Abstract
Glycosylation refers to the co- and post-translational modification of protein and lipids by monosaccharides or oligosaccharide chains. The surface of mammalian cells is decorated by a heterogeneous and highly complex array of protein and lipid linked glycan structures that vary significantly between different cell types, raising questions about their roles in development and disease pathogenesis. This review will begin by focusing on recent findings that define roles for cell surface protein and lipid glycosylation in pluripotent stem cells and their functional impact during normal development. Then, we will describe how patient derived induced pluripotent stem cells are being used to model human diseases such as congenital disorders of glycosylation. Collectively, these studies indicate that cell surface glycans perform critical roles in human development and disease.
Keywords: congenital disorders of glycosylation, glycosylation, pluripotent stem cells
Introduction
Virtually all secreted cell surface proteins are glycosylated. These glycosylated proteins can be divided into two main groups based on the type of linkage between the glycan and its acceptor. O-linked glycans are attached post-translationally at serine and threonine residues whereas N-linked glycans are added to specific asparagine residues in a co-translational or post-translational fashion [1, 2]. While these glycans represent the major types found on cell surface glycoproteins, some proteins also bear C-mannosylation, an ER-based post-translational modification involved in developmental processes [3]. Another major class of glycosylation found in cells is O-GlcNAcylation, the dynamic modification of many nuclear and cytosolic proteins by a single N-acetylglucosamine residue. This modification has been shown to play an important role in numerous biological processes including nutrient sensing and disease progression [4, 5].
The biosynthesis and elaboration of glycans found on secreted and cell surface glycoproteins occurs at the cytosolic and lumenal face of the endoplasmic reticulum (ER) and within the Golgi apparatus through the coordinated action of glycosidases, nucleotide-sugar transporters and glycosyltransferases that synthesize and remodel N-glycans as they proceed through the secretory pathway. This process begins with the biosynthesis of a 14-sugar lipid-linked oligosaccharide precursor that is used by the oligosaccharyltransferase (OST) complex to add N-glycans on nascent polypeptides in the ER (Fig. 1). Nucleotide-sugar transporters are responsible for the transport of high-energy sugar donors from the cytosol into the lumenal compartments of the Golgi where they are utilized by specific glycosyltransferases to produce structural diversity on glycosylated proteins. Several factors contribute to the diversity of glycan structures found on different cell types including differential expression of genes encoding glycosylation enzymes, organization within the Golgi complex and the availability of specific glycoprotein acceptors [6].
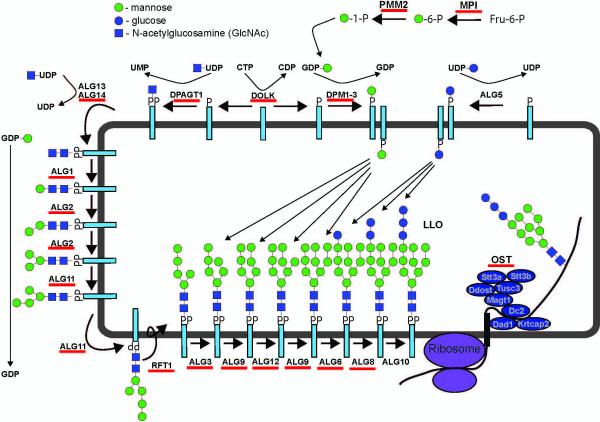
Figure 1.
Biosynthesis of the lipid-linked oligosaccharide precursor for N-linked glycosylation and known defects in this pathway. Scheme depicting the enzymes and proteins involved in the biosynthesis of the 14-sugar lipid-linked oligosaccharide (LLO) precursor for N-glycosylation. This precursor is then transferred to nascent polypeptides by the oligosaccharyltransferase (OST) enzyme complex in a co- and post-translational manner. Red lines under the gene names denote those genes associated with known CDG defects. Mutations in the OST genes STT3A, STT3B, DDOST, TUSC3 and MAGT1 have been identified in human patients.
Roughly 50% of the cellular proteins that are glycosylated are secreted. The glycans on these proteins are critical for protein folding and quality control. The sugar chains on cell surface glycoproteins and glycolipids play numerous roles including the regulation of protein-protein interactions and receptor-ligand binding that impact intracellular signaling pathways. Glycans also dictate how long glycoproteins reside at the cell surface and the rate at which they are internalized and degraded. This also has important implications for cell signaling and cell function.
The presence or absence of specific glycans is known to impact developmental processes and changes in protein and lipid glycosylation correlate with the onset and progression of many disease states, including diabetes and cancer [7, 8]. Mutations in genes that directly or indirectly impact glycosylation have been identified in human patients with clinical disorders, underscoring the potential importance of glycan composition in disease pathogenesis. Several inherited disease states linked to the mutation of genes involved in protein and lipid glycosylation have been described and together are referred to as congenital disorders of glycosylation (CDGs) [9, 10] (Fig. 1). The relevance of glycans to human health also extends to immunity and infectious diseases where cell surface glycans serve as the basis for attachment and entry of bacteria, fungi and viruses into cells.
Much of our understanding about how glycosylation impacts development, tissue homeostasis and disease has been derived from genetic manipulation of glycosylation-related genes in rodents [11, 12]. More recently however, the development of human stem cell-based systems and a new generation of genome editing technologies has greatly accelerated our understanding in this area. Likewise, the advent of modern glycomics and the optimization of techniques such as mass spectrometry [13, 14], capillary electrophoresis [15], and liquid chromatography [16] have made it possible to rapidly profile the glycan diversity in cells and tissues (Fig. 2). The ability to monitor how global glycan structures change in the context of different cell types in different disease states will continue to improve our understanding of the glycome and its function.
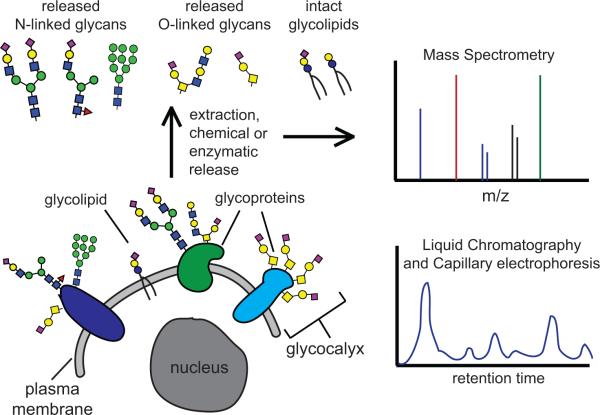
Figure 2.
Advances in glycomics allow the rapid profiling of cell-associated glycans. The surfaces of all cells are coated with a rich diversity of glycans including glycoproteins, glycolipids and glycosaminoglycans. This coat is called the glycocalyx and serves multiple vital functions for the cell. New advances in glycomics such as tandem mass spectrometry (MSn), capillary electrophoresis (CE) and high performance and ultra performance liquid chromatography (HPLC and UPLC) provide the opportunity to rapidly profile the diversity and abundance of different glycan structures. This profiling has facilitated the identification of glycan signatures in pluripotent and differentiated lineages.
This review will specifically focus on how pluripotent stem cells have been used as a model to understand the roles of glycans in mammalian development and disease. The ability of specific glycan structures to serve as signatures of pluripotency and differentiated lineages will be discussed. We will also highlight the emerging utility of induced pluripotent stem cell technology in the modeling of human glycosylation disorders.
Glycans as tools to identify and characterize pluripotent stem cells
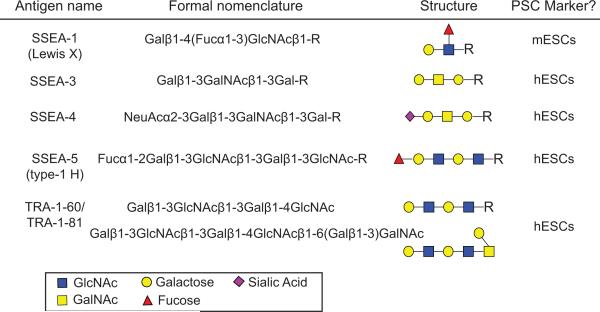
A major challenge in cell biology is to develop tools that will allow for the identification and isolation of different cell types based on their cell surface characteristics. These tools potentially have utility in diagnostic testing, basic research and in a clinical setting where pure cell populations are required for transplantation. A key requirement for this is the identification of specific cell surface markers that allow for isolation of specific cell populations. One practical example of this is the isolation of human hematopoietic stem cells from bone marrow that enables them to be used for autologous transplantation into cancer patients [17]. Here, hematopoietic stem cells are marked on their cell surface by the sialomucin glyoprotein CD34. Given their abundance on the cell surface and vast structural diversity between cell types, carbohydrate structures have been used as epitopes to discriminate between different cell types using affinity reagents including antibodies and sugar binding proteins such as lectins. As a result, many cell surface markers used to define pluripotent stem cells (PSCs) are in fact carbohydrate-based epitopes (Fig. 3). For example, stage specific embryonic antigen 1 (SSEA-1) was one of the first markers used to identify mouse embryonal carcinoma cells; this antigen corresponds to the lacto-series Lewis X antigen (Galβ1-4(Fucα1-3)GlcNAcβ1-R) [18, 19]. Murine embryonic stem cells (ESCs) display Lewis X highly in the eight cell stage embryo and through to the blastocyst stage but lose antigenicity during early germ layer specification [19, 20]. In contrast, human PSCs do not express the Lewis X antigen and are negative for SSEA-1 but are instead identified by antibodies against SSEA-3 and SSEA-4, which recognize related globo-series ganglioside antigens [18, 21, 22]. Monoclonal antibodies against tumor rejection antigens 1-60 and 1-81 (TRA-1-60 and TRA-1-81) (Fig. 3) are also commonly used for identifying hPSCs through recognition of terminal type 1 terminal lactosamine structures (Galβ1–3GlcNAc) [23, 24]. These epitopes are carried by the heavily glycosylated membrane protein podocalyxin and are down-regulated upon differentiation [25]. Each of these antibodies identifies glycan epitopes that are highly expressed in pluripotent cells and together have been used as major tools in the characterization of cells with an embryonic origin.
Figure 3.
Nomenclature and structures of common glycan-based epitopes used as pluripotency markers for mouse and human ESCs. SSEA-1 and SSEA-5 belong to the lactoseries (Lewis, ABH antigens) and are most commonly found on glycolipids but can also be present on glycoproteins. SSEA-3 and SSEA-4 belong to the globoseries (P blood group antigens). The carbohydrate structures of TRA-1-60 and TRA-1-81 antigens are defined as repeating lactosamine units and are most commonly present on glycolipids and mucin-type O-glycans.
Despite their utility in PSC characterization, the glycan epitopes described are sometimes found on the surface of other cell types [26]. To identify glycans that are more specifically associated with the cell surface of PSCs recent efforts have utilized high-throughput screening technologies to identify novel glycan epitopes that offer more specificity and therefore, greater utility. Such reagents are potentially critical in clinical situations where tumor-forming PSCs must be removed from populations to be used for transplantation. One such example is the antigen SSEA-5, recognized by a monoclonal antibody identified from a hybridoma library that specifically detects undifferentiated cells and the equivalent cells in blastocyst-stage embryos [27]. The antibody against SSEA-5 recognizes the H-type 1 epitope, a tri-saccharide structure (Fucα1-2Galβ1-3GlcNAcβ-R) most frequently found as a terminal modification of O-glycans (Fig. 3). This epitope is highly expressed in pluripotent cells and likely to be carried by multiple cell surface proteins but is lost rapidly upon differentiation. The SSEA-5 glyco-epitope rapidly decreases as cells exit the pluripotent state and offers improved sensitivity and resolution compared to other glyco-markers such as SSEA-3, SSEA-4, and TRA-1-81 [27]. In conjunction with antibodies that recognize two other cell surface markers, CD9 and CD90, antibodies against SSEA-5 have been successfully used to eliminate residual pluripotent cells prior to transplantation in a murine model and prevented teratoma formation [17]. Understanding the cell surface glycome in human cells therefore has potential utility in cell-based therapies.
Lectins as tools to identify and characterize pluripotent stem cells
In addition to the use of antibodies, several reports have described the use of plant lectins to characterize subclasses of PSCs and their differentiated derivatives. Lectins are highly specific sugar-binding proteins that have diverse roles in processes such as immunity and cell adhesion. Advantages to using lectins for cell characterization include their glycan sensitivity and specificity, utility across species, reversible binding to the cell surface and much lower expense compared to antibodies.
Pluripotent stem cells in culture exist in multiple forms, representative of the different pluripotent stem cell populations found at different stages of peri-implantation development [28]. An early report describing the utility of lectins in PSC biology addressed the question of whether differential cell surface glycan patterns could discriminate between ‘primed’ and ‘naive’ PSCs [29]. Here, a panel of eighteen lectins with different sugar binding specificities was used to screen for glycan changes as ‘naive’ mESCs convert to the ‘primed’ state. Nash and co-workers showed that the lectin Dolichos biflorus agglutinin recognizes epitopes on the surface of murine ESCs that are rapidly removed as they transition to the 'primed' state. Dolichos biflorus agglutinin recognizes α-N-acetylgalactosamine, typically found in N-linked glycan structures and can be used to effectively isolate mESC subpopulations as well as hPSCs.
Several other groups have exploited the sugar binding specificities of different lectins for characterization of PSCs. One notable example is the use of a high-density lectin microarray to identify novel sugar epitopes on the surface of PSCs. Using this platform, the lectin rBC2LCN was found to recognize epitopes on the surface of over one hundred PSC lines but not to a panel of somatic cells that were tested [30]. Originally derived from the bacterium Burkholderia cenocepacia, rBC2LCN recognizes the epitope of SSEA-5 (H-type 1) but also binds to related epitopes H-type 3 and 4 (Fucα1-2Galβ1-3GlcNAc/GalNAc-R) [30, 31]. These glycan structures are found on podacalyxin, the same cell surface protein that carries TRA-1-60 and TRA-1-81 antigens, suggesting that rBC2LCN recognizes multiple elements commonly associated with PSC identity [25, 32]. rBC2LCN also has utility for depletion of PSCs from mixed cell populations and may prove useful in a clinical setting [33]. In a similar experiment, Wang et al. identified three lectins that could specifically identify PSCs via recognition of fucosylated and sialylated glycans [34]. For example, the fucose-binding lectin UEA-1 shows low reactivity toward differentiated progenitors and can effectively deplete (>99.5% efficiency) PSCs from mixed populations of differentiated cells [18, 19, 34]. Induced pluripotent stem cells (iPSCs) can also be efficiently isolated from mixed cell populations using UEA-1 conjugated magnetic beads. Purified cells can be propagated and then differentiated to all three germ layers[34]. The depletion and isolation strategies outlined, using UEA-1 based reagents as a tool, highlights how knowledge of cell surface glycans can be used for practical purposes.
Pluripotent cells are enriched with proteins carrying simple N-glycan structures
Global cell surface glycan profiles vary considerably between cell types and several reports show that PSCs display their own characteristic glycome. Among the most prominent features of the hPSC glycan signature is the abundance of high mannose N-glycans [19, 20, 35-38]. This contrasts considerably with the vast majority of N-glycan structures in adult cell lineages and human serum fractions that have considerably greater complexity [23, 24, 35, 38-40]. High mannose structures are the core building blocks for all N-linked glycans and become processed enzymatically into more complex structures in the Golgi. The increased relative abundance of high mannose glycans, which represent up to 85% of the total N-glycome in PSCs, may reflect the expansion of the ER in these cells or decreased processing within the Golgi [18, 21, 22, 35]. The latter is less likely based on the transcript abundance analysis of mouse ES cells and differentiated lineages which shows equivalent expression of many glycosyltransferases involved in early N-glycan processing [13].
Fucosylated glycans are a strong indicator of the pluripotent state
Fucose is a deoxyhexose monosaccharide that is involved in a variety of biological processes in eukaryotic organisms including cell adhesion, signaling and embryonic development [27, 41]. Fucosylation of acceptor proteins occurs in the Golgi and is catalyzed by a family of thirteen fucosyltransferases that catalyze the addition of fucose onto N- and O-linked glycan structures. Direct protein fucosylation, defined as the direct linkage of a fucose monosaccharide to serine or threonine residues, can also occur in the ER but to a lower extent compared to the Golgi [27, 42]. Unlike other monosaccharides, which form the core elements of carbohydrate structures, fucose is primarily utilized as a terminal modification to alter the properties of cell surface glycans. The distinction between ABO blood groups is the most prominent example of this [30, 41]. Fucose is also commonly found attached to the chitobiose core of N-glycans.
The high abundance of α1-2 fucosylated glycans is one of the most striking factors that distinguishes hPSCs from differentiated cell types [30, 31, 34, 36, 38, 43, 44]. Increased expression of FUT1 and FUT2 genes, which encode enzymes that catalyze the linkage between fucose and its acceptor protein through an α1-2 linkage, accounts for elevated fucosylation although other regulatory steps are likely to contribute as well [25, 30, 32, 34, 43, 44]. These findings contrast with previous studies in murine PSCs where α1-2 fucosylation is not prevalent, highlighting potential differences in glycosylation patterns between the two species [13, 33]. Alternatively, rather than being indicative of differences at the species level this apparent conflict may indicate that ‘naive’ (murine ESCs) and ‘primed’ PSCs (human ESCs) have a different cell surface glycome, consistent with the earlier work of Nash and co-workers [29].
Interestingly, stem cell markers SSEA-5, rBC2LCN, and UEA-1 all recognize α1-2 fucosylated glycan structures [27, 30, 34]. Together, this indicates that α1-2 fucosylation may be a defining carbohydrate moiety associated with the pluripotent state. Although this suggests that fucosylation should be important for pluripotency, previous studies show that FUT1 and FUT2 deficient mice develop normally and that α1-2 fucosylation is not critical [45]. Other work is required to establish if a redundancy mechanism exists to compensate for loss of this specific glycan modification. It is also unclear if fucosylation is important in human cells and if there are species-specific roles for glycans in development.
Cell surface sialylation is critical for maintenance of pluripotency
N-acetyl-neuraminic acid, commonly known as sialic acid, is a nine-carbon sugar with an acidic carboxyl group attached to the anomeric carbon. Similar to fucose, sialic acid is also a terminal modification of complex carbohydrate structures and often serves as a cap on the non-reducing ends of mature N- and O-linked glycans. Because of this, sialic acid residues are extensively displayed on the surface of mammalian cells with tens of millions residues present on a typical cell [46]. Sialylation of glycoproteins and glycosphingolipids occurs late within the secretory apparatus by a family of Golgi-localized sialyltransferases that most commonly catalyze α2-3 or α2-6 linkages to galactose or the α2-8 linkage to sialic acid in oligo or polysialic acid structures [47]. This process is critical for mammalian development as sialylated glycans coat the surface of cells and perform a multitude of biological functions [47-49]. In addition, sialylated glycans on the surface play major roles in regulating immune and inflammatory responses and in host recognition by viruses, which often recognize specific sialic acid linkages to gain entry into cells [50].
Recent studies examining the glycome have revealed major changes in sialylation as cells transition from pluripotency to differentiated progenitors. hPSCs for example display elevated α2-6 linked sialic acid on their cell surface [34, 36, 38, 51]. In contrast, differentiated derivatives of hPSCs and terminally differentiated somatic cells present α2-3 linked sialic acid on their surface [34, 38]. Similarly, reprogramming of human dermal fibroblasts to iPSCs dramatically reverted the sialic acid linkages back to the α2-6 form [38]. Expression profiles of sialyltransferases match the pattern of sialylation on the cell surface of PSCs. For example, ST6GAL1 is highly expressed in PSCs and probably generates most α2-6 linked structures in human ESCs and human iPSCs [30, 52].
New reports have shown that α2-6 sialylation plays functional roles in pluripotency. Alisson-Silva et al. demonstrated that enzymatic removal of sialic acid from the cell surface triggers differentiation along the ectoderm lineage [51]. Their results also indicated an increase in terminal β-galactopyranoside residues, a structure characteristic of differentiated cells, following the removal of sialic acid that the authors hypothesize could be sensed by cell-surface carbohydrate binding proteins to induce spontaneous differentiation [51]. Alternatively, these results could suggest that α2-6 sialylation is critical for suppressing pro-ectoderm signaling cues present in the stem cell niche. Another recent study showed that loss of ST6GAL1 activity following RNA interference or pharmacological inhibition results in down-regulation of the core pluripotency factor OCT4, increased expression of developmental genes, and reduced reprogramming efficiency [52]. Together, these observations point toward α2-6 sialylation playing important roles in maintaining and establishing the pluripotent state. The mechanism by which α2-6 sialylation contributes to pluripotency is unclear but could be related to receptor function on the cell surface [53, 54].
Cell surface polysialylation is required during early PSC differentiation
Polysialic acid (PSA) is an unusual glycan modification characterized by repeating α2-8 sialic acid residues typically found at the end of N-glycan chains and can form extended polymer structures exceeding hundreds of residues in length. Two enzymes, encoded by ST8SIA2 (STX) and ST8SIA4 (PST), are capable of linking PSA to acceptor proteins in the Golgi. Several PSA acceptor proteins have been identified but neural cell adhesion molecule (NCAM) is generally the most heavily modified and the best characterized [55, 56]. PST and STX polysialyltransferases are critical for normal development and have been shown to perform roles in cell signaling, cell migration and neurogenesis [57-62]. Interestingly, many invasive tumor cells express PSA on their cell surface and polysialylation is often used as a diagnostic determinant of metastatic potential [63].
Although the majority of studies regarding cell surface polysialylation have focused on developmental pathways following organogenesis, recent work has evaluated its role as cells exit pluripotency (Fig. 4A). Pluripotent stem cells are epithelial and display no polysialylated glycans on their surface. As cells transition to the three germ layers (ectoderm, mesoderm and endoderm) levels of polysialylated glycans dramatically increases on the cell surface [64]. Although increased polysialylation is a common event during germ layer specification, this is controlled through the activity of different polysialyltransferases in a germ-layer specific manner. ST8SIA4 (PST) is prevalent in endoderm and mesoderm while ST8SIA2 (STX) is increased in ectoderm, thereby identifying lineage specific use of different transferase genes for polysialylation during early human development. Polysialylation of the cell surface and expression of ST8SIA4 is part of a developmental program and involves the activity of transcriptional regulators such as GOOSECOID that directly targets the ST8SIA4 gene (Fig. 4B). Interestingly, GOOSECOID activates the migratory pathway of invasive tumor cells [65], indicating that metastasis could be driven by the GOOSECOID-dependent activation of polysialyltransferases. shRNA knockdown and CRISPR-Cas9 directed knockout of PST blocks polysialylation and definitive endoderm differentiation, linking glycan modifications to cell fate decisions. Cell surface polysialylation therefore seems to be required for the activation of developmental programs during hPSC differentiation [64]. NCAM seems to be the major polysialylated acceptor protein involved in early cell fate decisions but as stated earlier, different polysialyltransferases are involved in the different germ layers. The mechanism by which increased polysialylation promotes differentiation is currently unknown but is likely to involve regulation of cell signaling and cell-cell interactions [66-68]. Increased polysialylation on the cell surface was observed as PSCs differentiate to the three germ layers in murine and human cells, indicating that this process applies across species barriers [48].
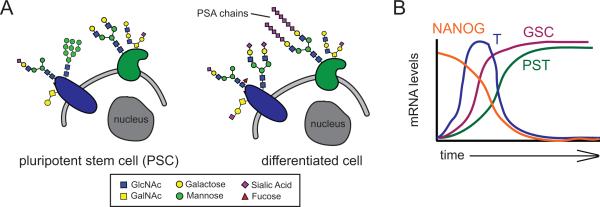
Figure 4.
Increased polysialylation is a hallmark of PSC differentiation. A: PSCs bear unique glycan signatures such as a high relative abundance of high mannose glycans that decrease in abundance upon differentiation. Other signatures such as polysialic acid (PSA) chains increase at the cell surface as cells move from the pluripotent state towards differentiated lineages. B: The increase in PSA expression upon differentiation is driven by increased expression of the polysialyltransferase PST and transcription factor GOOSECOID (GSC) along with a corresponding decrease in NANOG and BRACHYURY (T) expression.
Increased polysialylation upon exit from pluripotency, in spite of the apparent requirement for global sialylation to maintain pluripotency, likely reflects the effects of sialylation at the level of individual glycoproteins. In the case of polysialylation, the increase in this modification specifically on NCAM may drive the bulk of the effects on differentiation potential. More global increases in sialylation in PSCs may exert its effect at the level of multiple cell surface receptors, helping to regulate their residence and maintain the signaling that drives pluripotency. Unraveling these mechanisms represents an important area of future investigation and is likely to uncover new functions for glycans.
Glycosaminoglycans regulate the differentiation of pluripotent cells
Glycosaminoglycans (GAGs) are linear glycan polymers consisting of repeating disaccharide units that make up the extracellular matrix and play key roles in cell adhesion and signaling [69-71]. Hyaluronan, chondroitin sulfate/dermatan sulfate, keratin sulfate and heparan sulfate (HS) represent the major classes of GAGs and are each synthesized by separate glycosyltransferase networks in the Golgi or on the cell surface. The abundance of GAGs on the cell surface is very different between pluripotent and differentiated cells. mESCs display low levels of GAGs on the surface, however significant increases of hyaluronan, chondroitin sulfate/dermatan sulfate and HS are observed upon differentiation and is likely due to increased expression of GAG biosynthetic enzymes and core proteins [72]. Additionally, GAGs exhibit vastly different sulfation patterns upon differentiation indicating that major changes to GAG structures occur during embryonic development [73].
Perhaps the most interesting case of GAGs in stem cell biology is the role of heparan sulfate in differentiation. HS is the most abundant GAG in PSCs, accounting for up to 80% of total GAG content and is generated with surprisingly low levels of sulfation [72-74]. Upon differentiation, total HS content and HS sulfation increase as a result of increased transcript levels for the HS biosynthetic enzymes and sulfo-transferases [72, 73, 75]. Surprisingly, ablation of HS synthesis in murine ESCs causes a dramatic differentiation defect as cells are unable to form any of the three germ layers and retain pluripotent markers [75-77]. This effect is due to aberrant cell signaling through fibroblast growth factor and Wingless pathways, both of which are modulated by direct HS binding to ligands in the extracellular space to facilitate signal transduction, and highlights a critical role for GAGs in developmental processes [78, 79].
Induced pluripotent stem cells as models for glycosylation-related disorders
Over the past decade, iPSCs have proven valuable for modeling a wide array of diseases and genetic disorders [80-83]. The use of iPSCs allows for the study of cellular and molecular disease mechanisms within the patient's own genetic background. The differentiation capabilities of iPSCs makes it possible to investigate such mechanisms in a cell-type specific manner and their proliferative capacity permits unlimited expansion of cells from disease samples that are very rare and difficult to obtain (Fig. 5). Additionally, the ability to manipulate iPSCs using genome-editing technologies, such as CRISPR-Cas9, provides opportunities to determine the direct impact of a patient's genetic profile on disease pathology and potentially correct the disorder in vitro. All of these benefits make iPSC technology an extremely valuable tool for studying human diseases, including those that are caused by defects in the biosynthesis or turnover of glycans.
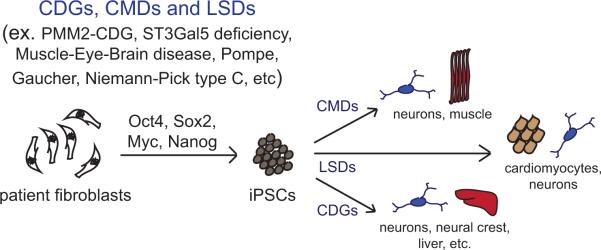
Figure 5.
iPSCs and iPSC-derived cell types as tools for disease modeling. Recent efforts have lead to the development of induced pluripotent stem cells (iPSCs) for several glycosylation-related disorders including the congenital disorders of glycosylation (CDGs), congenital muscular dystrophies (CMDs), and lysosomal storage disorders (LSDs). iPSCs are generated by reprogramming patient fibroblasts with factors that drive pluripotency. These iPSCs can then be directed to differentiate into various disease-relevant specialized cell types such as neurons or muscle precursors.
Congenital disorders of glycosylation (CDGs) and lysosomal storage diseases (LSDs) are two broad classes of disorders that are caused by either defects in glycan biosynthesis or degradation, respectively [84]. LSDs have been recognized since the early 20th century and the genetics of these disorders are well characterized. CDGs occur rarely and are not well studied but advances in technology have led to the discovery of over 100 glycosylation related disorders in the last decade [9, 85]. For both CDGs and LSDs, certain tissues are affected more than others, highlighting the notion that different organ systems are uniquely sensitive to the same underlying defect. Skin fibroblasts are the most accessible cell type available from patients and have been used extensively in the diagnosis of both LSDs and CDGs but have limited utility in exploring pathogenesis. For CDGs, this is primarily due to the fact that these cells often do not manifest defects in glycosylation that are found on glycoproteins in serum and other tissues. Likewise, skin pathology is only found in a subset of CDG patients. Other challenges include the fact that the genetic mutations causing a particular glycosylation disorder can vary from patient to patient. iPSCs provide the ability to study multiple cell types from a single individual while allowing for consistency with regards to genetic background. This approach allows researchers to investigate cell-type specific changes that may generate the disorder's pathology and help explain why some tissues show underglycosylation of glycoproteins whereas others do not. This represents an important emerging area of research in the field of glycosylation related disorders that is set to expand in the coming years. Efforts to apply iPSC technology to the study of CDGs and LSDs are discussed below.
Congenital disorders of glycosylation: modeling PMM2-CDG with hiPSCs
The most common and most studied neurological CDG is phosphomannomutase 2 deficiency, or PMM2-CDG [86]. PMM2-CDG, also known as CDG-1a, is caused by a mutation in PMM2 that encodes the enzyme phosphomannomutase, which catalyzes the conversion of mannose-6-phosphate to mannose-1-phosphate prior to synthesis of GDP-mannose in the cytosol (see Fig. 1). As a result, mutations in PMM2 limits the amount of GDP-mannose available and causes a deficiency in the supply of lipid-linked oligosaccharides available for the production of N-linked glycans [87]. Complete loss of N-linked glycosylation is lethal; therefore, studies of PMM2-CDG cannot be done using complete knockout of the gene [84]. There are several animal models of the disease, including Drosophila, zebrafish and mouse [88-90]. However, the hypomorphic mouse model exhibits embryonic lethality unless the pregnant dam is fed mannose supplementation [90, 91].
To circumvent some of the current limitations associated with animal models and to establish a human model that may be more representative of the clinical picture, multiple research groups have generated and characterized iPSCs from PMM2-CDG patients. Losfeld et al. [73] utilized iPSCs generated from two PMM2-CDG patients to test a fluorescent reporter construct that serves as a functional readout for decreases in N-glycan site occupancy. Their results indicate that PMM2 iPSCs exhibit a similar reduction in N-glycan occupancy compared to the patient fibroblasts, suggesting that the iPSCs accurately reflect the disease phenotype in vitro [92]. More recently, Thiesler et al. generated two iPSC lines from a single PMM2 patient-derived fibroblasts to characterize how PMM2-CDG affects stem cell glycosylation and pluripotency. Their results show that the PMM2-CDG iPSCs exhibit decreased PMM2 activity like the patient fibroblasts and confirm previous studies that report reduced N-glycan site occupancy in PMM2 patient cells, but no change in the profile of remaining N-glycan structures [9, 93]. Interestingly, decreased PMM2 activity in the iPSCs yielded up to 40% reduction in the abundance of high mannose glycans compared to WT PSCs but this reduction did not affect the cells ability to differentiate to all three germ layers [93]. Despite the reduction, these cells still exhibit the common hPSC pattern and bear mainly high mannose glycans. PMM2-iPSCs also exhibited similar patterns of sialylation to wild-type iPSCs as N-glycans were preferentially α2-6 sialylated but not α2-3 sialylated [93].
Modeling lysosomal storage disorders with patient-derived iPSCs
Another emerging area of iPSC disease modeling involves lysosomal storage disorders (LSDs) including Hurler syndrome [94], Pompe disease [95-97], Gaucher disease [98-103], Fabry disease [104], and Niemann-Pick Type C [105]. Each of these storage disorders result from mutations in proteins that facilitate the degradation of particular glycan products. Absence of these proteins results in a build-up of glycan fragments and leads to significant developmental and physiological effects [106]. LSDs often exhibit cell-type specific phenotypes that can best, and perhaps only, be elucidated by differentiating iPSCs towards the most afflicted cell-type.
Using this approach, an investigation of Pompe disease using patient-derived iPSCs recently revealed a novel glycosylation deficit in cardiomyocytes that may contribute to Pompe cardiomyopathy [97]. This finding is significant because it provides a potential mechanism to explain how accumulation of glycogen in lysosomes, a hallmark feature of Pompe disease, results in physiological effects and may highlight new therapeutic targets. Similarly, Maetzel et al. utilized iPSCs to study the effects of lysosomal cholesterol accumulation in hepatic and neural cells from Niemann-Pick Type C disease patients [94]. In their report, they found that defects in autophagic flux were associated with mutations in the NPC1 gene and identified an autophagy-inducing compound via small molecule screen that led to increased cell viability in NPC1 deficient cells [94]. Lastly, iPSC-derived neurons were generated from Gaucher patient fibroblasts and used to demonstrate altered Transcription Factor EB (TFEB)-mediated lysosomal biogenesis and electrophysiological responses as a consequence of glucosylceramide storage [99, 100]. In separate studies, iPSC-derived macrophages were also developed, revealing increased expression of inflammatory mediators, reduced production of reactive oxygen species and impaired chemotaxis [101, 102]. These recent works, among others, highlight the immense potential of iPSCs as a model system for in vitro disease modeling of LSDs and also highlight the application of these new cell systems for therapeutic assessment.
Challenges and opportunities with the application of CDG iPSCs
Despite their utility, multiple challenges still exist with the use of iPSCs to model CDGs [93, 96]. To date, the biggest issue lies in reprogramming efficiency. Thiesler et al. reported 0.008% efficiency of reprogramming PMM2-fibroblasts using standard methods, much lower than reprogramming rates typically achieved using wild type human fibroblasts. This effect may be tied to perturbations in glycosylation pathways and suggest that proper glycosylation is important for reprogramming. It may also be challenging to manipulate culture conditions for these cells (e.g. lower glucose concentration) without also influencing their differentiation potential. Lastly, it remains to be seen whether robust cellular phenotypes will emerge in specialized cell types derived form CDG iPSCs.
For most CDGs, the reason why glycosylation defects only manifest in specific tissues or cell types is unknown. iPSC and iPSC-derived cell lines from CDG patients should prove useful in defining the tissue-specific mechanisms that account for this effect. For those CDGs that impact the biosynthesis of glycan precursors, is it possible that the unique metabolism of some specialized cells increases the sensitivity of those cells to the underlying CDG defect? Or perhaps the increased production and secretion of glycoproteins in certain cell types such as hepatocytes places greater stress on the compromised glycosylation machinery? Of further interest will be the investigation of whether tissue-specific changes in glycosylation in this context will correspond to specific cellular or molecular phenotypes. Lastly, the development of these cell-based models for CDG should ultimately allow for the identification of glycoproteins whose stability or surface residence is sensitive to abnormal glycosylation. Such glycoproteins would represent high priority targets for the study of pathogenesis or therapy development.
Conclusion and outlook
Glycans not only serve as useful markers for stem cell pluripotency but also regulate the ability of these cells to differentiate towards specific lineages. As our understanding of the glycome in these cell types increases, further insights into how glycosylation controls cell fate decisions are likely to emerge. The ability to study these same questions in the context of glycosylation-related disorders should spur the development of new knowledge into the molecular and cellular bases for these disorders and hold the promise of identifying targets for therapeutic intervention. This work is already beginning to uncover new clues into the disease process for lysosomal storage disorders. The ability to track specific phenotypes in disease relevant cell types will provide researchers with valuable new cell models for use in the high throughput screening of potential drugs. We see similar potential for the use of iPSCs in the study of CDGs as a way to finally break free of the constraints associated with patient-derived skin fibroblasts.
Acknowledgments
The authors acknowledge support from the National Institutes of Health, Institute of General Medical Sciences (P01 GM75334 and P41 GM103490) and for helpful suggestions from Michael Tiemeyer.
Abbreviations
- CDGs
congenital disorders of glycosylation
- ESCs
embryonic stem cells
- GAGs
glycosaminoglycans
- HS
heparan sulfate
- iPSCs
induced pluripotent stem cells
- LSDs
lysosomal storage diseases
- NCAM
neural cell adhesion molecule
- PSA
polysialic acid
- PSCs
pluripotent stem cells
- SSEA
stage specific embryonic antigen
- TRA
tumor rejection antigens
References
- 1.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–64. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136:272–83. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niwa Y, Suzuki T, Dohmae N, Simizu S. Identification of DPY19L3 as the C-mannosyltransferase of R-spondin1 in human cells. Mol Cell Biol. 2016;27:744–56. doi: 10.1091/mbc.E15-06-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardivillé S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20:208–13. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–84. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi N, Kizuka Y. In Glycosylation and Cancer. Academic Press; 2015. Chapter Two - Glycans and Cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. pp. 11–51. [DOI] [PubMed] [Google Scholar]
- 9.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–51. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 10.Hennet T, Cabalzar J. Congenital disorders of glycosylation: a concise chart of glycocalyx dysfunction. Trends Biochem Sci. 2015;40:377–84. doi: 10.1016/j.tibs.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Stanley P. What have we learned from glycosyltransferase knockouts in mice? J Mol Biol. 2016;428:3166–3182. doi: 10.1016/j.jmb.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allende ML, Proia RL. Simplifying complexity: genetically resculpting glycosphingolipid synthesis pathways in mice to reveal function. Glycoconj J. 2014;31:613–22. doi: 10.1007/s10719-014-9563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nairn AV, Aoki K, Rosa dela M, Porterfield M, et al. Regulation of glycan structures in murine embryonic stem cells: combined transcript profiling of glycan-related genes and glycan structural analysis. J Biol Chem. 2012;287:37835–56. doi: 10.1074/jbc.M112.405233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North SJ, Hitchen PG, Haslam SM, Dell A. Mass spectrometry in the analysis of N-linked and O-linked glycans. Curr Opin Struct Biol. 2009;19:498–506. doi: 10.1016/j.sbi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoldoš V, Horvat T, Lauc G. Glycomics meets genomics, epigenomics and other high throughput omics for system biology studies. Omics. 2013;17:34–40. doi: 10.1016/j.cbpa.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Stöckmann H, Adamczyk B, Hayes J, Rudd PM. Automated, high-throughput IgG-antibody glycoprofiling platform. Anal Chem. 2013;85:8841–9. doi: 10.1021/ac402068r. [DOI] [PubMed] [Google Scholar]
- 17.Furness SGB, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res. 2006;34:13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- 18.Gooi HC, Feizi T, Kapadia A, Knowles BB, et al. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981;292:156–8. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- 19.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci USA. 1978;75:5565–9. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennington JE, Rastan S, Roelcke D, Feizi T. Saccharide structures of the mouse embryo during the first eight days of development. Inferences from immunocytochemical studies using monoclonal antibodies in conjunction with glycosidases. J Embryol Exp Morphol. 1985;90:335–61. [PubMed] [Google Scholar]
- 21.Kannagi R, Cochran NA, Ishigami F, Hakomori S, et al. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–61. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damjanov I, Fox N, Knowles BB, Solter D, et al. Immunohistochemical localization of murine stage-specific embryonic antigens in human testicular germ cell tumors. Am J Pathol. 1982;108:225–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews PW, Banting G, Damjanov I, Arnaud D, et al. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984;3:347–61. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- 24.Natunen S, Satomaa T, Pitkanen V, Salo H, et al. The binding specificity of the marker antibodies Tra-1-60 and Tra-1-81 reveals a novel pluripotency-associated type 1 lactosamine epitope. Glycobiology. 2011;21:1125–30. doi: 10.1093/glycob/cwq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schopperle WM, DeWolf WC. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells. 2006;25:723–30. doi: 10.1634/stemcells.2005-0597. [DOI] [PubMed] [Google Scholar]
- 26.Brimble SN, Sherrer ES, Uhl EW, Wang E, et al. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2006;25:54–62. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- 27.Tang C, Lee AS, Volkmer J-P, Sahoo D, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–34. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–92. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Nash R, Neves L, Faast R, Pierce M, et al. The lectin dolichos biflorus agglutinin recognizes glycan epitopes on the surface of murine embryonic stem cells: a new tool for characterizing pluripotent cells and early differentiation. Stem Cells. 2007;25:974–82. doi: 10.1634/stemcells.2006-0224. [DOI] [PubMed] [Google Scholar]
- 30.Tateno H, Toyota M, Saito S, Onuma Y, et al. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011;286:20345–53. doi: 10.1074/jbc.M111.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SulAk O, Cioci G, Delia M, Lahmann M, et al. A TNF-like trimeric lectin domainfrom burkholderia cenocepacia with Specificityfor Fucosylated Human histo-blood group antigens. Structure. 2010;18:59–72. doi: 10.1016/j.str.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Tateno H, Matsushima A, Hiemori K, Onuma Y, et al. Podocalyxin is a glycoprotein ligand of the human pluripotent stem cell-specific probe rBC2LCN. Stem Cells Transl Med. 2013;2:265–73. doi: 10.5966/sctm.2012-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tateno H, Onuma Y, Ito Y, Minoshima F, et al. Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem Cell Rep. 2015;4:811–20. doi: 10.1016/j.stemcr.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y-C, Nakagawa M, Garitaonandia I, Slavin I, et al. Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res. 2011;21:1551–63. doi: 10.1038/cr.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An HJ, Gip P, Kim J, Wu S, et al. Extensive determination of glycan heterogeneity reveals an unusual abundance of high mannose glycans in enriched plasma membranes of human embryonic stem cells. Mol Cell Proteomics. 2012;11:M111.010660–0. doi: 10.1074/mcp.M111.010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satomaa T, Heiskanen A, Mikkola M, Olsson C, et al. The N-glycome of human embryonic stem cells. BMC Cell Biol. 2009;10:42. doi: 10.1186/1471-2121-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujitani N, Furukawa J-I, Araki K, Fujioka T, et al. Total cellular glycomics allows characterizing cells and streamlining the discovery process for cellular biomarkers. Proc Natl Acad Sci USA. 2013;110:2105–10. doi: 10.1073/pnas.1214233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasehira K, Tateno H, Onuma Y, Ito Y, et al. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol Cell Proteomics. 2012;11:1913–23. doi: 10.1074/mcp.M112.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao SC, Li Y, Zhou J, Qian J, et al. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology. 2008;18:761–9. doi: 10.1093/glycob/cwn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu CS, Niñonuevo MR, Clowers BH, Perkins PD, et al. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9:1939–51. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 42.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 43.Liang Y-J, Kuo H-H, Lin C-H, Chen Y-Y, et al. Switching of the core structures of glycosphingolipids from globo- and lacto- to ganglio-series upon human embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2010;107:22564–9. doi: 10.1073/pnas.1007290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojima T, Shibata E, Saito S, Toyoda M, et al. Glycolipid dynamics in generation and differentiation of induced pluripotent stem cells. Sci Rep. 2015;5:14988. doi: 10.1038/srep14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domino SE, Zhang L, Gillespie PJ, Saunders TL, et al. Deficiency of reproductive tract alpha (1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha (1,2)fucosyltransferase locus. Mol. Cell. Biol. 2001;21:8336–45. doi: 10.1128/MCB.21.24.8336-8345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins BE, Blixt O, DeSieno AR, Bovin N, et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci USA. 2004;101:6104–9. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varki A, Schauer R. Sialic Acids. In Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 48.Schwarzkopf M, Knobeloch K-P, Rohde E, Hinderlich S, et al. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–70. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weidemann W, Klukas C, Klein A, Simm A, et al. Lessons from GNE-deficient embryonic stem cells: sialic acid biosynthesis is involved in proliferation and gene expression. Glycobiology. 2009;20:107–17. doi: 10.1093/glycob/cwp153. [DOI] [PubMed] [Google Scholar]
- 50.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alisson-Silva F, de Carvalho Rodrigues D, Vairo L, Asensi KD, et al. Evidences for the involvement of cell surface glycans in stem cell pluripotency and differentiation. Glycobiology. 2014;24:458–68. doi: 10.1093/glycob/cwu012. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y-C, Stein JW, Lynch CL, Tran HT, et al. Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells. Sci Rep. 2015;5:13317. doi: 10.1038/srep13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–18. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J-J, Yi JY, Jin YB, Lee Y-J, et al. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem Pharmacol. 2012;83:849–57. doi: 10.1016/j.bcp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Angata K, Fukuda M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie. 2003;85:195–206. doi: 10.1016/s0300-9084(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 56.Hildebrandt H, Mühlenhoff M, Gerardy-Schahn R. Structure and function of the neural cell adhesion molecule NCAM. Springer; New York: 2009. Polysialylation of NCAM. pp. 95–109. [Google Scholar]
- 57.Colley KJ, Kitajima K, Sato C. Polysialic acid: Biosynthesis, novel functions and applications. Crit Rev Biochem Mol Biol. 2014;49:498–532. doi: 10.3109/10409238.2014.976606. [DOI] [PubMed] [Google Scholar]
- 58.Angata K, Fukuda M. Roles of polysialic acid in migration and differentiation of neural stem cells. Meth Enzymol. 2010;479:25–36. doi: 10.1016/S0076-6879(10)79002-9. [DOI] [PubMed] [Google Scholar]
- 59.Fujimoto I, Bruses JL, Rutishauser U. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J Biol Chem. 2001;276:31745–51. doi: 10.1074/jbc.M104525200. [DOI] [PubMed] [Google Scholar]
- 60.Ono S, Hane M, Kitajima K, Sato C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J Biol Chem. 2012;287:3710–22. doi: 10.1074/jbc.M111.276618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinhold B, Seidenfaden R, Röckle I, Mühlenhoff M, et al. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–7. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 62.Hildebrandt H, Mühlenhoff M, Weinhold B, Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103:56–64. doi: 10.1111/j.1471-4159.2007.04716.x. [DOI] [PubMed] [Google Scholar]
- 63.Holst S, Wuhrer M, Rombouts Y. Glycosylation and Cancer. Academic Press; 2015. Chapter Six - Glycosylation Characteristics of Colorectal Cancer. pp. 203–56. [DOI] [PubMed] [Google Scholar]
- 64.Berger RP, Sun YH, Kulik M, Lee JK, et al. ST8SIA4 dependent polysialylation is part of a developmental program required for germ layer formation from human pluripotent stem cells. Stem Cells. 2016;34:1742–52. doi: 10.1002/stem.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartwell KA, Muir B, Reinhardt F, Carpenter AE, et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci USA. 2006;103:18969–74. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiermaier E, Moussion C, Veldkamp CT, Gerardy-Schahn R, et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science. 2016;351:186–90. doi: 10.1126/science.aad0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eggers K, Werneburg S, Schertzinger A, Abeln M, et al. Polysialic acid controls NCAM signals at cell-cell contacts to regulate focal adhesion independent from FGF receptor activity. J Cell Sci. 2011;124:3279–91. doi: 10.1242/jcs.084863. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Dai G, Cheng YB, Qi X, et al. Polysialylation promotes neural cell adhesion molecule-mediated cell migration in a fibroblast growth factor receptor-dependent manner, but independent of adhesion capability. Glycobiology. 2011;21:1010–8. doi: 10.1093/glycob/cwr020. [DOI] [PubMed] [Google Scholar]
- 69.Lander AD, Selleck SB. The elusive functions of proteoglycans: in vivo veritas. J Cell Biol. 2000;148:227–32. doi: 10.1083/jcb.148.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kjellén L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–75. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 71.Linhardt RJ, Toida T. Role of glycosaminoglycans in cellular communication. Acc Chem Res. 2004;37:431–8. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- 72.Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, et al. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–87. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gasimli L, Hickey AM, Yang B, Li G, et al. Changes in glycosaminoglycan structure on differentiation of human embryonic stem cells towards mesoderm and endoderm lineages. Biochim Biophys Acta. 2014;1840:1993–2003. doi: 10.1016/j.bbagen.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin X, Wei G, Shi Z, Dryer L, et al. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224:299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- 75.Kraushaar DC, Dalton S, Wang L. Heparan sulfate: a key regulator of embryonic stem cell fate. Biol Chem. 2013;394:741–51. doi: 10.1515/hsz-2012-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraushaar DC, Yamaguchi Y, Wang L. Heparan sulfate is required for embryonic stem cells to exit from self-renewal. J Biol Chem. 2010;285:5907–16. doi: 10.1074/jbc.M109.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson CE, Crawford BE, Stavridis M, Dam Ten G, et al. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells. 2007;25:1913–23. doi: 10.1634/stemcells.2006-0445. [DOI] [PubMed] [Google Scholar]
- 78.Lanner F, Lee KL, Sohl M, Holmborn K, et al. Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. Stem Cells. 2010;28:191–200. doi: 10.1002/stem.265. [DOI] [PubMed] [Google Scholar]
- 79.Kraushaar DC, Rai S, Condac E, Nairn A, et al. Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J Biol Chem. 2012;287:22691–700. doi: 10.1074/jbc.M112.368241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–36. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cherry ABC, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med. 2013;64:277–90. doi: 10.1146/annurev-med-050311-163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet. 2011;20:R109–15. doi: 10.1093/hmg/ddr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cramer AO, MacLaren RE. Translating induced pluripotent stem cells from bench to bedside: application to retinal diseases. Curr Gene Ther. 2013;13:139–51. doi: 10.2174/1566523211313020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freeze HH, Schachter H. In Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; 2009. Genetic disorders of glycosylation. [PubMed] [Google Scholar]
- 85.Freeze HH, Chong JX, Bamshad MJ, Ng BG. Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet. 2014;94:161–75. doi: 10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaeken J. Handbook of Clinical Neurology. Elsevier; 2013. Chapter 179 - Congenital disorders of glycosylation. pp. 1737–43. [DOI] [PubMed] [Google Scholar]
- 87.Westphal V, Peterson S, Patterson M, Tournay A, et al. Functional significance of PMM2 mutations in mildly affected patients with congenital disorders of glycosylation Ia. Genet Med. 2001;3:393–8. doi: 10.1097/00125817-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Cline A, Gao N, Flanagan-Steet H, Sharma V, et al. A zebrafish model of PMM2-CDG reveals altered neurogenesis and a substrate-accumulation mechanism for N-linked glycosylation deficiency. Mol Cell Biol. 2012;23:4175–87. doi: 10.1091/mbc.E12-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parkinson WM, Dookwah M, Dear ML, Gatto CL, et al. Synaptic roles for phosphomannomutase type 2 in a new Drosophila congenital disorder of glycosylation disease model. Dis Model Mech. 2016;9:513–27. doi: 10.1242/dmm.022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schneider A, Thiel C, Rindermann J, DeRossi C, et al. Successful prenatal mannose treatment for congenital disorder of glycosylation-Ia in mice. Nat Med. 2011;18:71–3. doi: 10.1038/nm.2548. [DOI] [PubMed] [Google Scholar]
- 91.Thiel C, Lübke T, Matthijs G, Figura von K, et al. Targeted disruption of the mouse phosphomannomutase 2 gene causes early embryonic lethality. Mol Cell Biol. 2006;26:5615–20. doi: 10.1128/MCB.02391-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Losfeld M-E, Soncin F, Ng BG, Singec I, et al. A sensitive green fluorescent protein biomarker of N-glycosylation site occupancy. FASEB J. 2012;26:4210–7. doi: 10.1096/fj.12-211656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thiesler CT, Cajic S, Hoffmann D, Thiel C, et al. Glycomic characterization of induced pluripotent stem cells derived from a patient suffering from phosphomannomutase 2 congenital disorder of glycosylation (PMM2-CDG). Mol Cell Proteomics. 2016;15:1435–52. doi: 10.1074/mcp.M115.054122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tolar J, Park I-H, Xia L, Lees CJ, et al. Hematopoietic differentiation of induced pluripotent stem cells from patients with mucopolysaccharidosis type I (Hurler syndrome). Blood. 2011;117:839–47. doi: 10.1182/blood-2010-05-287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higuchi T, Kawagoe S, Otsu M, Shimada Y, et al. The generation of induced pluripotent stem cells (iPSCs) from patients with infantile and late-onset types of Pompe disease and the effects of treatment with acid-α-glucosidase in Pompe's iPSCs. Mol Genet Metab. 2014;112:44–8. doi: 10.1016/j.ymgme.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Huang H-P, Chen P-H, Hwu W-L, Chuang C-Y, et al. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Hum Mol Genet. 2011;20:4851–64. doi: 10.1093/hmg/ddr424. [DOI] [PubMed] [Google Scholar]
- 97.Raval KK, Tao R, White BE, De Lange WJ, et al. Pompe disease results in a Golgi-based glycosylation deficit in human induced pluripotent stem cell-derived cardiomyocytes. J Biol Chem. 2015;290:3121–36. doi: 10.1074/jbc.M114.628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panicker LM, Miller D, Park TS, Patel B, et al. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc Natl Acad Sci USA. 2012;109:18054–9. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Awad O, Sarkar C, Panicker LM, Miller D, et al. Altered TFEB-mediated lysosomal biogenesis in Gaucher disease iPSC-derived neuronal cells. Hum Mol Genet. 2015;24:5775–88. doi: 10.1093/hmg/ddv297. [DOI] [PubMed] [Google Scholar]
- 100.Sun Y, Florer J, Mayhew CN, Jia Z, et al. Properties of neurons derived from induced pluripotent stem cells of Gaucher disease type 2 patient fibroblasts: potential role in neuropathology. PLoS One. 2015;10:e0118771. doi: 10.1371/journal.pone.0118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aflaki E, Stubblefield BK, Maniwang E, Lopez G, et al. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 2014;6:240ra73. doi: 10.1126/scitranslmed.3008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panicker LM, Miller D, Awad O, Bose V, et al. Gaucher iPSC-derived macrophages produce elevated levels of inflammatory mediators and serve as a new platform for therapeutic development. Stem Cells. 2014;32:2338–49. doi: 10.1002/stem.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tiscornia G, Vivas EL, Matalonga L, Berniakovich I, et al. Neuronopathic Gaucher's disease: induced pluripotent stem cells for disease modelling and testing chaperone activity of small compounds. Hum Mol Genet. 2013;22:633–45. doi: 10.1093/hmg/dds471. [DOI] [PubMed] [Google Scholar]
- 104.Kawagoe S, Higuchi T, Otaka M, Shimada Y, et al. Morphological features of iPS cells generated from Fabry disease skin fibroblasts using Sendai virus vector (SeVdp). Mol Genet Metab. 2013;109:386–9. doi: 10.1016/j.ymgme.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Maetzel D, Sarkar S, Wang H, Abi-Mosleh L, et al. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Stem Cell Rep. 2014;2:866–80. doi: 10.1016/j.stemcr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grabowski GA. Gaucher disease and other storage disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:13–8. doi: 10.1182/asheducation-2012.1.13. [DOI] [PubMed] [Google Scholar]