: an extensible image processing suite for electron microscopy. Journal of Structural Biology. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Trabuco LG, Villa E, Mitra K, Frank J, Schulten K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure. 2008;16:673–683. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- Wu JW, Cocina AE, Chai J, Hay BA, Shi Y. Structural analysis of a functional DIAP1 fragment bound to grim and hid peptides. Molecular Cell. 2001;8:95–104. doi: 10.1016/s1097-2765(01)00282-9. [DOI] [PubMed] [Google Scholar]
- Yan N, Huh JR, Schirf V, Demeler B, Hay BA, Shi Y. Structure and activation mechanism of the Drosophila initiator caspase Dronc. J Biol Chem. 2006;281:8667–8674. doi: 10.1074/jbc.M513232200. [DOI] [PubMed] [Google Scholar]
- Yan N, Wu JW, Chai J, Li W, Shi Y. Molecular mechanisms of DrICE inhibition by DIAP1 and removal of inhibition by Reaper, Hid and Grim. Nature Structural and Molecular Biology. 2004;11:420–428. doi: 10.1038/nsmb764. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang L, Acehan D, Wang X, Akey CW. Three-dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer. Journal of Molecular Biology. 2006;355:577–589. doi: 10.1016/j.jmb.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Yu X, Acehan D, Menetret JF, Booth CR, Ludtke SJ, Riedl SJ, Shi Y, Wang X, Akey CW. A structure of the human apoptosome at 12.8 Å resolution provides insights into this cell death platform. Structure. 2005;13:1725–1735. doi: 10.1016/j.str.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Yuan S, Akey CW. Apoptosome structure, assembly, and procaspase activation. Structure. 2013;21:501–15. doi: 10.1016/j.str.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Yu X, Topf M, Dorstyn L, Kumar S, Ludtke SJ, Akey CW. Structure of the Drosophila apoptosome at 6.9Å resolution. Structure. 2011a;19:128–140. doi: 10.1016/j.str.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Yu X, Asara JM, Heuser JE, Ludtke SJ, Akey CW. The holo-apoptosome: activation of procaspase-9 and interactions with procaspase-3. Structure. 2011b;19:1084–1096. doi: 10.1016/j.str.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Yu X, Topf M, Ludtke SJ, Wang X, Akey CW. Structure of an apoptosome-procaspase-9 CARD complex. Structure. 2010;18:571–583. doi: 10.1016/j.str.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Song Z, Tittel J, Steller H. HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol. Cell. 1999;4:745–755. doi: 10.1016/s1097-2765(00)80385-8. [DOI] [PubMed] [Google Scholar]
- Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan C, Scheres SH, Shi Y. Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes & Development. 2015;29:2349–2361. doi: 10.1101/gad.272278.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.