Abstract
Epstein-Barr virus (EBV) is one of the most common viruses in humans, capable of causing life-threatening infections and cancers in immunocompromised individuals. Although CD8+ T cells provide key protection against EBV, the persistence and dynamics of specific T-cell receptor (TCR) clones during immunosuppression in transplant patients is largely unknown. For the first time, we used a novel single-cell TCRαβ multiplex-nested reverse transcriptase PCR to dissect TCRαβ clonal diversity within GLCTLVAML (GLC)-specific CD8+ T cells in healthy individuals and immunocompromised lung transplant recipients. The GLC peptide presented by HLA-A*02:01 is one of the most immunogenic T-cell targets from the EBV proteome. We found that the GLC-specific TCRαβ repertoire was heavily biased toward TRAV5 and encompassed five classes of public TCRαβs, suggesting that these clonotypes are preferentially utilized following infection. We identified that a common TRAV5 was diversely paired with different TRAJ and TRBV/TRBJ genes, in both immunocompetent and immunocompromised individuals, with an average of 12 different TCRαβ clonotypes/donor. Moreover, pre-transplant GLC-specific TCRαβ repertoires were relatively stable over 1 year post transplant under immunosuppression in the absence or presence of EBV reactivation. In addition, we provide the first evidence of early GLC-specific CD8+ T cells at 87 days post transplant, which preceded clinical EBV detection at 242 days in an EBV-seronegative patient receiving a lung allograft from an EBV-seropositive donor. This was associated with a relatively stable TCRαβ repertoire after CD8+ T-cell expansion. Our findings provide insights into the composition and temporal dynamics of the EBV-specific TCRαβ repertoire in immunocompromised transplant patients and suggest that the early detection of EBV-specific T cells might be a predictor of ensuing EBV blood viremia.
Epstein-Barr virus (EBV), a member of the human herpesvirus family, infects ~95% of the developed world's population. Transmission during infancy occurs via saliva and is generally asymptomatic. However, adolescents have a higher chance of developing acute infectious mononucleosis, known as glandular fever, compared with the general population. In Western countries, the estimated incidence rate of infectious mononucleosis is 320–370/1 00 000 per annum in adolescents (15–19 years) compared with a general rate of 45/1 00 000 per annum.1, 2 EBV causes opportunistic infection in immunocompromised individuals, including HIV-infected and post-solid organ transplant patients undergoing maintenance immunosuppression.3, 4 CD8+ T cells have a crucial role in controlling EBV infection by providing persistent immunosurveillance. One of the most immunogenic T-cell targets from EBV is the GLCTLVAML (GLC) peptide derived from the lytic BMLF1 protein (residues 280–288). GLC is restricted to the HLA-A*02:01 allele, the most prevalent HLA-I molecule in Caucasians, with a frequency of 35–40%. In the peripheral blood of healthy EBV-positive individuals, circulating GLC-specific CD8+ T cells constitute between 0.5–2.2% of total CD8+ T cells during latent infection. During primary infection and in the elderly, this single antigen-specific population can increase to up to 10% of total circulating CD8+ T cells.5, 6
GLC-specific CD8+ T cells are characterized by a highly skewed T-cell receptor (TCR) repertoire, with identical (public) or near-identical TCRs within and between individuals.7, 8, 9, 10 This repertoire is relatively stable over time.11, 12 TCRs that bind the A2-GLC complex are composed of membrane-bound alpha (α) and beta (β) chains generated from the random recombination of variable (V), diversity (D), joining (J) and constant (C) genes. Further diversity is created by pairing the α and β chains with nucleotides additions and deletions between the V(D)J junctions specifically at the complementary-determining region 3 (CDR3).13 Given the potential TCR diversity of >1015 thymic14 and >107 peripheral different TCR combinations,15 it is surprising that the GLC-specific CD8+ TCR repertoire is biased to an average of ~9 unique clonotypes (range 3–20) per individual.7, 16
Structural studies between the TCR, peptide (p) and MHC (reviewed)17, 18 have provided insights into how TCRs are selected to preferentially bind to their cognate pMHC and thus shape the overall immune response (reviewed).10 The most abundant TCR clonotype within GLC-specific CD8+ T cells has been identified,7, 8, 9, 19 named AS01.20 AS01 consists of TRAV5/TRAJ31/TRBV20-1/TRBJ1-2 genes with CDR3α-DNNARL and CDR3β-RDGTGNGY sequences. Structurally and thermodynamically, AS01-TCR was preferentially selected by drawing on germline residues to uniquely engage the GLC/HLA-A*02:01 complex.20
Here, we use a new unbiased, single-cell TCRαβ multiplex-nested reverse transcriptase PCR21, 22 to quantitatively dissect clonotypic TCRαβ diversity within the GLC-specific CD8+ T cells and track the abundance of the AS01 clonotype in healthy individuals directly ex vivo. Furthermore, we explored the temporal dynamics of the GLC-specific CD8+ TCRαβ repertoire in a longitudinal cohort of lung transplant recipients under immunosuppressive conditions before and after EBV exposure. Extending previous studies, we observed that the GLC-specific CD8+ T-cell response was heavily dominated by TRAV5, yet was diversely paired with other TRAJ genes and TRBV/TRBJ genes, in both immunocompetent and immunocompromised individuals, with an average of 12 different TCRαβ clonotypes per individual (range 7–22 clonotypes). Our paired TCRαβ sequence analysis revealed four additional prominent public clonotypes, in addition to AS01, that were shared between donors. This bias had previously been underestimated because of the lack of paired TCRαβ analysis, low number of examined donors and/or limited cloning methodologies.
In the lung transplant cohort, we report for the first time, the detection of preclinical GLC-specific CD8+ T cells, using tetramer-associated magnetic enrichment (TAME), before the detectable onset of primary EBV infection (155 days earlier) in an EBV-seronegative patient, who received an EBV-seropositive donor lung allograft. Most strikingly, these preclinical GLC-specific CD8+ T cells exhibited prominent TRAV5 bias and encompassed public TCR clonotypes identified earlier in the healthy donors, suggesting that TCRαβ repertoires may have been genetically favorable prior to detectable viremia based on viral load titers and perhaps antigenic exposure. Importantly, the GLC-specific CD8+ TCRαβ repertoires within both EBV-seropositive and EBV-seronegative lung transplant recipients remained stable over time under immunosuppressive conditions in either the presence or absence of EBV-related clinical and viral titer events.
Results
GLC-specific T cells exhibit prominent TRAV5 gene bias with diverse TCRαβ repertoires in healthy donors
Historically, studies investigating GLC-specific CD8+ TCR repertoires to identify the dominant AS01-TCR used extensive cloning methodologies,8, 9 bulk sorting of GLC-specific CD8+ T cells for total mRNA9 or sequencing of individual CDR3α and/or CDR3β gene segments.7, 19 Here, we quantitatively characterized the GLC-specific TCR repertoire using a broader unbiased TCRαβ paired analysis,21 whereby TCRαβ transcripts from a single cell were simultaneously amplified following reverse transcriptase PCR and multiplex-nested PCR.22 GLC-specific CD8+ T cells from ex vivo peripheral blood mononuclear cell (PBMC) of ‘healthy' donors (that is, EBV-positive donors with no evidence of EBV reactivation) were used for TCRαβ analysis (Figure 1a).
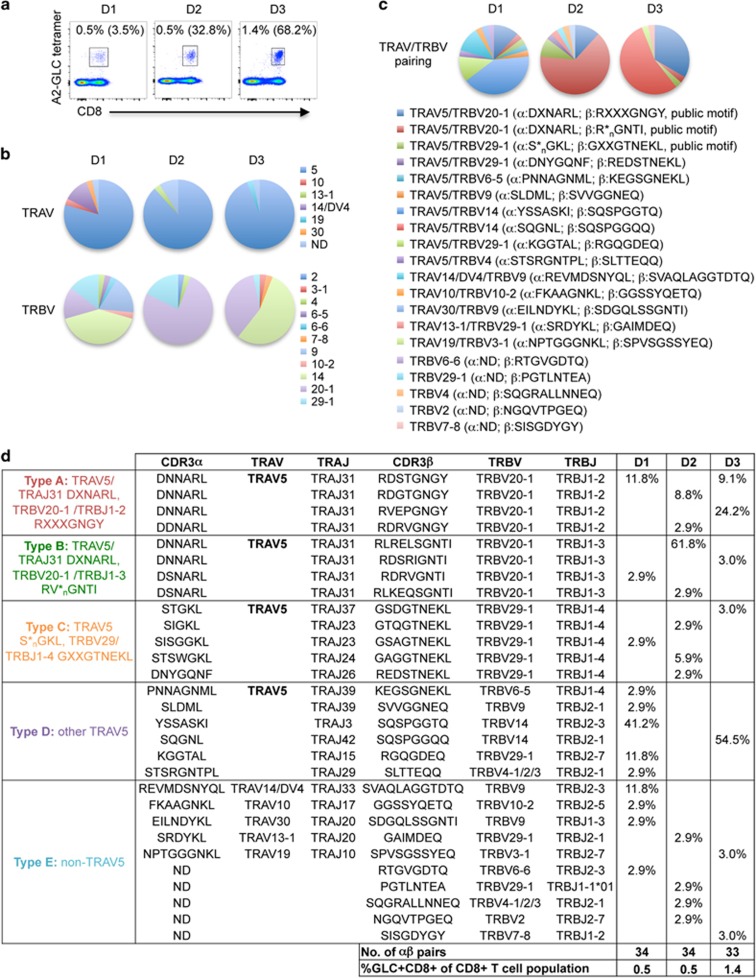
Figure 1.
TCRαβ repertoire of peripheral blood CD8+ T cells directed at the HLA-A*02:01-restricted EBV-GLC epitope in healthy individuals. Ex vivo stained GLC-specific CD8+ T cells from healthy donors D1-D3 were single-cell sorted for TCRαβ analysis. Representative FACS plots (a) of ex vivo detected GLC+CD8+ T cells. Cells were gated on live CD3+ T cells to compare the tetramer staining of CD8+ T cells against the background staining of CD8− T cells. Percentages are based on gated CD8+ T-cell population. Percentages of GLC+CD8+ T cells following in vitro expansion are shown in brackets. Frequency (b) of TRAV and TRBV gene usage for D1-3. Paired TRAV-TRBV clonotype frequencies are shown in c. Clonotypes A-E (d) were first grouped according to their TRAV5 gene usage followed by their pairing to TRAJ, TRBV and TRBJ genes to identify publicly paired motifs. Abbreviations: ND, not determined; X, different amino acid; *n, any number of amino acids.
The ex vivo GLC-specific CD8+ TCRαβ repertoire in healthy donors was dominated by TRAV5, representing 79.3–93.8% of the total TCRα repertoire. TRBV usage was more diverse between donors with TRBV14, TRBV20-1 and TRBV29-1 being the most frequently deployed genes (Figure 1b). Interestingly, paired TCRαβ analysis revealed that the three most dominant TRBV genes (TRBV14, TRBV20-1 and TRBV29-1) were preferentially paired with TRAV5, representing 58.8–93.8% of collective pairing (Figure 1c).
On average, the GLC-specific CD8+ TCRαβ repertoire consisted of 10 TCRαβ clonotypes (ranged 7–12) per individual. Paired TCRαβ clonotypes were grouped into five ‘classes' comprising of TRAV5 paired with dominant TRBV genes (Type A-C), other TRAV5 clonotypes (Type D) and non-TRAV5 clonotypes (Type E) (Figure 1d). In a pooled analysis, 49% of all TRAV5-bearing clonotypes were paired with TRAJ31 (Type A-B; Figure 1d) with identical (40/43 cells) or nearly identical (3/43 cells) CDR3α sequences to the public AS01-TCR (DXNARL, where ‘X' denotes amino-acid differences to the consensus sequence). In addition, these TRAV5/TRAJ31 clonotypes were paired with the public TRBV20-1 gene, but were not necessarily biased toward TRBJ1-2 (Type A; Figure 1d) and showed less similarity to AS01's CDR3β sequence (RXXXGNGY). Rather, >50% clonotypes within the TRAV5/TRAJ31/TRBV20-1 group contained the TRBJ1-3 gene (58%, Type B; Figure 1d). TRAV5 was also paired with the TRBV29-1/TRBJ1-4 genes but had variable CDR3α sequences and TRAJ gene usage (Type C; Figure 1d). Individually, 2/3 donors showed the most dominant pairing of TRAV5 with TRBV14 (D1: 41.2% and D3: 54.5%), whereas donor D2 had 61.8% of their GLC-specific TCR repertoire having a Type B pairing (TRAV5/TRAJ31/TRBV20-1/TRBJ1-3). Hence, type A and type B pairings may not necessarily be the most dominant TCR deployment across all individuals. Nevertheless, our paired single-cell analysis indicated a striking TRAV5 bias despite a highly diverse GLC-specific TCRαβ response in healthy EBV-positive donors.
GLC-specific TCRαβ repertoires in EBV-positive lung transplant patients with no detectable EBV reactivation are stable over time
We next examined whether similar TRAV5 bias was observed in clinical settings, and in particular, whether TCRαβ repertoires remained stable under immunosuppressive therapy following lung transplantation. EBV-positive patients Tx98 and Tx111 received bilateral lung allografts from EBV-negative donors and experienced no episodes of EBV reactivation, as clinically indicated or by viral load testing during the 12–13 months of follow-up post transplantation.
Owing to a limited number of patients' cells, PBMC from Tx98 and Tx111 were expanded for TCRαβ analysis (Figure 2a). Overall, the GLC-specific TCRαβ repertoire from patient Tx98 was dominated by TRAV5, which represented 46% of TCRαβ sequences (n=133). Clonotypes D, E, F and G bearing near-identical TRAV5/TRAJ31/TRBV20-1/TRBJ1-2 TCR sequences were present at pre-transplant (39%), which persisted after transplantation (21–34%) (Figures 2b and d). In addition, we observed another dominant non-TRAV5 TCR comprising TRAV16/TRAJ48/TRBV/TRBJ genes (Clonotype J) present at pre-transplant (18%), and observed in similar frequencies throughout the follow-up period (22–26%). The majority of dominant (D, J, K and L) and subdominant clonotypes (A, C and E) were observed pre- and post transplant (Figures 2b and d).
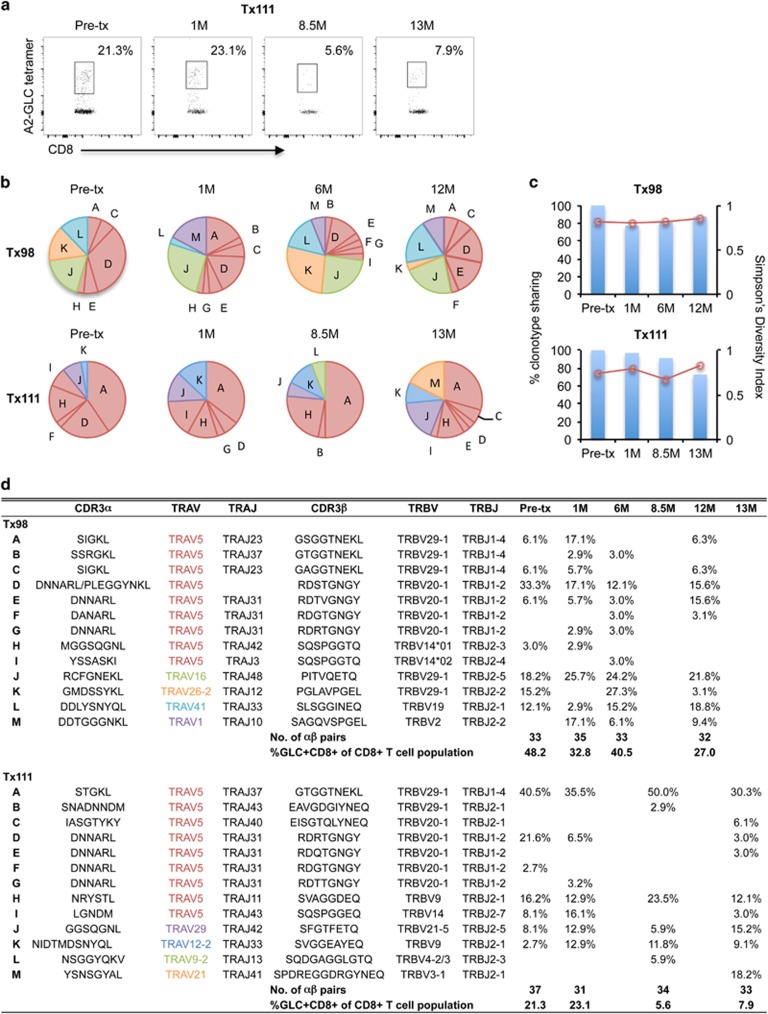
Figure 2.
Longitudinally stable GLC-specific TCRαβ repertoire in EBV-positive lung transplant recipients. GLC-tetramer+CD8+ T cells were single-cell sorted for TCRαβ analysis following in vitro expansion. Longitudinal dot plots (a) of expanded GLC+CD8+ T cells from patient Tx111. Frequency (b) of paired GLC-specific CD8+ CDR3αβ repertoires pre- and post transplant. Pie chart area shaded in red represent TRAV5 clonotypes. Percentage of TCRαβ clonotypes shared compared with Pre-transplant (bar graph, left y axis) and SDI values (line graph, right y axis) are shown in c. Frequencies of GLC+CD8+ T cells following expansion and clonotype details are listed in d.
Similarly, the GLC-specific TCRαβ repertoire from patient Tx111 was relatively stable from pre-transplant to 13 months post transplant, with the exception of clonotypes L and M, which emerged at 8.5 and 13 months, respectively (Figures 2b and d). Unexpectedly, the public AS01-TCRαβ was not observed in the GLC-specific TCRαβ repertoire from patient Tx98 and it was only detected once in a pre-transplant sample for Tx111 (clonotype F), even though the overall GLC-specific TCRαβ repertoire of patient Tx111 was dominated by TRAV5 (75%, n=135). Moreover, the most dominant TCRαβ in Tx111 was the pairing of TRAV5/TRAJ37 with TRBV29-1/TRBJ1-4 (clonotype A). Near-identical TRAV5/TRAJ37/TRBV29-1/TRBJ1-4 TCRs were also observed in patient Tx98 and healthy donor D3.
Analysis of clonotype sharing revealed that >77% common TCRαβ clonotypes (range 77.1–87.5%) were used for Tx98 at any time after the transplant and immunosuppression, whereas the TCRαβ clonotypes sharing was between 72.7 and 96.8% for Tx111 (Figure 2c). These results demonstrate a high degree of conservation of TCRαβ clonotypes during the standard post-lung transplant immunosuppression. Similarly, the Simpson Diversity Index (SDI) was stable at each time point for patients Tx98 and Tx111 from pre-transplant to post transplant (range 0.80–0.85 and 0.67–0.82, respectively; Figure 2c), which measures the richness (distinct numbers of clonotypes) and evenness (distribution of clonotypes) of the TCRαβ clonotypic repertoires.
Collectively, GLC-specific CD8+ T-cell responses were biased for TRAV5 in two lung transplant recipients, although to a lesser extent when compared with the healthy donors. Importantly, in the absence of detectable EBV reactivation, the GLC-specific TCRαβ repertoires remained stable over time under immunosuppressive conditions.
Detection of GLC-specific T cells prior to clinical primary EBV infection in EBV-negative lung transplant recipient receiving an EBV-positive allograft
To determine whether GLC-specific CD8+ T cells were biased for TRAV5 before antigen encounter or skewed toward TRAV5 usage after detectable EBV blood viremia, we examined GLC-specific CD8+ T cells in an EBV-negative lung transplant patient (Tx101) receiving an EBV-positive allograft prior to development of the blood EBV viremia. Using novel TAME methodology, we have identified naive GLC-specific CD8+ T-cell precursors in cord blood.23 However, which clonotypes are recruited has not been investigated by tracking the same individual before and after EBV clinical diagnosis.
The first clinical indication of EBV infection in patient Tx101 was corroborated with a viral load titre of 20 000 copies per ml in the blood on day (d)242 post transplant following routine clinical follow-up for primary EBV-mismatched patients. EBV viral loads were negative on days 33, 70 and 87. Continuing low levels of viremia were detected in the blood until d376 before a massive titre peak on d461 (2 500 000 copies per ml; Figure 3). Throughout the follow-up period (until day 1,860), patient Tx101 had repeated episodes of high EBV reactivation events based on viral load titers, which aligned with clinical manifestations, including upper respiratory tract infection, sore throat, malaise, nasal congestion, muscle aches/pain and severe lethargy. Symptoms were further exacerbated and prolonged owing to a secondary bacterial infection. Antiviral therapy with oral valganciclovir (900 mg daily) was implemented for 6 months post transplant as the patient was a primary EBV mismatch, but continued on owing to viral detection and clinical symptoms. EBV serology results showed that the patient was immunoglobulin (Ig)M-negative/IgG-negative on d87 post transplant. The only other available EBV serology test result was on day 519, which was IgM-negative and IgG-positive for EBV.
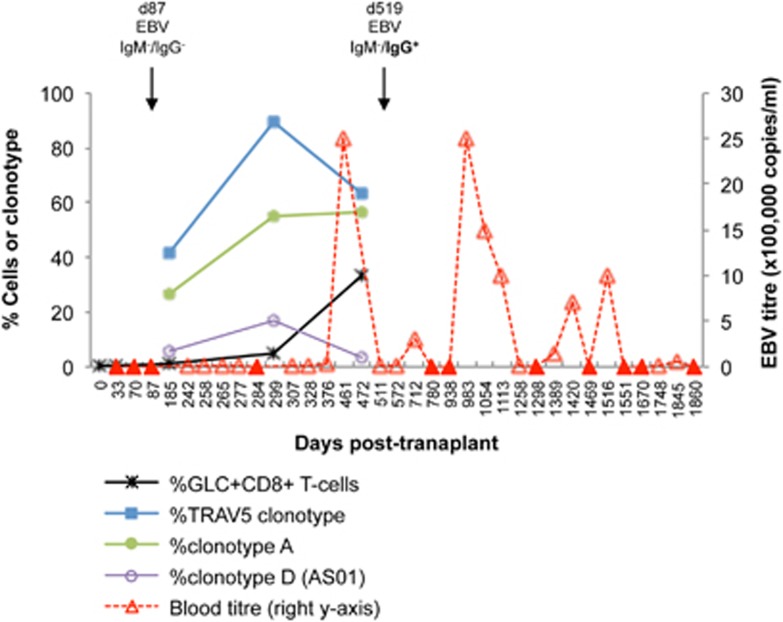
Figure 3.
GLC-specific CD8+ T-cell response precedes measurable EBV load in a EBV-negative lung transplant recipient receiving an EBV-positive allograft. Frequencies of GLC+CD8+ T cells of the total CD8+T-cell population following expansion and TCRαβ clonotype frequencies were represented on the left y axis. TCR CDR3αβ sequence details of TRAV5 clonotypes, clonotype A and D are listed in Figure 4f. Patient Tx101's EBV viral load (right y axis) was clinically measured in the blood up to d1840 post transplant (closed triangles, EBV viral load=0). EBV serology test results are indicated by the drop down arrows.
GLC-specific CD8+ T cells were expanded from PBMC (~4–8 × 106) at pre-transplant and on d32, d185, d299 and d472 post transplant with A2-GLC-tetramer+ frequencies of 0.37, 0.10, 0.90, 4.80 and 33.00% based on the CD8+ T-cell population (Figure 3). GLC-specific TCRαβ analysis was successfully performed on d185, d299, d472 post transplant, which had readily detectable tetramer+CD8+ T-cell populations and sufficient T-cell numbers for single-cell sorting. At pre-transplant and d32, insufficient T-cell numbers following T-cell expansion and negligible tetramer+ frequencies precluded cell sorting and TCR analysis. Nevertheless, expanded GLC-specific CD8+ T-cell populations from the blood were detected on d185 (0.90%) just before detectable EBV viral load (d242), continued to increase in expansion frequencies on d299 (4.80%), and markedly increased on d472 (33.00%) following very high EBV viral load levels (Figure 3).
In parallel with in vitro amplification, a more sensitive TAME approach was performed on PBMC from pre-transplant (~13 × 106 PBMC) and d32 post transplant (~8 × 106 PBMC), and no tetramer+ CD8+ T cells were detected (data not shown), which was not surprising given limited cells. However, when TAME was performed on ~40 × 106 PBMC from d87, tetramer+ CD8+ T cells were clearly detected post enrichment (24.38% of CD3+ T cells; Figure 4a) and single-cell sorted for TCRαβ analysis. Pre-enriched and flowthrough control samples generated negligible tetramer+ CD8+ T-cell frequencies of 0.07 and 0.00%. Interestingly, there was a defined population of tetramer+CD8low/− T cells detected post-enrichment (10.30%), but were not single-cell sorted with the tetramer+CD8+ T cells for TCRαβ analysis due to sorting limitations. Further characterization of these post-enriched tetramer+ cells showed that the tetramer+CD8+ T cells were a mixture of CD45RA+CD27+ and CD45RA−CD27+ cells and the tetramer+CD8low/− T cells consisted of a CD45RA−CD27− and CD45RA+CD27− phenotype (Figure 4b). We speculate that the CD45RA+CD27+ tetramer+CD8+ T cells contain a population of naive cells, but without CCR7 staining, we cannot further define the double-positive CD45RA+CD27+ cells as being truly naive. Based on the mixed CD45RA/CD27 expression profiles, it is possible that the GLC-specific CD8low T cells detected prior to clinically indicated EBV primary infection could represent activated T cells that recently encountered EBV antigen following recent virus infection. Further, these cells might have recently seen antigen but at very low, undetectable levels (owing to antiviral therapy and/or sensitivity of viral load measurements), and possibly at anatomical sites other than blood.
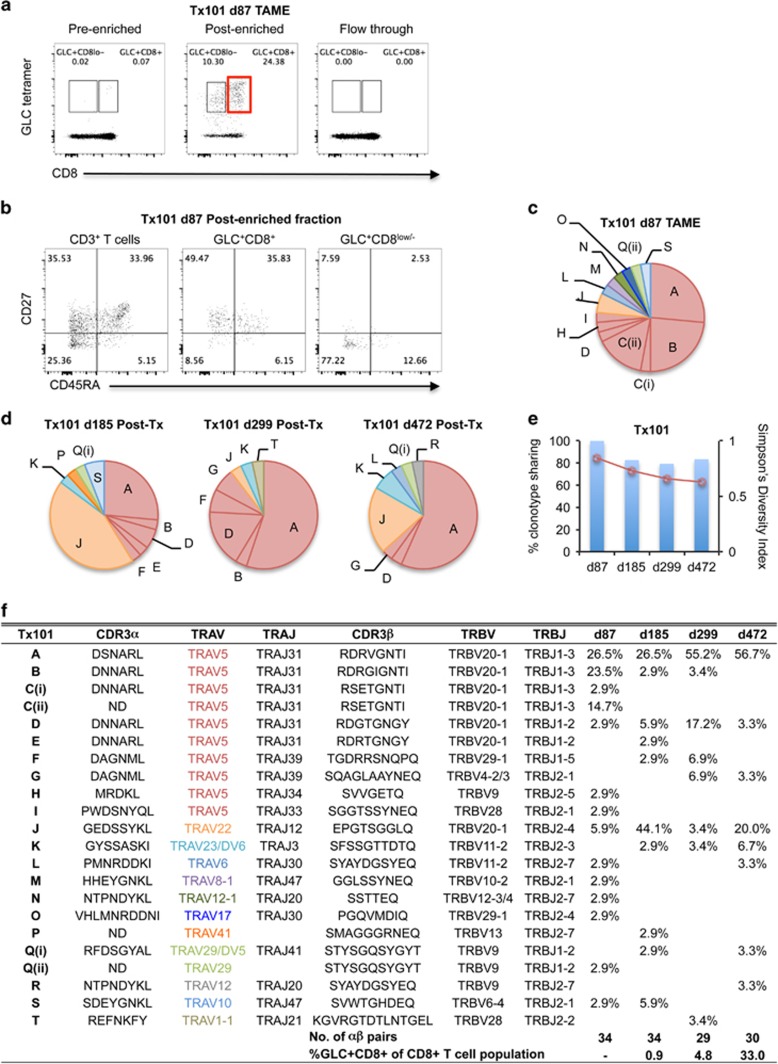
Figure 4.
Early detection of TRAV5-biased GLC-specific CD8+ T cells in patient Tx101. PBMC from patient Tx101 (a) at 87 days post transplant underwent tetramer-associated magnetic enrichment (TAME). Pre-enriched, post-enriched and the flowthrough fractions were collected and analyzed based on GLC-tetramer and anti-CD8 antibody staining. Percentages are based on CD3+ T cells. CD3+ T cells, GLC-tetramer+CD8+ and GLC-tetramer+CD8low/− cells from the post-enriched fraction (b) were phenotypically analyzed based on CD45RA and CD27 expression. Pie charts of paired GLC-specific CD8+ TCRαβ clonotypes following TAME (d87) (c) or following in vitro expansion (d185, d299 and d472) (d) are shown. Pie chart area shaded in red represent TRAV5 clonotypes. Percentage of TCRαβ clonotypes shared compared to TAME (d87) (bar graph, left y axis) and SDI values (line graph, right y axis) are shown in e. Frequencies of GLC+CD8+ T cells following expansion and clonotype details are listed in f (ND; not determined).
Diversity of GLC-specific CD8+ TCRαβ repertoire prior to and after detection of EBV DNA in the blood
In patient Tx101, TCRαβ analysis on d87 post transplant using ex vivo TAME enrichment for preclinical GLC-specific CD8+ T cells revealed 14 different paired clonotypes with the majority expressing TRAV5 (76%) (Figures 4c and f). Intriguingly, three co-dominant TCRαβ clonotypes were found (A-26%, B-24% and C-18%), which consisted of TRAV5/TRAJ31/TRBV20-1/TRBJ1-3 genes with near-identical CDR3α and CDR3β sequences. Nucleotide sequence alignments comparing individual cells were identical within the dominant clonotypes (A–B). Similarly, these near-identical TCRαβ clonotypes were found in three healthy donors (Figure 1d). The widely published TRAV5/TRAJ31/TRBV20-1/TRBJ1-2 AS01-TCR was at low frequency using ex vivo TAME combined with single-cell TCRαβ analysis (1/34 cells), which may have been undetected using older cloning strategies.
Next, we examined the longitudinal GLC-specific TCRαβ repertoire using expanded T-cell lines. TRAV5-biased TCRs were less abundant on d185 post transplant (41%), which then increased to 90% on d299 post transplant following primary EBV blood viremia before declining to 63% on d472 post transplant (Figure 3). The decline in TRAV5 usage was attributed to a non-TRAV5 TCR comprising TRAV22/TRAJ12/TRBV20-1/TRBJ2-4 genes (clonotype J), which was dominant on d185 post transplant but subdominant at other time points (d299 and d472 post transplant). Of interest was the TCRαβ repertoire dynamics of clonotype A, which was subdominant on d185 post transplant (26%), but dominant by d299 post transplant (55%) and up to d472 post transplant (57%) (Figures 3,4d and f), suggesting that this TCRαβ clonotype might contribute toward controlling early EBV infection and possibly subsequent reactivations. Conversely, AS01-TCR was subdominant, peaking at 17% on d299 post transplant (clonotype D; Figures 3,4d and f).
Analysis of clonotype sharing revealed that >79% common TCRαβ clonotypes (range 79.4–83.4%) were used on d185, d299 and d472 as compared to d87, demonstrating high conservation level of TCRαβ signatures during the primary detectable blood EBV viremia and immunosuppression. This occurred despite the fact that different detection methods were used for GLC-specific CD8+ T cells on d87 (preclinical; TAME) and days 185–472 (in vitro amplification). The SDI of EBV-specific CD8+ T cells in Tx101 (with the exception of d87 for patient Tx101, as those data were acquired with a different method and so not directly comparable to the other data sets) was lower at all time points measured than the SDI of responses in patient Tx98. SDIs of the responses in patient Tx111 were more variable, but were on average also higher than those of Tx101. An ANOVA analysis found a significant difference between the average SDI of the three patients (P=0.009), which we followed up by Tukey's Honest Significant Difference post-test and found a significant difference between Tx98 and Tx101 (P=0.007), whereas the P-values for the Tx111-Tx101 comparison and the two non-EBV reactivated patients (Tx98-Tx111 comparison) were not significant (P=0.088 and P=0.2, respectively).
Overall, we successfully captured the early GLC-specific TCRαβ repertoire in an ex vivo immunocompromised setting using TAME technology. The TCRαβ repertoire was heavily biased for TRAV5 and was recapitulated in our clinical analysis at later time points, where we observed a significant dynamic skewing and reorganization in the order of TCRαβ dominance hierarchy prior to onset of symptoms of EBV infection and following subsequent EBV reactivations.
Identification of novel paired public CDR3αβ motifs
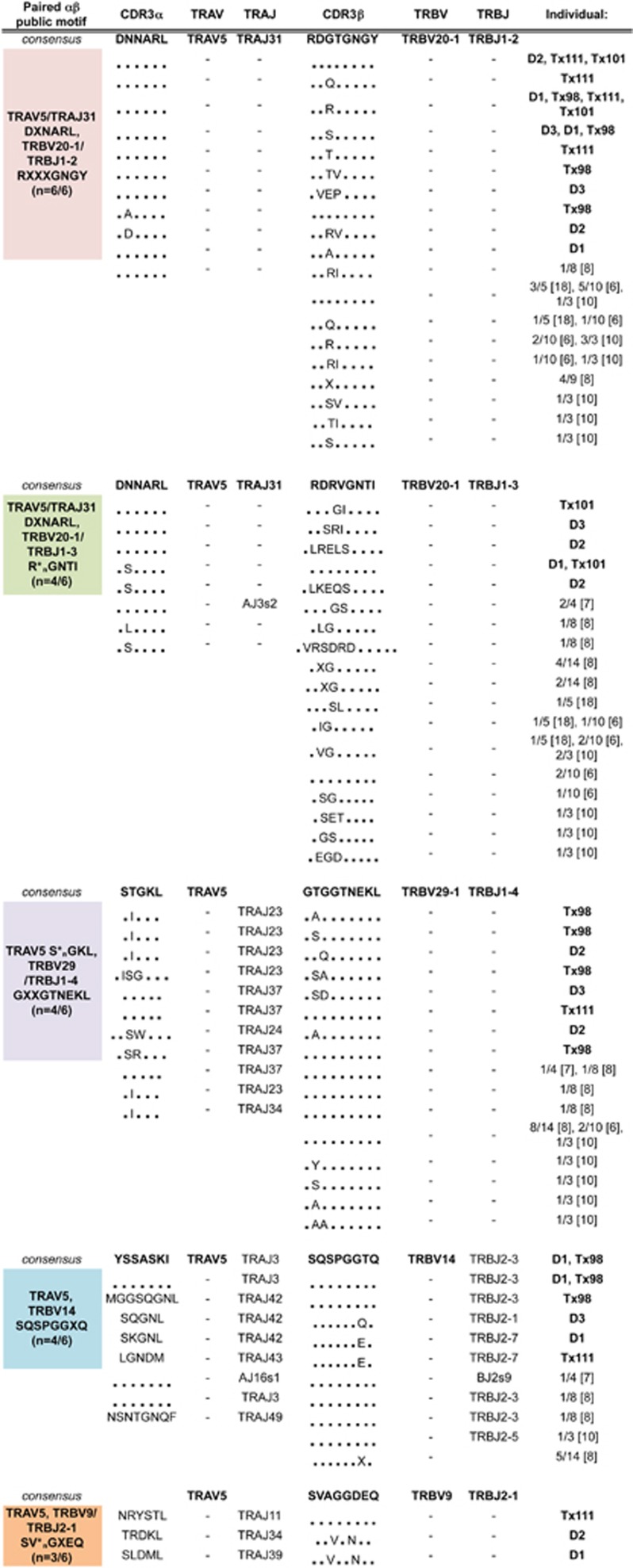
We have classified four new-paired public TCR CDR3αβ genes and motifs, in addition to the public AS01-TCR, using single-cell multiplex reverse transcriptase PCR, which simultaneously analyses CDR3α and CDR3β chains from a single cell. All TRAV5-TCRs generated from three healthy donors and three lung transplant patients were collated to assess which TCRs were identical or near-identical and observed between donors (Figure 5). As expected, all six donors had identical (3/6) or near-identical (6/6) TCRs to AS01 and the paired-motif was generalized as TRAV5/TRAJ31 DXNARL, TRBV20-1/TRBJ1-2 RXXXGNGY. The other paired motifs identified in our study were not strictly TRAV5/TRAJ31/TRBV20-1/TRBJ1-2 gene pairings and included TCRαβ gene sequence combinations of: TRAV5/TRAJ31_DXNARL/TRBV20-1/TRBJ1-3_R*nGNTI (n=4/6) (‘*n' denotes any number of amino-acid residues), TRAV5_S*nGKL/TRBV29-1/TRBJ1-4_GXXGTNEKL (n=4/6), TRAV5/TRBV14_SQSPGGXQ (n=4/6) and TRAV5/TRBV9/TRBJ2-1_SV*nGXEQ (n=3/6). Limited TCRαβ paired and unpaired TCR sequences from older studies were also collated (Figure 5) to further highlight the publicly paired motifs, which were not originally identified, possibly due to the lack of paired TCRαβ analysis, lack of donors or extensive cloning methodologies. Thus, our data show that the GLC-specific TCRαβ repertoire included a number of highly prominent and paired public clonotypes and clonotype motifs that had been previously underestimated.
Figure 5.
TRAV5-biased GLC-specific CD8+ TCRαβ repertoires between different individuals reveal new public paired genes and motifs. TCRαβ clonotype sequences from our study were grouped into consensus sequences and common motifs. Sequences from previously published literature were collated under each group (X, different amino acid; *n, any number of amino acid).
Discussion
Human EBV-GLC-specific CD8+ T cells are oligoclonal with an average of nine unique clonotypes per individual, ranging between 3 and 20 per individual.7, 16 In HLA-A*02:01 individuals, GLC-specific CD8+ TCRαβ clonotypes bearing TRAV5/TRBV20-1 were described as public, that is, observed across different individuals, and found as the most dominant pairing.7, 8, 9 Here, we quantitatively compared the abundance and prevalence of public TCRαβ repertoires within the EBV-GLC-specific CD8+ T cells between healthy individuals and a longitudinal cohort of lung transplant recipients under immunosuppressive conditions. We report, for the first time, the TCRαβ repertoires of GLC-specific CD8+ T cells in a previously EBV-seronegative lung transplant patient prior to and following symptomatic EBV infection and detectable viral load in the blood.
In support, the GLC-specific CD8+ T-cell response was quite diverse in healthy and immunocompromised individuals, with an average of 12 TCRαβ clonotypes per individual (range: 7–22 clonotypes). Second, the GLC-specific CD8+ TCR repertoire was dominated by TRAV5/TRAJ31, which included clusters of paired public TCRs with either TRBV20-1/TRBJ1-2 or TRBV20-1/TRBJ1-3 β-chains. Interestingly, rarely were multiple clusters observed within the same individual. In addition, we found dominant, previously unrecognized public pairings of TRAV5 with TRBV29-1/TRBJ1-4, TRBV14 and TRBV9/TRBJ2-1. These pairings were only observed in a single individual in other studies8, 9 and were not identified as public TCRαβ pairings previously.
In the EBV-seronegative patient Tx101, whom had received an HLA-A2−/EBV-seropositive lung allograft, we detected a small population of GLC-specific CD8+ T cells at pre-transplant (0.37%) and d32 post transplant (0.10%) following in vitro culture, but not ex vivo using the TAME methodology. Despite insufficient cell numbers for TCR analysis and the discrepancy in starting cell numbers for TAME (8–13 × 106 PBMCs compared with 40 × 106 PBMCs used at d87 post transplant), it is possible that naive GLC-specific CD8+ T cells that had never encountered antigen before transplantation. Interestingly, the successful detection of ex vivo GLC-specific CD8+ T cells on d87 post transplant, prior to EBV DNA detection in the blood (d242 post transplant), did reveal two populations of GLC-specific CD8+ T cells, a tetramer+CD8+ and a tetramer+CD8low/− population, and these were phenotypically analyzed based on CD45RA and CD27 expression. The tetramer+CD8+ population showed both CD45RA+CD27+ (mainly naive phenotype) and CD45RA−CD27+ (predominantly memory) phenotypes whereas, the tetramer+CD8low/− population consisted mainly of a CD45RA−CD27− phenotype. It is possible that these GLC-specific CD8+ T cells may not be naive and have recently encountered antigen at low undetectable levels or have been primed at local sites other than blood. On the other hand, the GLC-specific CD8+ T cells in our seropositive and asymptomatic donors (D1, D2, D3) and patients (Tx98 and Tx111) most likely represent memory rather than naive or effector CD8+ T cells. In support, we have previously routinely characterized ex vivo GLC-specific CD8+ T cells from a range of healthy, asymptomatic EBV-positive donors following TAME (Supplementary Figures 1a and b) and show that those EBV+CD8+ T cells are predominantly of a CD27+CD45RA− phenotype (average±s.d.: 74.79%±9.10%, n=7) and chiefly represent memory CD8+ T cells.
Our striking finding was that patient Tx101's early GLC-specific CD8+ TCRαβ repertoires were mainly dominated by TRAV5 gene and consisted of public TCRαβ clonotypes identified in our study (TRAV5/TRAJ31/TRBV20-1/TRBJ1-3 and TRAV5/TRAJ31/TRBV20-1/TRBJ1-2 public TCRs) and others.8, 9 In fact, the majority of dominant and subdominant GLC-specific CD8+ TCR clonotypes in the preclinical setting (following TAME) resembled those at later time points leading to onset of symptomatic EBV infection and at subsequent time points (bulk T-cell lines), suggesting that these repertoires were established very early on and perhaps genetically favored upon antigenic exposure. In support of our hypothesis, a seminal study24 comparing naive and immune CD8+ TCR repertoires toward dominant influenza peptides (DbNP366/DbPA224) in B6 mice showed that the immune TCR repertoire was directly determined by the naive TCR repertoires.
Our structural analysis of the public AS01-TCR bearing TRAV5/TRAJ31/TRBV20-1/TRBJ1-2 genes20 exposed how the combination of these TCR genes were exquisitely designed to bind to the A2-GLC complex through unique germline residues at crucial contact points between the TCR and pMHC.20 Similarly, other groups have found that the GLC-specific CD8+ T cells were highly skewed toward the TRBV20-1 gene linked to the non-germline aspartate residue at CDR3β position four.7, 8, 9, 19 Here, TRBV29-1 was always associated with glycine at CDR3β position 4 and was replaced with serine for TRBV14 and TRBV9. Further structural studies are needed to confirm whether the paired public TCRαβ clusters contain unique germline and non-germline contact residues that are structurally and biophysically optimal when engaging the A2-GLC complex. Our findings are similar to those described earlier for MelanA-specific T cells,25 in which we also found a strong V-alpha chain bias (TRAV12-2), which could pair with different TRBV chains and structural evidence has shown this is because the TRAV12-2 CDR1 is predominantly responsible for the recognition of the MART-1 pHLA complex. In addition, this study also showed the high frequency of naive epitope-specific CD8+ T cells directed toward this epitope owing to a lack of negative selection.25
Longitudinal analysis of the GLC-specific CD8+ T cells in our three lung transplant patients revealed the relative stability of the TCRαβ repertoire during early EBV infection, convalescence and immunosuppression. Especially, in the absence of detectable EBV reactivation based on viral load testing (patients Tx98 and Tx111), the GLC-specific TCRαβ repertoires remained stable over time under immunosuppression. Previously, GLC-specific TCR usage of CD8+ T-cell clones between primary (7–15 days of clinical symptoms in acute infectious mononucleosis patients) and memory EBV responses (~26 months later) were compared in three donors.8 Dominant primary TCR clonotypes were not observed or were less dominant in the memory response. Conversely, previous longitudinal studies showed stable EBV-specific TCR repertoires following EBV infection.12, 26 However in these studies, the changes in the TCR repertoire cannot be interpreted quantitatively due to the biases in long term in vitro cloning methodologies and bulk RNA TCR analyses. In our study, we acknowledge that our viral load measurements are limited by the sensitivity of the assay, yet these methods are standardly used in our hospitals for clinical diagnostic purposes.
Our recent analysis of antiviral T-cell responses and their TCRαβ repertoires in lung transplant recipients using limited PBMCs for T-cell expansion22, 27 showed a gradual increase in CMV-specific CD8+ T cells and a skewing of the TCRαβ repertoire leading up to CMV reactivation, which then decreased and diversified following cessation of viremia.22, 27 Here, we found a modest increase in the number of detectable GLC-specific CD8+ T cells after 2 weeks of culture, which preceded EBV DNA detection in the blood and the onset of clinical manifestation. In the past, we have also shown early detection of ex vivo blood and BAL antiviral T cells prior to measurable levels of viral activation in two primary CMV-mismatched lung transplant recipients.28 Based on our current and previous observations, the detection of virus-specific T cells using tetramer staining seems to be a more sensitive technique to detect early signs of viral activation from seronegative patients receiving EBV+ donor grafts, compared with the routine in-house qPCR methods to detect EBV viral load, which is typically designed for high throughput screening. This method of viral load measurement has been well established using plasmid DNA to detect <1–50 EBV genomes from 106 B-lymphocytes or 1 genome from 150 000 cells.29 However, sensitivity is much lower when extracting DNA from whole-blood specimens, as other cell populations (that is, mainly neutrophils) dilute the EBV DNA from B cells. On the other hand, tetramer staining is highly specific and allows for detection of very rare antigen-specific T cells, such as naive populations using tetramer staining and other enrichment strategies. However, we should acknowledge that there are differences in the timing and frequency of PBMC collection and viral load measurements (often when clinically indicated), in which both are sometimes not performed on the same day owing to logistical constraints.
In conclusion, our findings provide unique insights into the stability of the GLC-specific TCRαβ repertoire in immunocompromised transplant patients and that early detection of preclinical GLC-specific CD8+ T cells and their TCRαβ repertoire dynamics can predict clinical EBV reactivation in transplant recipients.
Methods
Human ethics
Experiments were conducted according to the Declaration of Helsinki and conformed to the NHMRC Code of Practice. All subjects provided written informed consent and ethics approval was granted from University of Melbourne, the Alfred Hospital and Monash University Human Research Ethics Committees.
Subjects and PBMCs
PBMCs were isolated from buffy packs (Australian Red Cross Blood Service; VIC, Australia). Peripheral blood from HLA-A*02:01-positive bilateral lung transplant recipients Tx98, Tx111 and Tx101 were collected in heparinized tubes. Patients received standard triple-therapy immunosuppression and underwent routine surveillance bronchoscopy at ~14, 30, 60, 90, 180, 270 and 365 days post transplant or as indicated.28 PBMC were isolated by Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation and cryopreserved. HLA molecular typing was performed by the Victorian Transplantation and Immunogenetics Service (VIC, Australia).
EBV serology and monitoring
EBV IgM and IgG (against early antigen and viral capsid antibody) serology was determined by the Alfred Hospital Pathology Service where indicated (VIC, Australia). Patients Tx98 and Tx111 were EBV-seropositive and received EBV-seronegative donor lung allografts. Patient Tx101 was EBV-seronegative at the time of transplantation and received an EBV-seropositive donor lung allograft. Blood EBV viral loads were measured when clinically indicated (Victorian Infectious Diseases Reference Laboratory and Women's Centre for Infectious Diseases, The Royal Women's Hospital, VIC, Australia) using an in-house automated MagNA Pure LC (Roche Diagnostics, Indianapolis, IN, USA) to extract DNA, followed by qPCR to detect EBV, as previously described.29
Tetramer and generation of T-cell lines
HLA-A*02:01/GLCTLVAML (A2-GLC) monomer (ImmunoID, University of Melbourne) was tetramerized with streptavidin-phycoerythrin (BD Biosciences, San Jose, CA, USA). For peptide pulsing, PBMCs were incubated with the GLC peptide (1–10 μm; Genscript, Piscataway Township, NJ, USA) for 1–1.5 h at 37 °C, then washed twice before priming.30, 31 PBMCs were stimulated with GLC-pulsed autologous PBMCs for 10–13 days (37 °C, 5% CO2) at a 2:1 ratio in cRF10 media plus 10 U ml−1 recombinant interleukin-2.32
Paired TCRαβ analysis of EBV-specific CD8+ T cells
T-cell lines were stained with A2-GLC-tetramer-PE for 1h at room temperature. Cells were washed twice, then stained with anti-CD3-PE-Cy7 (#563423), anti-CD8-PerCP-Cy5.5 (#341051), anti-CD14-APC-Cy7 (#560180), anti-CD19-APC-Cy7 (#560177) and LIVE/DEAD Fixable NIR (Molecular Probes, Life Technologies, Eugene, Oregon, USA). Antibodies (their catalog number in brackets) were from BD Biosciences. CD3+CD8+tetramer+dump− single-cells were sorted (FACSAria III, BD) into 96-well PCR plates (Eppendorf, Hamburg, Germany). Analysis of paired CDR3α and CDR3β regions were performed by multiplex-nested reverse transcriptase PCR, then sequencing of TCRα and TCRβ products.21, 22 Sequences were analyzed according to the IMGT/V-QUEST web-based tool.33, 34
TAME of preclinical EBV-specific CD8+T cells
PBMCs from Tx101 at d87 post transplant were thawed and rested overnight before incubating with anti-human FcR block (Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min on ice. Cells were stained with A2-GLC-tetramer-PE, washed, incubated with anti-PE microbeads (Miltenyi Biotec) before passing through LS columns (Miltenyi Biotec) to enrich for tetramer-stained cells.24, 35 Cells were stained with surface antibodies mentioned above, as well as CD45RA-FITC (#555488) and CD27-APC (#558664) (both from BD Biosciences) or CD27-AF700 (#56-0279-42, eBioscience, San Diego, CA, USA) for phenotypic analysis, and then single-cell sorted for TCRαβ analysis.
Statistical analysis
TCRαβ repertoires from patients Tx98, Tx101, and Tx111 were analyzed by SDI using the ‘vegan' package in R. An ANOVA with Tukey's honest significant difference as a post-test was run on the SDIs obtained, with the P-values indicated.36, 37 In our previous study,22 >20 TCRαβ sequence pairs per sample have been sufficient to ensure adequate power for statistical analysis.
Acknowledgments
We gratefully acknowledge the generous support of all the clinicians, nurses and allied health professionals associated with the Lung Transplant Service at The Alfred Hospital, especially the patients recruited for this study. KK is supported by the Australian National Health and Medical Research Council (NHMRC) Program Grant (ID 1071916) and is an NHMRC SRFB Fellow (ID1102792). EJG was a recipient of an NHMRC Aboriginal and Torres Strait Islander Health Research Scholarship and Douglas and Lola Douglas Scholarship in Medical Science and is supported by an NHMRC CJ Martin Fellowship.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Elliott SL, Suhrbier A, Miles JJ, Lawrence G, Pye SJ, Le TT et al. Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J Virol 2008; 82: 1448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetham MM, Roberts KB. Infectious mononucleosis in adolescents. Pediatr Ann 1991; 20: 206–213. [DOI] [PubMed] [Google Scholar]
- Wong JY, Tait B, Levvey B, Griffiths A, Esmore DS, Snell GI et al. Epstein-Barr virus primary mismatching and HLA matching: key risk factors for post lung transplant lymphoproliferative disease. Transplantation 2004; 78: 205–210. [DOI] [PubMed] [Google Scholar]
- Davis JE, Sherritt MA, Bharadwaj M, Morrison LE, Elliott SL, Kear LM et al. Determining virological, serological and immunological parameters of EBV infection in the development of PTLD. Int Immunol 2004; 16: 983–989. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med 2002; 195: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol 2004; 173: 7481–7489. [DOI] [PubMed] [Google Scholar]
- Venturi V, Chin HY, Asher TE, Ladell K, Scheinberg P, Bornstein E et al. TCR beta-chain sharing in human CD8+ T cell responses to cytomegalovirus and EBV. J Immunol 2008; 181: 7853–7862. [DOI] [PubMed] [Google Scholar]
- Annels NE, Callan MF, Tan L, Rickinson AB. Changing patterns of dominant TCR usage with maturation of an EBV-specific cytotoxic T cell response. J Immunol 2000; 165: 4831–4841. [DOI] [PubMed] [Google Scholar]
- Lim A, Trautmann L, Peyrat MA, Couedel C, Davodeau F, Romagne F et al. Frequent contribution of T cell clonotypes with public TCR features to the chronic response against a dominant EBV-derived epitope: application to direct detection of their molecular imprint on the human peripheral T cell repertoire. J Immunol 2000; 165: 2001–2011. [DOI] [PubMed] [Google Scholar]
- Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol 2006; 6: 883–894. [DOI] [PubMed] [Google Scholar]
- Iancu EM, Corthesy P, Baumgaertner P, Devevre E, Voelter V, Romero P et al. Clonotype selection and composition of human CD8 T cells specific for persistent herpes viruses varies with differentiation but is stable over time. J Immunol 2009; 183: 319–331. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Islam SA, Noble MS, Lau C, Brander C, Altfeld MA et al. Clonotype tracking of TCR repertoires during chronic virus infections. Virology 2002; 304: 474–484. [DOI] [PubMed] [Google Scholar]
- Miles JJ, Douek DC, Price DA. Bias in the alphabeta T-cell repertoire: implications for disease pathogenesis and vaccination. Immunol Cell Biol 2011; 89: 375–387. [DOI] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature 1988; 334: 395–402. [DOI] [PubMed] [Google Scholar]
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science 1999; 286: 958–961. [DOI] [PubMed] [Google Scholar]
- Koning D, Costa AI, Hoof I, Miles JJ, Nanlohy NM, Ladell K et al. CD8+ TCR repertoire formation is guided primarily by the peptide component of the antigenic complex. J Immunol 2013; 190: 931–939. [DOI] [PubMed] [Google Scholar]
- Miles JJ, McCluskey J, Rossjohn J, Gras S. Understanding the complexity and malleability of T-cell recognition. Immunol Cell Biol 2015; 93: 433–441. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol 2015; 33: 169–200. [DOI] [PubMed] [Google Scholar]
- Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med 2005; 202: 1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JJ, Bulek AM, Cole DK, Gostick E, Schauenburg AJ, Dolton G et al. Genetic and structural basis for selection of a ubiquitous T cell receptor deployed in Epstein-Barr virus infection. PLoS Pathog 2010; 6: e1001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG. T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci Transl Med 2012; 4: 128ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Rowntree LC, Pellicci DG, Bird NL, Handel A, Kjer-Nielsen L et al. Recognition of distinct cross-reactive virus-specific CD8+ T cells reveals a unique TCR signature in a clinical setting. J Immunol 2014; 192: 5039–5049. [DOI] [PubMed] [Google Scholar]
- Neller MA, Ladell K, McLaren JE, Matthews KK, Gostick E, Pentier JM et al. Naive CD8(+) T-cell precursors display structured TCR repertoires and composite antigen-driven selection dynamics. Immunol Cell Biol 2015; 93: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K et al. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest 2010; 120: 1885–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Sommermeyer D, Michel C, Wilde S, Schendel D, Uckert W et al. Misinitiation of intrathymic MART-1 transcription and biased TCR usage explain the high frequency of MART-1-specific T cells. Eur J Immunol 2014; 44: 2811–2821. [DOI] [PubMed] [Google Scholar]
- Levitsky V, de Campos-Lima PO, Frisan T, Masucci MG. The clonal composition of a peptide-specific oligoclonal CTL repertoire selected in response to persistent EBV infection is stable over time. J Immunol 1998; 161: 594–601. [PubMed] [Google Scholar]
- Nguyen TH, Westall GP, Bull TE, Meehan AC, Mifsud NA, Kotsimbos TC. Cross-reactive anti-viral T cells increase prior to an episode of viral reactivation post human lung transplantation. PLoS ONE 2013; 8: e56042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall G, Kotsimbos T, Brooks A. CMV-specific CD8 T-cell dynamics in the blood and the lung allograft reflect viral reactivation following lung transplantation. Am J Transplant 2006; 6: 577–584. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Bein G, Bitsch A, Kirchner H. Detection and quantification of latently infected B lymphocytes in Epstein-Barr virus-seropositive, healthy individuals by polymerase chain reaction. J Clin Microbiol 1992; 30: 2826–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras S, Kedzierski L, Valkenburg SA, Laurie K, Liu YC, Denholm JT et al. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci USA 2010; 107: 12599–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Parra S, Grant E, Loh L, Nguyen TH, Campbell KA, Tong SY et al. Preexisting CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc Natl Acad Sci USA 2014; 111: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud NA, Purcell AW, Chen W, Holdsworth R, Tait BD, McCluskey J. Immunodominance hierarchies and gender bias in direct T(CD8)-cell alloreactivity. Am J Transplant 2008; 8: 121–132. [DOI] [PubMed] [Google Scholar]
- Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 2008; 36: W503–W508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch G, Scaviner D, Contet V, Lefranc MP. Protein displays of the human T cell receptor alpha, beta, gamma and delta variable and joining regions. Exp Clin Immunogenet 2000; 17: 205–215. [DOI] [PubMed] [Google Scholar]
- Cukalac T, Valkenburg SA, La Gruta NL, Turner SJ, Doherty PC, Kedzierska K. Multiplexed combinatorial tetramer staining in a mouse model of virus infection. J Immunol Methods 2010; 360: 157–161. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB et al vegan: Community Ecology Package. R package version 2.3-0. http://CRAN.R-project.org/package=vegan2015.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.