Abstract
Background
Based on the recently sequenced gene coding for the Trypanosoma evansi (T. evansi) RoTat 1.2 Variable Surface Glycoprotein (VSG), a primer pair was designed targeting the DNA region lacking homology to other known VSG genes. A total of 39 different trypanosome stocks were tested using the RoTat 1.2 based Polymerase Chain Reaction (PCR).
Results
This PCR yielded a 205 bp product in all T. evansi and in seven out of nine T. equiperdum strains tested. This product was not detected in the DNA from T. b. brucei, T. b. gambiense, T. b. rhodesiense, T. congolense, T. vivax and T. theileri parasites. The Rotat 1.2 PCR detects as few as 10 trypanosomes per reaction with purified DNA from blood samples, i.e. 50 trypanosomes/ml.
Conclusion
PCR amplification of the RoTat 1.2 VSG gene is a specific marker for T. evansi strains, except T. evansi type B, and is especially useful in dyskinetoplastic strains where kDNA based markers may fail to amplify. Furthermore, our data support previous suggestions that some T. evansi stocks have been previously misclassified as T. equiperdum.
Background
Surra is an animal disease occurring in Africa, Asia and Latin America, caused by Trypanosoma evansi. T. evansi belongs to the subgenus Trypanozoon, together with T. equiperdum and T. brucei. The parasite can infect different host species and is mechanically transmitted by different biting flies such as Tabanidae and Stomoxys as well as by vampire bats such as Desmodus rotondus [1]. Camels and horses are very susceptible to the infection and death can occur within weeks or months. Moreover, T. evansi infections of cattle and buffaloes usually lead to a pronounced immunosuppression resulting in an increased susceptibility to other opportunistic diseases such as Pasteurella and anthrax [2].
Diagnosis of a T. evansi infection usually starts with clinical symptoms or the detection of antibodies to T. evansi. Conclusive evidence of T. evansi infection, however, relies on detection of the parasite in the blood or tissue fluids of infected animals. Unfortunately, parasitological techniques cannot always detect ongoing infections as the level of parasitaemia is often low and fluctuating, particularly during the chronic stage of the disease [3].
As an alternative to parasitological tests, DNA detection based on PCR has been proposed. Trypanozoon specific primers have been designed previously: TBR primers which target a 177 bp repeat [4], pMUTEC primers targeting a retrotransposon [5] and ORPHON primers which target the spliced leader sequence [6]. Most of them have been tested on cattle [7,8], water buffaloes [9] or goats [10]. PCR tests for diagnosis of T. congolense and T. vivax infections exist as well [11]. The development of a PCR test that would be able to differentiate between the different members of the Trypanozoon subgenus still remains a challenging issue. For T. evansi infections, the only specific test available so far is based on the detection of a kinetoplast DNA sequence [12,13]. However, the existence of dyskinetoplastic trypanosomes such as T. evansi RoTat 5.1 [14] and E152 [12] casts doubt about the diagnostic potential of such tests to detect all infections caused by T. evansi parasites. Recently, Ventura et al. [15] developed a PCR (PCR-Te664) for the detection of T. evansi based on a Random Amplified Polymorphic DNA (RAPD) fragment. The taxon specificity of this PCR remains uncertain since it was only tested on nine T. evansi strains, one T. equiperdum, two T. b. gambiense and one T. b. rhodesiense. Following evidence that the variable epitope of RoTat 1.2 VSG is expressed by all T. evansi strains tested so far [16], and that the gene encoding RoTat 1.2 VSG is present in all T. evansi but not in T. brucei isolates [17], we designed primers derived from the sequence of this VSG cDNA. In this article we will present and discuss the results obtained with these primers and compare them to the results we obtained using the PCR-Te664.
Results
PCR RoTat 1.2 : taxon specificity
The 39 different trypanosome stocks used in this study are listed in Table 1 [see Additional file 1]. They were derived from a wide range of hosts and from distinct geographical locations. In all PCR runs, RoTat 1.2 DNA was used as a positive control. As shown in Figure 1, the RoTat 1.2 PCR yielded a 205 bp amplicon in the positive control (lane 1) as well as in all other T. evansi populations (lanes 3–8). Moreover, the same fragment was found in seven out of the nine T. equiperdum populations tested. The T. equiperdum BoTat 1.1 was PCR negative (lane 10), while the T. equiperdum OVI strain yielded a PCR product shorter than 205 bp (lane 11) probably due to mispriming. All other tested trypanosome populations, including six T. b. brucei, eight T. b. gambiense, five T. b. rhodesiense, two T. congolense, one T. vivax and one T. theileri, were negative. (lanes 18–40). As a negative control, a PCR-mix without template DNA was included (lane 2). Sequencing of the positive samples revealed that all amplicon were identical (data not shown). The weak band in OVI did not yield sufficient material to enable sequencing.
Figure 1.
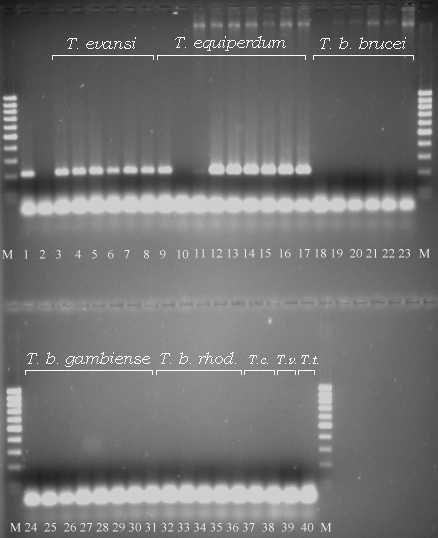
PCR specificity results for the different Trypanosoma (T.) species and subspecies in this study. Lane 1 pos. control RoTat 1.2, Lane 2 neg. control, Lanes 3–8 (T. evansi) are, respectively, AnTat 3.1, STIB 816, Zagora I.17, Colombia, Merzouga 56, CAN 86 K; Lanes 9–17 (T. equiperdum) are, respectively, AnTat 4.1, BoTat 1.1, OVI, STIB 818, Alfort, Hamburg, SVP, Am. Strain, Can. Strain ; Lanes 18–23 (T.b.brucei) are, AnTat 1.8, AnTat 2.2, AnTat 5.5, KETRI 2494, TSW 196, STIB 348; Lanes 24–31 (T.b.gambiense) are, respectively, AnTat 9.1, AnTat 11.6, AnTat 22.1, NABE, SEKA, ABBA, LIGO, LiTat 1.6; Lanes 32–36 (T.b. rhodesiense) are STIB 884, STIB 850, AnTat 25.1/S, Etat 1.2/S, AnTat 12.1/S ; Lanes 37–38 (T. congolense) are IL1180, TRT 17; Lane 39 (T. vivax) is ILRAD 700 and Lane 40 (T. theileri) is MELSELE ; Lanes M 100 bp molecular marker (MBI Fermentas, Germany).
PCR RoTat 1.2 : analytical sensitivity
A tenfold dilution series (105 trypanosomes down to 1 trypanosome per 200 μl sample) of RoTat 1.2 trypanosomes in mouse blood was prepared to determine the analytical sensitivity of the PCR. As shown in figure 2, the PCR was able to detect as few as 10 trypanosomes per PCR reaction, which corresponds with a lower detection limit of 50 trypanosomes per ml. In principle, this limit can still be lowered if a blood sample of 200 μl extracted with the QIAamp DNA mini kit is eluted in less than 200 μl.
Figure 2.
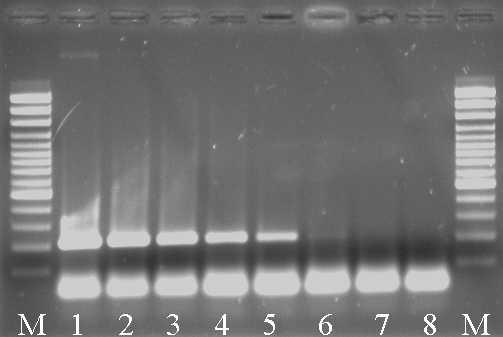
Analytical sensitivity of the RoTat 1.2 PCR. Lanes M 100 bp molecular marker (MBI Fermentas, Germany); lane 1: 105 trypanosomes, lane 2: 104 trypanosomes, lane 3: 103 trpyanosomes, lane 4: 102 trypanosomes, lane 5: 10 trypanosomes, lane 6: 1 trypanosome, lane 7: 0.1 trypanosome, lane 8: negative control.
PCR-Te664 : taxon specificity
To evaluate the RoTat 1.2 diagnostic system alongside other published methods, we compared our method to the PCR-Te664 method as published by Ventura et al. [15] using the same trypanosome stocks. The PCR-Te664 method yielded the expected amplicon in all seven T. evansi strains and in seven out of nine T. equiperdum. As with the RoTat 1.2 PCR only T. equiperdum strains OVI and BoTat 1.1 remained negative. Unexpectedly, four out of six T. b. brucei (AnTat 2.2, AnTat 5.2, TSW 196 and KETRI 2494) and two T. b. gambiense type II strains (ABBA and LIGO) tested positive in this PCR (data not shown).
Discussion
This study was initiated to develop a specific PCR test that would be able to distinguish T. evansi from the other members of the Trypanozoon subgenus. The study is an extension of the initial observation that the RoTat 1.2 VSG gene only is found in T. evansi and not in T. brucei strains [17]. This study mainly focused on the presence and expression of the RoTat 1.2 VSG gene in T. evansi rather than the use of this VSG in diagnosis of Salivarian trypanosomes.
Previously, other research groups have used VSG genes as target sequences for PCR detection of T.b. gambiense infections (sleeping sickness). In these studies, five different primers derived from VSG genes, AnTat 11.17, LiTat 1.3, 117, 2 K and U2 were used in PCR screening of different trypanosome populations, originating from distinct geographical locations [18-20]. AnTat 11.17 based PCR tests were capable of distinguishing T.b. gambiense from T.b. brucei parasites from most foci of sleeping sickness in countries such as Nigeria, Cameroon, Côte d'Ivoire, R. P. Congo/Brazza. and Sudan. However, populations originating from the Moyo focus in North-west Uganda and from Cameroon were shown to be negative in AnTat 11.17 and in LiTat 1.3 (2 K) PCRs respectively. According to Bromidge et al. [18], this might be due to antigenic variation and genetic evolution of the VSG genes. On the other hand, the presence of 117 and U2 genes was shown to be a common feature among all T. brucei populations tested. In T. evansi, a similar phenomenon may occur in certain Kenyan isolates. A recent study by Ngaira et al. [21] pointed out that some T. evansi stocks in the Isiolo district in Kenya seem to lack the Rotat 1.2 VSG gene. It is believed that these stabilates belong to the T. evansi type B group. So far, this type of T. evansi has only been observed in this specific region in Kenya [22,23]. To our knowledge, all other T. evansi isolated elsewhere, are from the classical T. evansi type A group. Thus, we assume that, except for these few Kenyan strains belonging to the type B group, our PCR is specific for T. evansi.
Compared to the PCR-Te664 presented by Ventura et al. [15], the PCR RoTat 1.2 seems to have a higher taxon specificity, since no reaction with T. b. brucei, nor with T. b. gambiense type II was observed. However, regarding T. equiperdum, both PCR test positive for the same seven T. equiperdum strains and are both negative for the BoTat 1.1 and OVI strains. Since the RAPD fragment (AF397194) shares no homology with the Rotat 1.2 VSG gene (AF317914) and is not found within the expression site of trypanosomes, both sequences can be considered as independent molecular markers. Based on the observations with both markers, it appears that on the genomic level the Botat 1.1 and the OVI strains are different from the other T. equiperdum and T. evansi strains. The observed analytical sensitivity with the RoTat 1.2 PCR is comparable to what was reported for the Te664 PCR (25 cells per reaction) [15].
The presence of a RoTat 1.2 specific DNA sequence in some T. equiperdum strains corresponds with the serological evidence that rabbits experimentally infected with these strains develop RoTat 1.2 specific lytic antibodies within 30 days post infection [24]. In contrast, rabbits infected with the BoTat 1.1 clone and the OVI strain, which are negative in the present PCR, did not produce specific antibodies to the RoTat 1.2 clone when tested in immune trypanolysis. This might be explained by the loss of the RoTat 1.2 gene in the OVI and the BoTat 1.1 strain. It is also possible that there has been a sequence drift at the sites where these primers could bind. However, we hypothesize that RoTat 1.2 VSG truly is T. evansi specific and that RoTat 1.2 PCR positive T. equiperdum strains are actually T. evansi and not T. equiperdum. Indeed, in a previous molecular characterization study using Random Amplified Polymorphic DNA (RAPD) and the Multiplex-endonuclease Genotyping Approach (MEGA) it appeared that the T. equiperdum collection is not as homogenous as previously believed and that the generally followed concept that T. equiperdum is very closely related to T. evansi and more distant from T. b. brucei, seems incorrect. From the cluster analysis on the available strains, it appeared that only two clusters can be identified: a homogeneous T. evansi/T. equiperdum cluster and a more heterogeneous T. b. brucei/T. equiperdum cluster [25]. Interestingly, all strains of that homogeneous T. evansi/T. equiperdum cluster are all PCR RoTat 1.2 VSG positive while the strains found in the more heterogeneous T. b. brucei/T. equiperdum cluster, in casu BoTat 1.1 and OVI are PCR RoTat 1.2 VSG negative.
Conclusions
PCR amplification of the RoTat 1.2 VSG gene is a specific marker for T. evansi strains, except T. evansi type B, and is especially useful in dyskinetoplastic strains where kDNA based markers may fail to amplify. Furthermore, our data support previous suggestions that some T. evansi stocks have been previously misclassified as T. equiperdum.
Methods
Trypanosome populations
A total of 39 different trypanosome populations were used in this study. They belong to 39 stocks and six species, isolated from a variety of host species at distinct geographical locations (Table 1 [see Additional file 1]). Only three T. equiperdum strains, BoTat 1.1, OVI and STIB 818 are well documented, i.e. known origin and host. The other six are putative T. equiperdum, based on publications or on their use as reference strains in different national dourine reference laboratories [26-30].
Preparation of trypanosome DNA
Procyclic trypanosome populations were grown in vitro in Cunningham's medium [31] and in the Kit for In Vitro Isolation (KIVI) [32]. Pure procyclic trypanosomes were obtained by repeated centrifugation (20 min., 2000 g) and sediment washes with Phosphate Glucose Sacharose buffer (PGS) (38 mM Na2HPO4.2H20, 2 mM NaHPO4, 80 mM glucose, 100 mM sacharose, pH 8.0). Bloodstream form trypanosomes were expanded in mice and rats and were purified from the blood by di-ethyl-amino-ethyl (DEAE) chromatography [33], followed by repeated centrifugation (20 min., 2000 g) and sediment washes with Phosphate Buffered Saline Glucose (PSG) (38 mM Na2HPO4.2H20, 2 mM NaHPO4, 80 mM glucose, 29 mM NaCl, pH 8.0). Trypanosome sediments were subsequently stored at -80°C.
Twenty μl of trypanosome sediment (approximately 2.107 cells) were resuspended in 200 μl of Phosphate Buffered Saline (PBS) (8.1 mM Na2HPO4.2H20, 1.4 mM NaHPO4, 140 mM NaCl, pH 7.4) and the trypanosome DNA was extracted using the commercially available QIAamp DNA mini kit (Westburg, Leusden, The Netherlands), resulting in pure DNA in 200 μl of TE buffer. The typical yield of DNA extracted from a 20 μl pellet was 150 ng/μl or 30 μg total DNA. The extracts obtained were diluted 200 times in water and divided into aliquots of 2 ml in microcentrifuge tubes for storage at -20°C.
For trypanosome dilution series, 180 μl of each heparinized blood sample were mixed with an equal volume of the Qiagen AS-1 storage buffer and subsequently extracted using the QIAamp DNA blood mini kit (Westburg, Leusden, The Netherlands) resulting in 200 μl of extracted DNA in Millipore water. Manipulation was performed according to the manufacturer's instructions.
PCR RoTat 1.2
Primers were derived from the RoTat 1.2 VSG sequence (AF317914), recently cloned and sequenced by Urakawa et al. [17]. Using DNA sequence homology search programs to interrogate databases at TIGR (The Institute for Genomic Research) and GenBank, primer sequences were identified within the region (608–812 bp) lacking homology with any other known VSG sequence present in the databases. Primer sequences (in 5'-3' direction) and annealing temperatures were as follows: RoTat 1.2 Forward GCG GGG TGT TTA AAG CAA TA, Tann. 59°C and RoTat 1.2 Reverse ATT AGT GCT GCG TGT GTT CG, Tann. 59°C.
Twenty μl of extracted DNA were mixed with 30 μl of a PCR-mix containing: 1 U Taq DNA recombinant polymerase (Promega, UK), PCR buffer (Promega, UK), 2.5 mM MgCl2 (Promega, UK), 200 μM of each of the four dNTPs (Roche, Mannheim, Germany) and 0.8 μM of each primer (Gibco BRL, UK).
All amplifications were carried out in a Biometra® Trio-block thermocycler. Cycling conditions were as follows: denaturation for 4 min. at 94°C, followed by 40 amplification cycles of 1 min. denaturation at 94°C, 1 min. primer-template annealing at 59°C and 1 min. polymerization at 72°C. A final elongation step was carried out for 5 min. at 72°C.
Twenty μl of the PCR product and ten μl of a 100 bp size marker (MBI Fermentas, Germany) were subjected to electrophoresis in a 2 % agarose gel (25 min. at 100 V). Gels were stained with ethidium bromide (0.5 μg/ml) (Sigma, USA) and analyzed on an Imagemaster Video Detection System (Pharmacia, UK).
PCR Te-664
PCR on purified DNA samples was performed using primers and PCR conditions according to Ventura et al. [15]. Only the Taq DNA polymerase was purchased from another distributor, i.e. Promega (UK) instead of Gibco BRL (UK).
Competing interests
None declared.
Authors' contributions
FC carried out the molecular work and drafted the manuscript. MR and TU participated in the molecular analysis. PM, BG and PB participated in the design and co-ordination of the study. All authors read and approved the final manuscript.
Supplementary Material
Table 1. Data on the different Trypanosoma (T.) populations used in this study
Acknowledgments
Acknowledgements
This study received financial support from the International Livestock Research Institute in Nairobi, Kenya and the Institute for the Promotion of Innovation by Science and Technology in Flanders, Belgium (IWT). Trypanosoma spp. stabilates were kindly provided by: T Baltz, University of Bordeaux II, France; T De Waal, Onderstepoort Veterinary Institute, South Africa; R Brun, Swiss Tropical Institute Basel, Switzerland; P-H Clausen, Free University Berlin, Germany; J Hagebock†, National Veterinary Services Laboratories, United States Department of Agriculture, USA; Z Lun, Zhongshan University, P.R. China; and V Zablotsky, All-Russian Research Institute for Experimental Veterinary Medicine (VIEV), Russia.
Contributor Information
Filip Claes, Email: fclaes@itg.be.
Magda Radwanska, Email: mradwanska@itg.be.
Toyo Urakawa, Email: toyo.urakawa@nifty.ne.jp.
Phelix AO Majiwa, Email: pmajiwa@cgiar.org.
Bruno Goddeeris, Email: bruno.goddeeris@agr.kuleuven.ac.be.
Philip Büscher, Email: pbuscher@itg.be.
References
- Hoare CA. The trypanosomes of mammals. Oxford, Blackwell Scientific Publications; 1972. pp. 1–749. [Google Scholar]
- Stephen LE. In: Trypanosomiasis A veterinary perspective. StephenLE, editor. Oxford, Pergamon Press; 1986. [Google Scholar]
- Nantulya VM. Trypanosomiasis in domestic animals: the problems of diagnosis. Rev Sci Tech. 1990;9:357–367. doi: 10.20506/rst.9.2.507. [DOI] [PubMed] [Google Scholar]
- Moser DR, Cook GA, Ochs DE, Bailey CP, McKane MR, Donelson JE. Detection of Trypanosoma congolense and Trypanosoma brucei subspecies by DNA amplification using the polymerase chain reaction. Parasitology. 1989;99:57–66. doi: 10.1017/s0031182000061023. [DOI] [PubMed] [Google Scholar]
- Wuyts N, Chokesajjawatee N, Panyim S. A simplified and highly sensitivity detection of Trypanosoma evansi by DNA amplification. Southeast Asian J Trop Med Public Health. 1994;25:266–271. [PubMed] [Google Scholar]
- Pereira de Almeida PJL, Ndao M, Van Meirvenne N, Geerts S. In: Diagnostic evaluation of PCR in goats infected with Trypanosoma brucei brucei. IAEA, editor. Vienna, International Symposium on Diagnosis and Control of Livestock Diseases using Nuclear and Related Techniques; 1997. p. 68. [Google Scholar]
- Clausen P-H, Wiemann A, Patzelt R, Kakaire D, Poetzsch C, Peregrine A, Mehlitz D. Use of a PCR assay for the specific and sensitive detection of Trypanosoma spp. in naturally infected dairy cattle in peri-urban Kampala, Uganda. Ann N Y Acad Sci. 1998;849:21–31. doi: 10.1111/j.1749-6632.1998.tb11029.x. [DOI] [PubMed] [Google Scholar]
- Omanwar S, Rao JR, Basagoudanavar SH, Singh RK, Butchaiah G. Direct and sensitive detection of Trypanosoma evansi by polymerase chain reaction. Acta Vet Hung. 1999;47:351–359. doi: 10.1556/AVet.47.1999.3.9. [DOI] [PubMed] [Google Scholar]
- Holland WG, Claes F, My LN, Thanh NG, Tam PT, Verloo D, Büscher P, Goddeeris B, Vercruysse J. A comparative evaluation of parasitological tests and a PCR for Trypanosoma evansi diagnosis in experimentally infected water buffaloes. Vet Parasitol. 2001;97:23–33. doi: 10.1016/S0304-4017(01)00381-8. [DOI] [PubMed] [Google Scholar]
- Pereira de Almeida PJL, Ndao M, Goossens B, Osaer S. PCR primer evaluation for the detection of trypanosome DNA in naturally infected goats. Vet Parasitol. 1998;80:111–116. doi: 10.1016/S0304-4017(98)00205-2. [DOI] [PubMed] [Google Scholar]
- Majiwa PAO. DNA probe- and PCR-based methods for the detection of trypanosomes. In: SonesKR, editor. Twenty-second Meeting of the International Scientific Councel for Trypanosomiasis Research and Control (ISCTRC) Kampala, Uganda,25-29 October 1993. Nairobi, OAU/STRC; 1995. pp. 53–58. [Google Scholar]
- Masiga DK, Gibson WC. Specific probes for Trypanosoma (Trypanozoon) evansi based on kinetoplast DNA minicircles. Mol Biochem Parasitol. 1990;40:279–284. doi: 10.1016/0166-6851(90)90049-R. [DOI] [PubMed] [Google Scholar]
- Artama WT, Agay MW, Donelson JE. DNA comparisons of Trypanosoma evansi (Indonesia) and Trypanosoma brucei spp. Parasitology. 1992;104:67–74. doi: 10.1017/s0031182000060819. [DOI] [PubMed] [Google Scholar]
- Diall O. Camel trypanosomosis in Mali: contribution to the diagnosis and the epidemiology. Vrije Universiteit Brussel; 1993. pp. 1–91. [Google Scholar]
- Ventura RM, Takeda GF, Silva RAMS, Nunes VLB, Buck GA, Teixeira MMG. Genetic relatedness among Trypanosoma evansi stocks by random amplification of polymorphic DNA and evaluation of a synapomorphic DNA fragment for species-specific diagnosis. Int J Parasitol. 2001:1–11. doi: 10.1016/s0020-7519(01)00314-9. [DOI] [PubMed] [Google Scholar]
- Verloo D, Magnus E, Büscher P. General expression of RoTat 1.2 variable antigen type in Trypanosoma evansi isolates from different origin. Vet Parasitol. 2001;97:183–189. doi: 10.1016/s0304-4017(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Urakawa T, Verloo D, Moens L, Büscher P, Majiwa PAO. Trypanosoma evansi: cloning and expression in Spodoptera fugiperda insect cells of the diagnostic antigen RoTat 1.2. Exp Parasitol. 2001;99:181–189. doi: 10.1006/expr.2001.4670. [DOI] [PubMed] [Google Scholar]
- Bromidge T, Gibson W, Hudson K, Dukes P. Identification of Trypanosoma brucei gambiense by PCR amplification of variant surface glycoprotein genes. Acta Trop. 1993;53:107–119. doi: 10.1016/0001-706X(93)90023-5. [DOI] [PubMed] [Google Scholar]
- Dukes P, Gibson WC, Gashumba JK, Hudson KM, Bromidge TJ, Kaukus A, Asonganyi T, Magnus E. Absence of the LiTat 1.3 (CATT antigen) gene in Trypanosoma brucei gambiense stocks from Cameroon. Acta Trop. 1992;51:123–134. doi: 10.1016/0001-706X(92)90054-2. [DOI] [PubMed] [Google Scholar]
- Cross M, Taylor MC, Borst P. Frequent loss of the active site during variant surface glycoprotein expression site switching in vitro in Trypanosoma brucei. Mol Cell Biol. 1998;18:198–205. doi: 10.1128/mcb.18.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaira JM, Njagi ENM, Ngeranwa JJN, Olembo NK. PCR amplification of RoTat 1.2 VSG gene in Trypanosoma evansi isolates in Kenya. Vet Parasitol. 2004;120:23–33. doi: 10.1016/j.vetpar.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Gibson WC, Wilson AJ, Moloo SK. Characterisation of Trypanosoma (Trypanozoon) evansi from camels in Kenya using isoenzyme electrophoresis. Res Vet Sci. 1983;34:114–118. [PubMed] [Google Scholar]
- Borst P, Fase-Fowler F, Gibson WC. Kinetoplast DNA of Trypanosoma evansi. Mol Biochem Parasitol. 1987;23:31–38. doi: 10.1016/0166-6851(87)90184-8. [DOI] [PubMed] [Google Scholar]
- Claes F, Verloo D, De Waal DT, Urakawa T, Majiwa P, Goddeeris BM, Büscher P. Expression of RoTat 1.2 cross-reactive variable antigen type in Trypanosoma evansi and T. equiperdum. Ann N Y Acad Sci. 2002;969:174–179. doi: 10.1111/j.1749-6632.2002.tb04373.x. [DOI] [PubMed] [Google Scholar]
- Claes F, Agbo EC, Radwanska M, te Pas MFW, Baltz T, De Waal DT, Goddeeris BM, Claassen E, Büscher P. How does Trypanosoma equiperdum fit into the Trypanozoon group? A cluster analysis by RAPD and Multiplex-endonuclease genotyping approach. Parasitology. 2003;126:425–431. doi: 10.1017/S0031182003002968. [DOI] [PubMed] [Google Scholar]
- Barrowman PR. Experimental intraspinal Trypanosoma equiperdum infection in a horse. Onderstepoort J Vet Res. 1976;43:201–202. [PubMed] [Google Scholar]
- Van Meirvenne N. Immunologische aspekten van de relatie tussen salivaria trypanosomen en de zoogdiergastheer. Universitaire Instelling Antwerpen, Antwerpen; 1977. pp. 1–240. [Google Scholar]
- Baltz T. Etude de la variation antigénique chez Trypanosoma equiperdum. L'Université de Bordeaux II, Bordeaux; 1982. pp. 1–84. [Google Scholar]
- Hagebock JM, Chieves L, Frerichs WM, Miller CD. Evaluation of agar gel immunodiffusion and indirect fluorescent antibody assays as supplemental tests for dourine in equids. Am J Vet Res. 1993;54:1201–1208. [PubMed] [Google Scholar]
- Lun Z-R, Allingham R, Brun R, Lanham SM. The isoenzyme characteristics of Trypanosoma evansi and Trypanosoma equiperdum isolated from domestic stocks in China. Ann Trop Med Parasitol. 1992;86:333–340. doi: 10.1080/00034983.1992.11812675. [DOI] [PubMed] [Google Scholar]
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977;24:325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Aerts D, Truc P, Penchenier L, Claes Y, Le Ray D. A kit for in vitro isolation of trypanosomes in the field: first trial with sleeping sickness patients in the Congo Republic. Trans R Soc Trop Med Hyg. 1992;86:394–395. doi: 10.1016/0035-9203(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Lanham SM, Godfrey DG. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970;28:521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Data on the different Trypanosoma (T.) populations used in this study