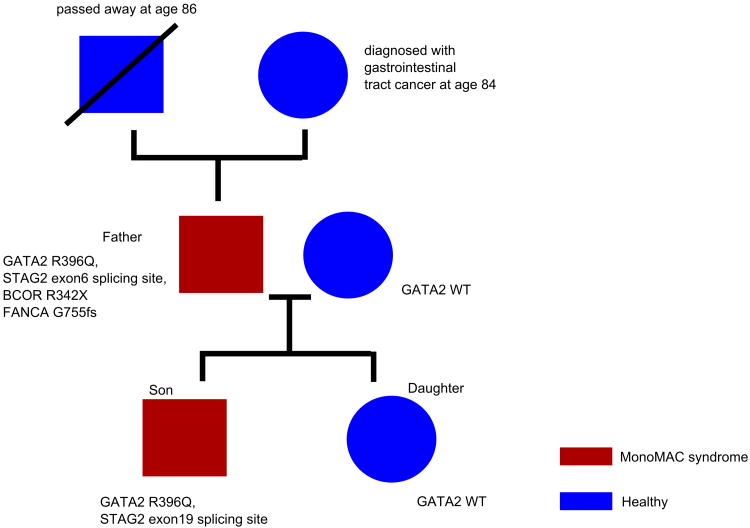
We performed whole genome and exome sequencing of a family with high risk myelodysplastic syndrome (MDS). Based on the sequencing results, the affected family members were diagnosed as having MonoMAC syndrome with a heritable germline GATA2 mutation (R396Q) as the causative factor of the disease (Fig. 1).
Fig 1.
Pedigree of family affected by the MonoMAC syndrome. Squares represent the male family members and circle represent the female family members.
MonoMAC is an autosomal-dominant syndrome and manifests as a deficiency of monocytes, B lymphocytes and NK cells. It is often accompanied with mycobacterial, fungal and viral infections1. This rare genetic disorder was initially described a few years ago2 and subsequent studies then revealed mutation of the transcription factor GATA2 as the cause of the syndrome3-7. Although targeted sequencing or exome sequencing has been performed recently and a number of cooperating mutations has been found in several studies3, 4, 6, 8, to our knowledge, the entire spectrum of genomic changes of this disease has not yet been characterized.
The studied family includes two disease-affected patients (father and son) and a disease-free daughter. Both father and son experienced repeated infections and were diagnosed with MDS at ages 18 and 17, respectively. They remained healthy after receiving hematopoietic stem cell transplantation from unrelated donors. Genomic DNA was extracted from the father's malignant paraffin embedded bone marrow before transplantation as well as from a swab of the mouth mucosa (germline control), the son's paraffin embedded bone marrow before transplantation and his mouth swab, as well as the healthy daughter's bone marrow together with her normal cells from the mouth swab. Whole genome sequencing was performed on the above samples using Illumina HiSeq X Ten (mean >30× coverage). Exome sequencing of the son's bone marrow sample was also performed using Illumina Hiseq 4000 (mean >200× coverage) to enhance the identification of potential subclonal mutations. The sequencing reads were aligned to the standard human genome (hg19) using BWA, and mutations were called using Mutect (for single nucleotide variants, SNVs), VarScan (for SNVs and indels), Pindel (for indels) and Delly (for structural variants). Results were filtered with dbSNP 131,1000 genome (cutoff >0.001), Esp5400 database (cutoff >0.001), and the repetitive and low complexity regions(repeats and genomic SuperDups track, UCSC Genome Browser). Inactivating germline mutations (nonsense, frameshift and splicing-site mutations) were further analyzed. All the germline variants of GATA2 gene, including coding region, intron, UTR and upstream/downstream were called and filtered with dbSNP 131, 1000 genome, Esp5400 database and further examined using ExAC exome database (http://exac.broadinstitute.org/).
The disease was featured with a paucity of somatic mutation. We found a germline missense variant R396Q within the GATA2 gene in all of the specimens of both the father and son (both swab and bone marrow), but not in the healthy daughter. This variant was previously reported as a mutation hotspot associated with MonoMAC disease4. The father's bone marrow (cytogenetic: 47XY, +8) also carried the well-appreciated inactivating mutations of MDS/acute myeloid leukemia (AML) genes: BCOR (stop-gain R342X, variant allele frequency (VAF)=92%, X-chr gene), STAG2 (splicing site, exon 6:C.289-2A>C, VAF=80%, X-chr gene), FANCA (indel, G755fs, Fanconi Anemia Complementation Group A, involved in DNA repair), as well as a missense mutation of MLL2 (P187S) (Fig. 1, Supplementary Table 1). Potential MDS related missense mutations include KDM4B (Histone H3K9 demethylase), DGKG (diacylglycerol kinases) and APIP (functions in the methionine salvage pathway).
We found 2 somatic mutations in the son's bone marrow sample (STAG2, RYR2, cytogenetic 46XY, der(16)t(1; 16)(q21; q22)). A STAG2 splicing-site mutation (exon 19:C.1821+1G>A) was found with low VAF (=22%), indicating its presence as a minor subclone. Notably, the STAG2 mutations found in father and son occurred in different position, indicating that these mutations were acquired independently, suggesting STAG2 may act as a cooperating-gene with GATA2 in the development of the disease. Collectively, our observation indicates that MonoMAC syndrome is a disease that develops with low mutational burden, emphasizing the crucial role of GATA2 mutation in this syndrome.
GATA2 encodes a hematopoietic transcription factor that controls the differentiation of myeloid lineage and is crucial for the proliferation and maintenance of hematopoietic stem cells and multipotential progenitors9, 10. Mutation of GATA2 has been found in AML and MDS and was discovered as a predisposing gene for familial and pediatric AML/MDS4, 11-15. Notably, analysis of the ExAC database (containing protein-coding genetic variation in 60,706 normal humans) revealed that GATA2 is strongly intolerant towards loss of function (LOF), with a probability of LOF intolerant (pLI) value 0.98 (pLI> 0.9 indicates an extreme intolerance to loss of function). Indeed, no frameshift or nonsense mutation can be found in the entire ExAC cohort (60,706 individual). This observation suggests that GATA2 is involved in the essential pathway of embryonic/developmental growth and survival and is indispensable for normal development. It will be interesting to follow-up those individuals who carry rare missense variant of GATA2 (around 110 individuals in ExAC cohort) to determine whether they display any indolent hematopoietic abnormality.
Supplementary Material
Acknowledgments
This research is supported by the National Research Foundation Singapore under its Singapore Translational Research (STaR) Investigator Award (NMRC/STaR/0021/2014) and administered by the Singapore Ministry of Health's National Medical Research Council (NMRC), the NMRC Centre Grant awarded to National University Cancer Institute of Singapore, the National Research Foundation Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiatives. This research is supported by the National Institutes of Health of the USA (R01CA026038-35), as well as a generous donation from the Melamed family.
Footnotes
Competing Financial Interests: The authors disclose no potential conflicts of interest.
References
- 1.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquet M, Bellanne-Chantelot C, Tavitian S, Prade N, Beaupain B, Larochelle O, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121:822–829. doi: 10.1182/blood-2012-08-447367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119:1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wlodarski MW, Hirabayashi S, Pastor V, Stary J, Hasle H, Masetti R, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387–1397. doi: 10.1182/blood-2015-09-669937. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Muramatsu H, Okuno Y, Sakaguchi H, Yoshida K, Kawashima N, et al. GATA2 and secondary mutations in familial myelodysplastic syndromes and pediatric myeloid malignancies. Haematologica. 2015;100:e398–401. doi: 10.3324/haematol.2015.127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicente C, Conchillo A, Garcia-Sanchez MA, Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82:1–17. doi: 10.1016/j.critrevonc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol. 2015;169:173–187. doi: 10.1111/bjh.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiba N, Funato M, Ohki K, Park MJ, Mizushima Y, Adachi S, et al. Mutations of the GATA2 and CEBPA genes in paediatric acute myeloid leukaemia. Br J Haematol. 2014;164:142–145. doi: 10.1111/bjh.12559. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micol JB, Abdel-Wahab O. Collaborating constitutive and somatic genetic events in myeloid malignancies: ASXL1 mutations in patients with germline GATA2 mutations. Haematologica. 2014;99:201–203. doi: 10.3324/haematol.2013.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J, Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2014;99:276–281. doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.