Abstract
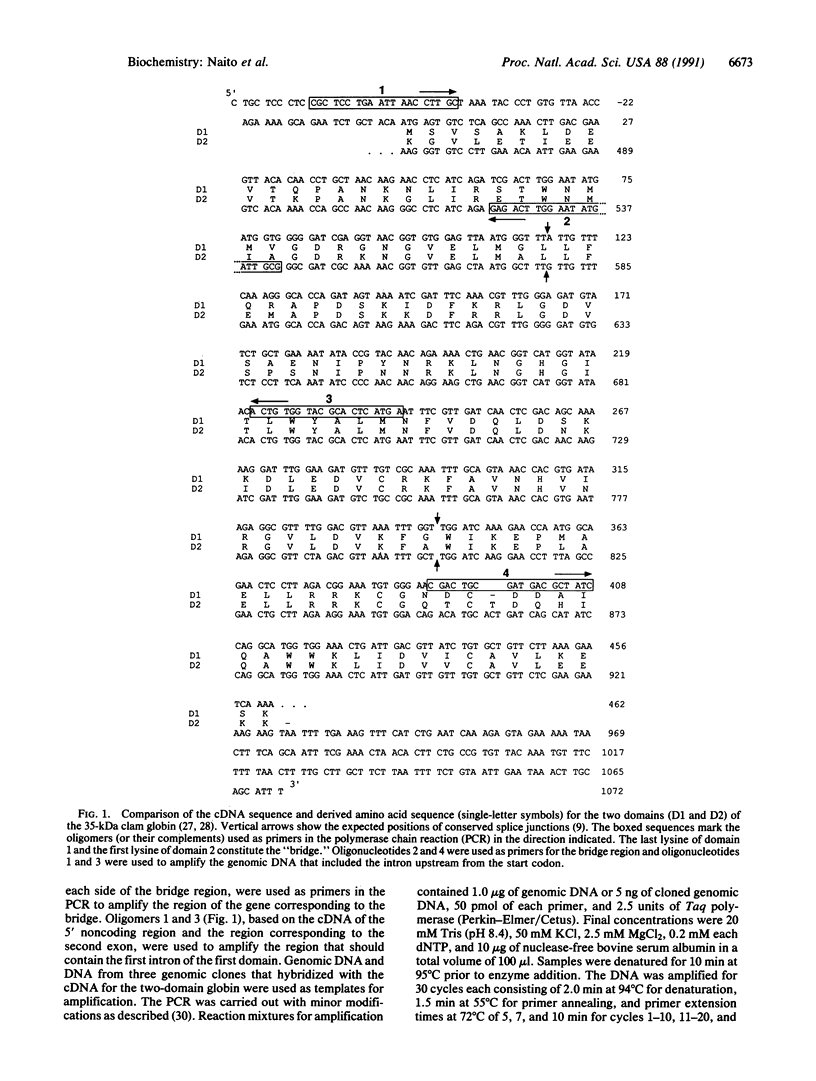
Red cells of the clam Barbatia reeveana express two hemoglobins, one composed of 16- to 17-kDa chains and the other of 35-kDa chains. The nucleotide sequence of the cDNA encoding the 35-kDa chain shows that the polypeptide has two very similar heme-binding domains, which are joined without use of an additional bridging sequence. Two novel introns occur in the gene for the two-domain globin: one, the "precoding" intron, is located two bases 5' from the start codon, and the other, a "bridge" intron, separates the DNA sequences encoding the two domains. Close correspondence exists between the 3' end of the precoding intron and the 3' end of the bridge intron and between parts of the 3' noncoding region of the cDNA for the two-domain globin and the 5' end of the bridge intron. These observations indicate that the bridge intron arose by unequal crossing-over between two identical or very similar genes for a single-domain globin. This conclusion, together with the proposal that exons were initially independent "minigenes" [Gilbert, W. (1987) Cold Spring Harbor Symp. Quant. Biol. 52, 901-905], suggests that many introns may have evolved from the 5' noncoding region of one gene and/or the 3' noncoding region of a second gene. This hypothesis implies that splice junctions would be associated with the original NH2 and COOH termini of proteins and provides an explanation for the observation that splice junctions usually map to protein surfaces. They do so because most NH2- and COOH-terminal residues are usually located on or near the surfaces of proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- Blanchetot A., Wilson V., Wood D., Jeffreys A. J. The seal myoglobin gene: an unusually long globin gene. Nature. 1983 Feb 24;301(5902):732–734. doi: 10.1038/301732a0. [DOI] [PubMed] [Google Scholar]
- Bogusz D., Appleby C. A., Landsmann J., Dennis E. S., Trinick M. J., Peacock W. J. Functioning haemoglobin genes in non-nodulating plants. Nature. 1988 Jan 14;331(6152):178–180. doi: 10.1038/331178a0. [DOI] [PubMed] [Google Scholar]
- Brändén C. I., Eklund H., Cambillau C., Pryor A. J. Correlation of exons with structural domains in alcohol dehydrogenase. EMBO J. 1984 Jun;3(6):1307–1310. doi: 10.1002/j.1460-2075.1984.tb01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J., Hwang P., Anderson L., Lebo R., Gorin F., Fletterick R. Intron/exon structure of the human gene for the muscle isozyme of glycogen phosphorylase. Proteins. 1987;2(3):177–187. doi: 10.1002/prot.340020303. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Buchman S. R., Beychok S. Characterization of globin domains: heme binding to the central exon product. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1384–1388. doi: 10.1073/pnas.77.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Buchman S. R., Beychok S. O2 binding properties of the product of the central exon of beta-globin gene. Nature. 1981 May 7;291(5810):87–90. doi: 10.1038/291087a0. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Choo Q. L., Swift G. H., Quinto C., MacDonald R. J., Rutter W. J. Structure of two related rat pancreatic trypsin genes. J Biol Chem. 1984 Nov 25;259(22):14255–14264. [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Sprang S., Fletterick R., Rutter W. J. Intron-exon splice junctions map at protein surfaces. Nature. 1982 Sep 9;299(5879):180–182. doi: 10.1038/299180a0. [DOI] [PubMed] [Google Scholar]
- De Sanctis G., Falcioni G., Giardina B., Ascoli F., Brunori M. Mini-myoglobin. The structural significance of haem-ligand interactions. J Mol Biol. 1988 Apr 20;200(4):725–733. doi: 10.1016/0022-2836(88)90483-4. [DOI] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Eaton W. A. The relationship between coding sequences and function in haemoglobin. Nature. 1980 Mar 13;284(5752):183–185. doi: 10.1038/284183a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. The exon theory of genes. Cold Spring Harb Symp Quant Biol. 1987;52:901–905. doi: 10.1101/sqb.1987.052.01.098. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Go M. Correlation of DNA exonic regions with protein structural units in haemoglobin. Nature. 1981 May 7;291(5810):90–92. doi: 10.1038/291090a0. [DOI] [PubMed] [Google Scholar]
- Gonda D. K., Bachmair A., Wünning I., Tobias J. W., Lane W. S., Varshavsky A. Universality and structure of the N-end rule. J Biol Chem. 1989 Oct 5;264(28):16700–16712. [PubMed] [Google Scholar]
- Gorin M. B., Cooper D. L., Eiferman F., van de Rijn P., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. I. A comparison of the primary amino acid sequences of mammalian alpha-fetoprotein and albumin. J Biol Chem. 1981 Feb 25;256(4):1954–1959. [PubMed] [Google Scholar]
- Grinich N. P., Terwilliger R. C. The quaternary structure of an unusual high-molecular-weight intracellular haemoglobin from the bivalve mollusc Barbatia reeveana. Biochem J. 1980 Jul 1;189(1):1–8. doi: 10.1042/bj1890001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhiang S. M., Garey J. R., Riggs A. F. Exon-intron organization in genes of earthworm and vertebrate globins. Science. 1988 Apr 15;240(4850):334–336. doi: 10.1126/science.2832953. [DOI] [PubMed] [Google Scholar]
- Jhiang S. M., Riggs A. F. The structure of the gene encoding chain c of the hemoglobin of the earthworm, Lumbricus terrestris. J Biol Chem. 1989 Nov 15;264(32):19003–19008. [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Manning A. M., Trotman C. N., Tate W. P. Evolution of a polymeric globin in the brine shrimp Artemia. Nature. 1990 Dec 13;348(6302):653–656. doi: 10.1038/348653a0. [DOI] [PubMed] [Google Scholar]
- Minghetti P. P., Ruffner D. E., Kuang W. J., Dennison O. E., Hawkins J. W., Beattie W. G., Dugaiczyk A. Molecular structure of the human albumin gene is revealed by nucleotide sequence within q11-22 of chromosome 4. J Biol Chem. 1986 May 25;261(15):6747–6757. [PubMed] [Google Scholar]
- Senapathy P. Possible evolution of splice-junction signals in eukaryotic genes from stop codons. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1129–1133. doi: 10.1073/pnas.85.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N. The structure of invertebrate extracellular hemoglobins (erythrocruorins and chlorocruorins). Comp Biochem Physiol B. 1985;82(1):1–15. doi: 10.1016/0305-0491(85)90120-8. [DOI] [PubMed] [Google Scholar]
- Weller P., Jeffreys A. J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984 Feb;3(2):439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


