Abstract
The effects of temperature and of the membrane-active protein CTII on the formation of nonbilayer structures in mitochondrial membranes were studied by 31P-NMR. Increasing the temperature of isolated mitochondrial fractions correlated with an increase in ATP synthase activity and the formation of nonbilayer packed phospholipids with immobilized molecular mobility. Computer modeling was employed for analyzing the interaction of mitochondrial membrane phospholipids with the molecular surface of CTII, which behaves like a dicyclohexylcarbodiimide-binding protein (DCCD-BP) of the F0 group in a lipid phase. Overall, our studies suggest that proton permeability toroidal pores formed in mitochondrial membranes consist of immobilized nonbilayer-packed phospholipids formed via interactions with DCCD-BP. Our studies support the existence of a proton transport along a concentration gradient mediated via transit toroidal permeability pores which induce conformational changes necessary for mediating the catalytic activity of ATP synthase in the subunits of the F0–F1 complex.
INTRODUCTION
The molecular mechanism of ATP synthesis in mitochondrial membranes has been the subject of intense research for the past 30 years [1]. The structure of group F1 subunits of the ATP synthase complex has been well characterized. However, the structure, organization, and dynamic movement of subunits of the F0 group, which is localized in the inner mitochondrial membrane has been less studied [2]. The mechanism of how H+ protons are transferred from the intermembrane space into the mitochondrial matrix to induce the structural changes that arise in the F0 subunits to trigger rotation of the rotor of the ATP synthase complex remain to be elucidated [3]. In this work, we analyzed the ATP-synthase activity of mitochondria and the changes in phospholipid packing in mitochondrial membranes in response to changes in temperature and upon treatment with the cobra snake venom membrane-active protein CTII, which interacts with phospholipids in a lipid phase similarly to the dicyclohexylcarbodiimide- binding protein (DCCD-BP) of the F0 group [4]. In addition, computer modeling was used to study the interaction of the molecular surface of CTII with lipids found in the mitochondrial membrane, including phosphatidylcholine, cardiolipin, phosphatidic acid, and phosphatidylserine.
MATERIALS AND METHODS
Bovine heart mitochondria and cobra snake- venom CTII were obtained as described previously [5]. DCCD-BP, which is a component of the F0 group and is involved in proton transport [6], was isolated from mitochondria as previously published [7]. The binding of DCCD-BP with dicyclohexylcarbodiimide was assayed as previously published [8]. ATP synthase activity of mitochondria was assayed as previously published [9]. To estimate the oligomycin sensitivity of ATP synthase activity, mitochondria were treated with 5 nM Streptomyces diastatochromogenes oligomycin (Sigma-Aldrich, United States; purity ≥95% by HPLC). Multilamellar liposomes (MLLs) containing natural phospholipids (65 mol% phosphatidylcholine, 6 mol% phosphatidic acid, 4 mol% phosphatidylserine, and 25 mol% cardiolipin) were generated as published [10]. To incorporate DCCD-BP and CTII in MLLs, the lipids and proteins in a water–methanol phase were dried in a vacuum and the resulting film was hydrated according to a published protocol [10]. 31P-NMR spectra of mitochondria and MLLs were recorded as described previously [5]. The spatial structure coordinates of CTII were retrieved from PDB (ID 1CB9); energy minimization of the CTII structure was performed as described previously [11]. Cardiolipin coordinates were obtained from a crystal structure of a bovine heart oxidoreductase–cardiolipin complex (PDB ID 1V54). Phosphatidylcholine coordinates were obtained from the crystal structure of the phosphatidylinositol transfer protein (PITP)–phoshatidylcholine complex (PDB ID 1T27). Phosphatidylserine coordinates were obtained from a crystal structure of T-cell immunoglobulin mucin protein 4 (Tim-4)–phosphatidylserine complex (PDB ID 3BIB). The spatial structure coordinates of phosphatidic acid were kindly provided by I.H. Shrivastava (Department of Computational and Systems Biology, University of Pittsburgh, Pennsylvania, United States). Computer simulated docking of the molecular surfaces of the phospholipids and CTII was performed using AutoDockVina v. 4.2 as previously published [5].
RESULTS
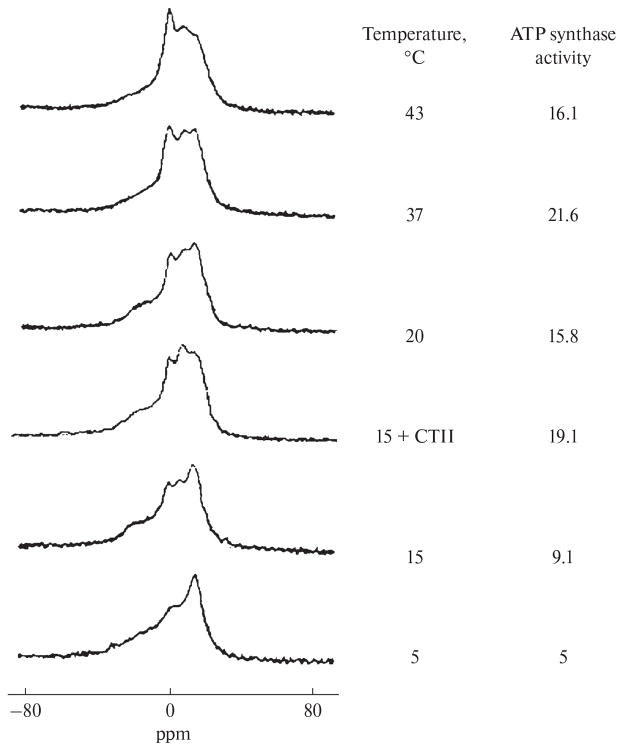
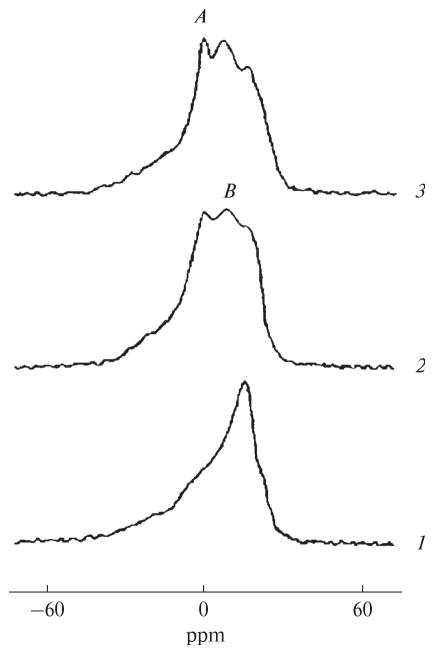
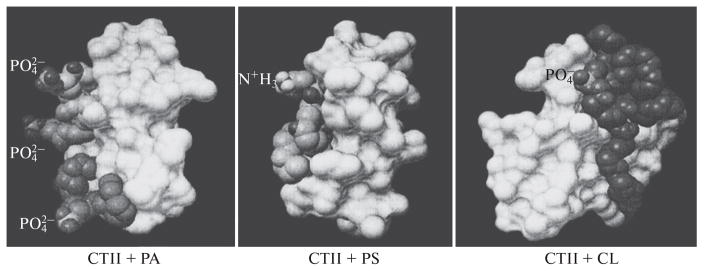
The 31P-NMR spectrum for mitochondria at 5°C shows a bilayer packing of phospholipids in mitochondrial membranes (Fig. 1). When the temperature was increased to 15°C, two nonbilayer signals were detected (signal A at 0 ppm and signal B shifted to a higher field from signal A), along with a threefold increase in ATP synthase activity. When 9 × 10−4 M CTII was added to mitochondria at 15°C, 31P-NMR signals A and B increased in intensity (especially signal B) and a two-fold increase was observed in ATP synthase activity. Adding 9 × 10−4 M DCCD-BP to mitochondria at 15°C did not change the 31P-NMR spectrum or ATP synthase activity (data not shown). An increase in temperature (15°C to 37°C) increased the intensity of the nonbilayer signals and ATP synthase activity in mitochondria in the absence of CTII and DCCD-BP (Fig. 1). At 43°C, signal A appreciably increased in intensity, while ATP synthase activity decreased. Adding oligomycin to mitochondria (5 nM) completely inhibited ATP synthesis, both in the absence or presence of DCCD-BP or CTII (data not shown). When the high-field 31P-NMR signal of the lamellar phase was suppressed with a DANTE pulse sequence [12], the lamellar phase signal and signal A disappeared, while signal B persisted (data not shown). This finding indicates that phospholipids responsible for signal B are not exchanged with phospholipids of the lamellar phase [5]. In CTII-free mitochondria, signal B that remained following exposure to a DANTE sequence increased by a factor of 1.7 at 20°C, 2.35 at 37°C, and 2.33 at 43°C as compared with that observed at 15°C. At 15°C, the integral intensity of signal B in CTII- containing mitochondria was twice as high as in CTII-free mitochondria. When the temperature increased from 15°C to 37°C, the increase in the integral intensity of signal B in mitochondria, with or without CTII, correlated with an increase in ATP synthase activity. Adding CTII or DCCD-BP in MLL membranes, which were comparable in phospholipid composition to the inner mitochondrial membrane [13], led to the detection of nonbilayer 31P-NMR signals A and B (Fig. 2). The integral intensity of signal B preserved in the spectrum following exposure to a DANTE pulse sequence was similar in MLLs containing CTII and in MLLs containing DCCD-BP. By using AutoDockVina software, docking phosphatidylcholine, phosphatidic acid, phosphatidylserine, and cardiolipin with CTII yielded nine energetically favorable binding conformations, wherein ionic, ion–dipole, hydrogen, and hydrophobic interactions were detected between the phospholipids and the protein. Unusual conformations were observed with acidic phospholipids, especially phosphatidic acid. The forces involved in facilitating the binding between alkyl chains of lipids and hydrophobic regions of the surface of CTII prevailed in these conformations. In addition, the polar groups of the lipid heads were observed to be perpendicular to the long axis of CTII and faced away from the surface of CTII (Fig. 3).
Fig. 1.
Polymorphic transitions of the phospholipid packing and ATP synthase activity in mitochondria in response to changes in temperature and by the treatment with 9 × 10−4 CTII. The phospholipid concentration in mitochondria was estimated at 6.3 × 10−2 M by comparing the integral intensities of the 31P-NMR signals between mitochondria and a control MLL sample. The variation of phospholipid concentration among mitochondrial samples was found to be no more than 8%. The 31P-NMR signals from nonlipid organic phosphates of mitochondria were suppressed by using the DANTE pulse sequence [12], which did not affect the 31P-NMR signals from phosphate groups of mitochondrial membrane phospholipids. ATP synthase activity was expressed as the concentration (μM) of ATP synthesized per minute per mg of protein. ATP activity is shown as the mean from three independent mitochondrial samples; the deviation from the mean was found to be no more than 4% for all estimates.
Fig. 2.
31P-NMR spectra of MLLs at 15°C. Curve 1, a control MLL sample; curve 2, MLLs containing DCCD-BP; curve 3, MLLs containing CTII. MLLs contained phosphatidylcholine, phosphatidic acid, phosphatidylserine, and cardiolipin at a molar ratio of 6.5 : 0.6 : 0.4 : 2.5, respectively. The total MLL phospholipid concentration was 65 mM. The protein–lipid molar ratio in MLL samples (2) and (3) was 0.005 as calculated from the molecular weights, 8 kDa for DCCD-BP and 7 kDa for CTII.
Fig. 3.
Models generated with AutoDockVina showing interactions of phosphatidic acid (PA), phosphatidylserine (PS), and cardiolipin (CL) with the molecular surface of membrane-active CTII. The interactions with CTII are shown for three phosphatidic acid molecules (right panel), one phosphatidylserine molecule (central panel), and one cardiolipin molecule (left panel). The molecular surface of CTII is shown in white.
DISCUSSION
We have previously shown that phospholipids with rapid isotropic mobility on the NMR time scale are responsible for giving rise to the 31P-NMR signal A, while immobilized phospholipids with an isotropic orientation of phosphate groups are responsible for generating the 31P-NMR signal B [4, 5]. Signal B was detectable for both mitochondria examined at 15°C or at higher temperatures (Fig. 1), and in MLLs containing DCCD-BP (Fig. 2). This observation suggests that isotropically-oriented phospholipids that are responsible for signal B are immobilized in both mitochondria and MLLs with DCCD-BP, presumably as a result of their interaction with DCCD-BP, a group F0 protein that is involved in proton transport. Signal B is detectable at 37°C, indicating that nonbilayer structures that are responsible signal B exist in mitochondrial membranes under physiological conditions. When the temperature was elevated, both ATP synthase activity in mitochondria and the integral intensity of signal B in their 31P-NMR spectra increased, presumably because more phospholipids interacted with group F0 DCCD-BP. A substantial increase in 31P-NMR signal A was observed at 43°C (Fig. 1), suggesting an increase in the formation of a sub-population of phospholipids with fast isotropic mobility. It is clear that this drastic change in temperature distorts the barrier properties of the mitochondrial membranes and decreases ATP synthase activity. An interesting finding is that treating mitochondria with CTII, which is an amphiphilic protein that is capable of entering the intermembrane space of mitochondria [4, 5], increased the intensity of nonbilayer signal B; this increase was accompanied by an increase in ATP synthase activity (Fig. 1). These two effects are most likely related to the interactions of CTII with acidic phospholipids that are in direct contact with F0 group proteins [14]. Such effects were not observed when DCCD-BP was added to mitochondria, possibly because hydrophobic DCCD-BP is incapable of interacting with mitochondrial membranes in an aqueous solution. However, MLLs containing DCCD-BP in a water–methanol solution have a 31P-NMR spectrum that is very similar to that of MLLs containing CTII (Fig. 2). Nearly identical integral signal B intensities were obtained for MLLs containing DCCD-BP and MLLs containing CTII. This finding indicates that DCCD-BP and CTII have similar regions on their molecular surfaces that bind phospholipids, and thereby produce a population of immobilized phospholipids with a nonbilayer packing. As we have demonstrated previously, CTII interacts with the lipid bilayer in such a manner that the long molecular axis is parallel to the alkyl chains of lipids and perpendicular to the membrane surface [4]. By using the Auto-DockVina program, we obtained the hypothetical conformations that result from interactions of acidic phospholipids with CTII. The data shows that the long axes of polar lipid heads are oriented perpendicularly to the long axis of CTII and facing away from the surface of CTII (this is most clearly seen in the case of phosphatidic acid, Fig. 3). Such conformations should destabilize the lipid bilayer packing and stimulate the formation of toroidal permeability pores [5, 15]. The structure of toroid permeability pores which contain a nonbilayer packing of lipids immobilized via interactions with CTII [5], is consistent with the presence of the 31P-NMR signal B in both mitochondria and MLLs. The presence of signal B in the 31P-NMR spectrum of MLLs that contain DCCD-BP indicates that DCCD-BP is also capable of inducing the formation of toroidal pores, which may serve as proton permeability channels in the inner mitochondrial membrane. Proton transport from the intermembrane space into the matrix of mitochondria via toroidal pores formed by lipids directly bound with group F0 of DCCD-BP may induce conformational changes in F0 group proteins and thereby trigger rotation of the rotor of the F0–F1 complex to release ATP from the active centers of the F1 complex [2]. The functioning of the electron transport chain of the inner mitochondrial membrane decreases the intermembrane space pH; presumably to facilitate the formation or opening of toroidal pores. Once open, toroidal pores pass H+ from the matrix to restore the initial pH of the intermembrane space and the bilayer packing of the inner mitochondrial membrane; i.e., the toroidal pores are thereby closed. Thus, the opening–closing cycles of toroidal pores are cycles of polymorphic transformations of the bilayer–nonbilayer packing of phospholipids and induce cyclic conformational changes in F0 group proteins, which explains the cyclic nature of how the ATP synthase work. The formation of toroidal pores is probably facilitated by the presence of certain proteins, such as DCCD-BP and CTII, in the membrane and the proton concentration gradient across the membrane. It should be noted that CTII facilitated the formation of the permeability pore, an event that is associated with ATP synthesis only at lower concentrations and at a lower temperature (15°C); presumably due to an induction of polymorphic transformations of lipids directly bound with F0 group proteins. At higher concentrations and a higher temperature (37°C), CTII causes membrane lysis and a drop in ATP synthase activity [4]. We acknowledge that the possible existence of proton permeability pores that regulate ATP synthesis in mitochondrial membranes requires direct experimental verification. However, hypothetical transit pores formed by lipids rather than proteins may explain that fact that numerous X-ray, NMR, luminescence, and other studies have failed to produce convincing detailed structural coordinates and organization of the complete ATP synthase proton channel [1, 2]. Therefore, our data warrants futures studies to elucidate the structural aspects of the lipid phase that directly interacts with F0 group proteins in mitochondrial membranes.
CONCLUSIONS
This study was the first to demonstrate that the formation of nonbilayer structures with immobilized phospholipids in the mitochondrial membrane is associated with an increase in ATP synthase activity. We hypothesize that phospholipids with a nonbilayer packing and hydrophobic interactions with an F0 group protein(s) in the inner mitochondrial membrane serve as structural elements of proton transport and facilitate the ATP synthase function.
Acknowledgments
This work was supported by the University of Nevada, Reno (International Activities Grant IAG-2014) and by grant from the National Institutes of Health (NIH grant no. GM103554) (United States).
References
- 1.Kocherginski N. Progr Biophys Mol Biol. 2009;99:20. doi: 10.1016/j.pbiomolbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Nakamoto RK, Scanlon JAB, Al-Shawi MK. Arch Biochem Biophys. 2008;476(1):43. doi: 10.1016/j.abb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber J. Biochim Biophys Acta. 2006;1757:1162. doi: 10.1016/j.bbabio.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasanov SE, Dagda RK, Rael ED. J Clinic Toxicol. 2014;4:181. doi: 10.4172/2161-0495.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasanov SE, Shrivastava IH, Israilov FS, et al. PLOS ONE. 2015;10(6):e0129248. doi: 10.1371/journal.pone.0129248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillingame RH. Annu Rev Biochem. 1980;49:1079. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- 7.Segal’ NK, Gasanov SE, Palamarchuk LA, et al. Biokhimiya. 1993;58(11):1812. [PubMed] [Google Scholar]
- 8.Sebald W, Graf T, Lukins HB. Eur J Biochem. 1979;93:587. doi: 10.1111/j.1432-1033.1979.tb12859.x. [DOI] [PubMed] [Google Scholar]
- 9.Hara KY, Mori H. J Biomol Screen. 2006;11(3):310. doi: 10.1177/1087057105285112. [DOI] [PubMed] [Google Scholar]
- 10.Gasanov SE, Vernon LP, Aripov TF. Arch Biochem Biophys. 1993;301(2):367. doi: 10.1006/abbi.1993.1157. [DOI] [PubMed] [Google Scholar]
- 11.Dagda RK, Gasanov SE, Zhang B, et al. J Biol Phys. 2014;40(2):193. doi: 10.1007/s10867-013-9339-3. doi:1007/s10867-013-9339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Kruijff B, Morris GA, Culliss PR. Biochim Biophys Acta. 1980;598:206. doi: 10.1016/0005-2736(80)90281-3. [DOI] [PubMed] [Google Scholar]
- 13.Schwertner HA, Biale JB. J Lipid Res. 1973;14(2):235. [PubMed] [Google Scholar]
- 14.Gasanov SE, Alsarraj MA, Gasanov NE, et al. J Membr Biol. 1997;150:132. doi: 10.1007/s002329900165. [DOI] [PubMed] [Google Scholar]
- 15.Wi S, Kim C. J Phys Chem B. 2008;112(36):1140. doi: 10.1021/jp801825k. [DOI] [PubMed] [Google Scholar]