Abstract
Rationale
Differences in sensitivity to the prepulse inhibition (PPI)-disruptive effects of D2-family agonists in Sprague–Dawley (SD) vs. Long Evans (LE) rats are heritable, reflect differential activation of DA signaling in the nucleus accumbens (NAC), and are associated with differences in expression of specific NAC genes. These differences may inform us about the biology of PPI deficits in disorders such as schizophrenia.
Objectives
After confirming these strain-based PPI differences, we measured expression of four genes in NAC and other regions that regulate PPI: medial prefrontal cortex and ventral hippocampus (VH).
Methods
Startle and PPI were assessed in SD and LE rats administered d-amphetamine (0 vs. 4.5 mg/kg, sc). Two weeks later, brain tissue was processed for comt, nrg1, grid2, and csnk1e expression; blood comt expression was also tested.
Results
Data confirmed expected PPI phenotypes. Gene expression levels differed across strains, sexes, and brain regions, with LE>SD expression in most genes and regions, and female>male expression for all NAC genes. Within any brain region, expression of the four genes was highly inter-correlated; across regions, correlations were less robust, reflecting distinct strain- or sex-based subgroups. PPI amphetamine sensitivity at 120 ms correlated significantly with NAC nrg1 expression, while amphetamine sensitivity for 30 ms PPI and startle magnitude correlated significantly with VH nrg1 and blood comt expression.
Conclusions
Rat strains differing in a schizophrenia-linked phenotype also differ in expression levels of genes associated both with that phenotype, and with schizophrenia, within brain regions associated with that phenotype and schizophrenia.
Keywords: Amphetamine, Medial prefrontal cortex, Nucleus accumbens, Prepulse inhibition, Schizophrenia, Ventral hippocampus
Introduction
“Prepulse inhibition” (PPI) is the suppression of the startle reflex when the startling stimulus is preceded by a weak prestimulus (Graham 1975). While the startle-inhibiting effect of the prepulse is mediated in the pons, the forebrain regulates pontine inhibitory “tone” to determine the degree to which the prepulse inhibits the reflex (cf. Swerdlow et al. 2001a). In rats, dopamine (DA) regulates PPI predominantly via D2 receptor activation in the forebrain, including the nucleus accumbens (NAC), and the resulting reduction in GABAergic release “downstream” in the ventral pallidum and elsewhere (Qu et al. 2009; cf. Swerdlow et al. 2001a). Changes in NAC D2-linked G-protein signaling appear to mediate the impact of DA agonists on PPI (Swerdlow et al. 2006, 2011). Studies are explicating the molecular genetics of PPI as a vehicle to understand the genetics of PPI deficits in human brain disorders, including schizophrenia (Braff et al. 1978; Greenwood et al. 2011, 2012).
PPI is highly heritable (Anokhin et al. 2003). PPI deficits in Huntington's Disease, 22q11 deletion, or fragile-X syndromes, and corresponding animal models (Swerdlow et al. 1995b; Frankland et al. 2004; Sobin et al. 2005) suggest that genes affected in these disorders modify brain circuitry that regulates PPI. Genes also regulate levels of PPI in healthy individuals. For example, within the Val158Met polymorphism of catechol-O-methyltransferase (COMT), the high COMT activity-conferring Val/Val allele is associated with low PPI levels, and the Met/Met allele with high PPI levels, in both healthy individuals and schizophrenia patients (Roussos et al. 2008; Quednow et al. 2010).
In rodents, PPI is heritable, as is sensitivity to DAergic regulation (Swerdlow et al. 2004c). Heritable differences in PPI sensitivity to DAergic manipulations may help us identify neural and genetic determinants of the DAergic regulation of sensorimotor gating. To the degree that there is convergence across species in the biology of PPI, these determinants might also contribute to differences in DA-linked PPI phenotypes in humans, including reduced PPI under conditions of DA dysregulation.
Based on this reasoning, we tested PPI DA agonist sensitivity in inbred and outbred rat strains from different vendors and facilities (e.g., Swerdlow et al. 2001b, 2004b) and determined that sensitivity of PPI to DA agonists in Sprague–Dawley (SD) rats was significantly different than that in hooded Long Evans (LE) rats. The stability of this phenotype was intact through >15 years of breeding stock (Swerdlow et al. 2001), with Mendelian patterns of inheritance in F1 and N2 generations that could not be explained by strain differences in maternal behavior (Swerdlow et al. 2004a, c) or regional drug levels (Swerdlow et al. 2002). Strain-related differences in PPI sensitivity to DA agonists were significantly associated with levels of D2-linked GTPγS binding (Swerdlow et al. 2006) and reduced CREB-phosphorylation in the NAC core (Swerdlow et al. 2011); they were evident from the earliest testable age (day 16) and were neurochemically specific (Swerdlow et al. 2003a). We reported highly significant differences in expression of specific NAC genes in SD vs. LE rats (Shilling et al. 2008), a disproportionate number of which contribute to DA signaling (p<10−5), synaptic long-term potentiation (p<10−11), or inositol phosphate metabolism (p<10−14); the expression of many genes in these pathways correlated significantly with PPI sensitivity to DA agonists in SD or LE rats. Furthermore, PPI-related endophenotypes in schizophrenia probands and normals in two separate samples (Greenwood et al. 2011, 2012) were significantly associated with many genes that also differed in NAC expression in SD vs. LE rats, including several associated with DAergic and glutamatergic function, such as COMT, NRG1, and GRID2 (Shilling et al. 2008).
This study extended this convergent/translational approach in several new ways. First, we measured expression levels of four genes, three of which (comt, neuregulin (nrg1) and glutamate receptor, ionotropic, delta 2 (grid2)) exhibit: (1) differences in SD vs. LE rats (Shilling et al. 2008); (2) associations with PPI-related phenotypes in humans (Green-wood et al. 2011, 2012); or (3) both. Second, while our previous gene expression study tested the phenotype of apomorphine-disrupted PPI (Shilling et al. 2008), this study instead utilized the indirect DA agonist, d-amphetamine (AMPH), to provide convergence with studies of the genetic regulation of AMPH-disrupted PPI in humans (Talledo et al. 2009). We previously reported heritable patterns of PPI AMPH sensitivity in SD and LE rats and their F1 and N2 offspring (Swerdlow et al. 2003a). Based on this change, we also examined expression of casein kinase 1 epsilon (csnk1e), which is associated with sensitivity to AMPH effects in mice and humans (Palmer et al. 2005; Veenstra-VanderWeele et al. 2006). Third, this study examined gene expression within two brain regions that regulate PPI and are implicated in the pathophysiology of schizophrenia—medial prefrontal cortex (mPFC) and ventral hippocampus (VH)—in addition to the NAC. This enabled us to examine correlated expression of PPI-relevant genes across key nodes of functionally and anatomically interconnected fronto-limbic-striatal circuitry. Fourth, this study included male and female rats, based on known similarities and differences in PPI and its AMPH-sensitivity across sexes (Swerdlow et al. 1993, 2003b; Talledo et al. 2009). Lastly, this study examined the relationship between peripheral and central expression of comt, which was previously found to show the strongest SD vs. LE strain difference in NAC expression levels (Shilling et al. 2008).
Methods and materials
Sixteen SD and 16 LE rats (M/F=1:1; 229–250/175–199 g) (Harlan Laboratories, Livermore, California) were housed and handled as in past reports (e.g., Shilling et al. 2008). All studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the UCSD Animal Subjects Committee (protocol #S01221).
PPI testing
Startle chambers (SR-LAB; San Diego Instruments, San Diego, CA) were in a sound-attenuated room (60 dB(A) ambient noise). A brief startle session was used to form balanced drug groups according to average percent PPI. Testing began 7 days later. Rats received either AMPH (4.5 mg/kg, sc) or saline vehicle 5 min before testing. This active dose of AMPH produces the two critical strain-specific “phenotypes”: PPI-enhancing effects of AMPH at short (30 ms) prepulse intervals in LE rats and PPI-disruptive effects of AMPH at long (120 ms) prepulse intervals in SD rats (Talledo et al. 2009); full dose–response effects of AMPH on long-interval PPI in SD and LE rats are found in Swerdlow et al. (2003a, 2007). Test sessions were as in Shilling et al. (2008). Testing was repeated 5 days later with AMPH dose reversed and treatment order balanced within and between strains.
Gene expression
A 14-day interval was used to ensure both drug washout and diminished acute stress effects resulting from startle testing. Thus, this study compared basal gene expression in SD vs. LE strains and not drug-induced gene activation patterns. Fourteen days after PPI testing, rat brains were removed and placed in ice-cold saline for 30 s. Coronal tissue slabs were cut with a wire tissue slicer, and the NAC, mPFC, and VH were removed bilaterally with either a tissue punch or free-hand razor dissection. Each bilateral tissue sample was placed in an RNAse-free tube and snap-frozen in liquid nitrogen. Simultaneously, 500 uL of trunk blood was collected in a RNAprotect Animal blood tube (Qiagen, 76554), inverted, and remained at room temperature for 2 h, stored at 20°C until RNA extraction. In this process, all utensils were cleaned in between each rat brain dissection with RNAlater (QIAGEN, Inc, Valencia, CA). One region (VH) from one rat was not retrieved.
Reverse transcription polymerase chain reaction
Total RNA was isolated from brain tissue using an RNeasy Mini kit (QIAGEN, Valencia, CA 91355) or from blood using RNeasy protect Animal Blood kit (QIAGEN) and protocols followed as per manufacturer (QIAGEN). Samples were spot-checked for quality using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), which provides a RNA Integrity Number (RIN). An RIN>7.0 indicates good quality RNA; the RIN for all samples analyzed was >8.50. To measure RNA concentration, the optical density of 1.5 μL of total RNA at 260 nm was measured in a spectrophotometer (NanoDrop, ND-1000, NanoDrop Technologies, Wilmington, DE). Equal amounts of RNA/sample were used to make cDNA after DNase treatment. First-strand cDNA was synthesized using qScript™ cDNA Super-Mix as per manufacturer (Quanta Biosciences, Gaithersburg, MD). Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using Applied Biosystems’ TaqMan Gene Expression Assays in an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Each 20-μL RT-PCR reaction contained 10 μL of 2× Universal PCR Master Mix, 1 μL of primer/probe mix (900 nM/250 nM final concentration), 4 μL of nuclease-free water, and 5 μL of cDNA template (40 ng). Reactions were performed in MicroAmp Optical 384-well reaction plates (Applied Biosystems) as per manufacturer. Genes and assay ID numbers (Applied Biosystems) included: comt rn00561037_m1; nrg1 rn01482165_m1; grid2 rn00515053_m1; csnk1e rn00581130_m1; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) rn01775763_g1. Assays were performed in duplicate. Data were analyzed using SDS 2.3 software from Applied Biosystems. Amplification efficiencies were validated and relative quantitation calculated after normalization to the rat GAPDH reference gene. Fold change (FC) values for all genes were calculated relative to levels within a single rat, to which FC values of 1.0 were assigned.
Statistical analyses
PPI was calculated as in Shilling et al. (2008) and analyzed by analysis of variance (ANOVA) with strain and sex as between factors and drug and prepulse interval as within factors. A measure of the magnitude of the AMPH effect (mean PPI (vehicle) minus mean PPI (AMPH)) was also used for strain comparisons; this value effectively detects differences in PPI drug sensitivity (Swerdlow et al. 2004c; Shilling et al. 2008). The 30- and 120-ms prepulse intervals were selected a priori for strain comparisons, and for correlational analyses, because these intervals are characterized by the most robust SD vs. LE differences in DA agonist sensitivity (LE being most sensitive to PPI-enhancing effects of DA agonists at 30 ms and SD most sensitive to PPI-reducing effects of DA agonists at 120 ms) (Swerdlow et al. 2004a; Shilling et al. 2008; Talledo et al. 2009).
Gene expression data (FC) were analyzed several ways. For each gene, a repeated-measures ANOVA was conducted with brain region as a within-subject factor and strain and sex as between-subject factors. Because robust and significant sex differences (but not sex×strain interactions) were detected in much of the expression data, pooling data from both sexes for unpaired t tests would obscure strain effects on gene expression levels. For this reason, separate post hoc comparisons assessed strain differences in each of the three brain regions, using one-factor ANOVAs, with strain and sex as between-subject factors, and alpha adjusted to 0.0167 for comparisons performed in the three brain regions. Correlations among the four genes within one brain region and among each gene across the three brain regions (and trunk blood, for comt) were examined via simple regressions. Lastly, structural equation modeling (SEM; Bollen 1989) was used in exploratory analyses via Mplus software v. 6.12 (Muthén and Muthén 1998). SEM can simultaneously estimate both a measurement model (confirmatory factor analysis) and a structural model (path/regression analyses) and can specify latent variables that reflect unmeasured constructs estimated by measured variables. Given the sample size, goodness of model fit was determined by specific guidelines—>0.90 by the Comparative Fit Index (CFI) (Bentler 1990); <0.08 by the standardized root mean residual (SRMR) (Hu and Bentler 1999); and a non-significant chi-square test of model fit.
Results
Behavior
PPI data are displayed in Fig. 1. Analysis of variance of percent PPI revealed the expected interaction of AMPH dose×strain×trial type (F=2.70; df, 4,112; p< 0.04), as well as a significant interaction of AMPH dose×sex×trial type (F=4.27; df, 4,112; p<0.005). There were expected significant effects of trial type and AMPH dose×trial type (p's<0.0001), and trial type×strain (p<0.007), but no other informative main or interaction effects. Inspection of the data confirmed the expected pattern of greater AMPH-enhanced PPI at short prepulse intervals in LE rats (e.g., Fig. 1, compare data within ovals: ANOVA of AMPH effect at 30-ms intervals; main effect of strain—F=8.20; df, 1,30; p<0.008), and greater AMPH-disrupted PPI at long prepulse intervals in SD rats (e.g., Fig. 1, compare data within rectangles—ANOVA of AMPH effect at 120-ms intervals; main effect of strain—F=6.99; df, 1,30; p<0.015). These two AMPH effects were significantly correlated (r=0.41, p<0.025). The significant three-way interaction of AMPH dose×sex×trial type reflected the fact that, compared with female rats, males tended to be both more sensitive to the PPI-enhancing effects of AMPH at 30-ms intervals (d=0.65) and more sensitive to the PPI-reducing effects of AMPH at 120-ms intervals (d=0.40), but neither of these effects independently reached statistical significance.
Fig. 1.
Startle phenotypes of SD vs. LE rats in the present study. Bottom: percent PPI over prepulse intervals of 10–120 ms in SD (left) and LE (right) rats after administration of saline vehicle (open circles) or AMPH (4.5 mg/kg, sc; filled circles). Oval highlights PPI-enhancing effects of AMPH with 30-ms prepulse intervals in LE (#), but not SD rats. Rectangle highlights greater PPI-disruptive effects of AMPH with 120-ms prepulse intervals in SD vs. LE rats (*). Inset shows startle magnitude on pulse-alone trials during PPI testing, with significant startle-potentiating effects detected only in male LE rats (*)
Analysis of startle magnitude on PULSE trials (Fig. 1, inset) revealed a significant effect of AMPH dose (F=5.92; df, 1,28; p<0.025) and significant interactions of AMPH dose×strain (F=12.63; df, 1,28; p<0.002), AMPH dose×sex (F=12.10; df, 1,28; p<0.002), and dose×strain×sex (F=8.73; df, 1,28; p<0.007). Post hoc comparisons revealed that AMPH significantly increased startle magnitude only in male LE rats, while its effect in SD and female LE rats was either null or to minimally reduce startle magnitude. Additional analyses (see Electronic supplementary materials) confirmed that AMPH effects on PA amplitude did not interact with its strain- and interval-specific effects on PPI.
Gene expression
Regional levels of gene expression in the NAC, mPFC, and VH, and blood levels of comt expression are seen in Fig. 2. ANOVAs were conducted for each gene, across the three brain regions, and for each brain region, across the four genes. F values for main effects, statistically significant p values, and detailed analyses of interaction effects are found in Electronic supplementary material Table 3. Among the four individual genes, ANOVAs revealed differential expression patterns across brain regions, some of which were strain- and/or sex-dependent.
Fig. 2.
Relative levels of expression (fold change) of csnk1e (a), grid2 (b), nrg1 (c), and comt (d) in the NAC, VH, and mPFC, in male and female SD (open bars) and LE (solid bars) rats. e Shows comt expression levels in blood. Patterns of strain- and sex-differences differed across genes and brain regions, as described in the text, with a predominant pattern of LE>SD and female>male expression (level of LE>SD expression within each brain region marked as: *, **, ***, ****=p<0.05, 0.01, 0.001, and 0.0001, respectively). Levels of significance for sex differences are as follows: csnk1e and grid2, F>M, NAC (p<0.0001); nrg1, F>M NAC (p<0.0002), and M>F VH (p<0.002); comt, F>M NAC (p<0.0001) and VH (p<0.0002), and M>F blood (p<0.05)
Analyses of gene expression within each brain region revealed differential levels of expression of the four genes, some of which were strain- and/or sex-dependent. In the NAC, ANOVA of gene expression levels revealed significant effects of strain (LE>SD; p=0.0005) and sex (F>M; p<0.0001); there were significant differences in overall expression levels among the four genes (p<0.0001), and significant interactions of gene×strain (p<0.0001) and gene×sex (p<0.0001), but no significant interaction of gene × strain × sex. Among the 4 genes (csnk1e, grid2, nrg1, and comt), all exhibited the same pattern of greater expression in LE vs. SD (p's<0.0161, 0.0002, 0.0001 and 0.009, respectively) and in female vs. male rats. In the mPFC, ANOVA revealed a significant effect of strain (LE>SD; p<0.009) and gene (p<0.0001), and a significant interaction of gene × strain (p<0.0009). Among the 4 genes, LE>SD differences were greatest for nrg1 (p<0.0001), and least for csnk1e (ns), with intermediate differences noted for grid2 (p<0.022, exceeding the adjusted alpha of 0.0167) and comt (p< 0.011). In the VH, ANOVA revealed a significant effect of strain (LE>SD; p<0.0003) and gene (p<0.0001), and significant interactions of gene × strain (p<0.0001) and gene × sex (p<0.0001), but no significant interaction of gene× strain×sex. Among the four genes, LE>SD strain differences were evident for csnk1e (p<0.0004), nrg1 (p<0.0001), and comt (p<0.0002). Opposite patterns of sex differences in gene expression were noted for nrg1 (M>F) and comt (F>M).
Blood levels of comt expression exhibited significant effects of strain (LE>SD; p<0.0001) and sex (M>F; p< 0.05); the sex difference reflected M>F comt expression in SD and not LE rats (Fig. 2e).
Correlations among levels of gene expression
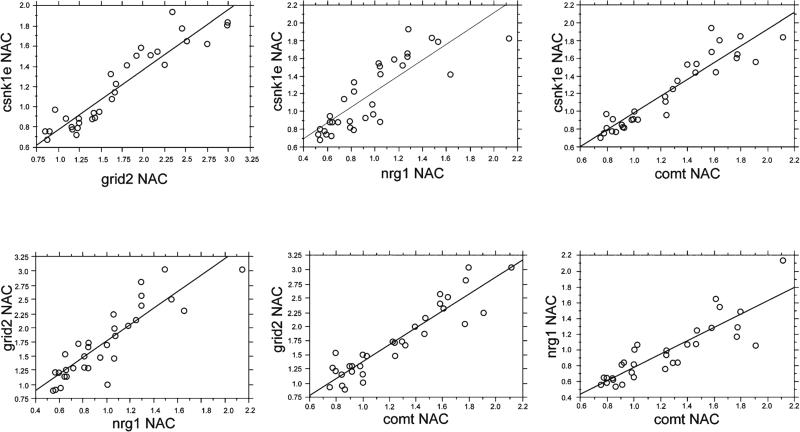
We next examined correlations of gene expression levels across the three brain regions (Table 1). Almost all observed correlations were positive, i.e., greater expression of one gene was generally associated with greater expression of another gene, and no “negative correlations” reached statistical significance. Based on a conservative value of corrected alpha (p<0.001; approximate value of alpha corrected for 61 possible contrasts), numerous significant correlations were detected for the expression of different genes within one brain region (e.g., NAC csnk1e vs. NAC grid2 (p<0.0001), NAC nrg1 (p<0.0001), and NAC comt (p<0.0001); NAC grid2 vs. NAC nrg1 (p<0.0001) and NAC comt (p<0.0001), and NAC nrg1 vs. NAC comt (p<0.0001) (Fig. 3). Correlations among expression levels of one gene across different brain regions were generally weaker: only the correlation of mPFC comt vs. VH comt reached corrected levels of significance (p<0.0001); other correlations approached but did not reach adjusted p values (e.g., VH csnk1e in vs. mPFC csnk1e, p<0.01; NAC nrg1 vs. mPFC nrg1, p<0.01; NAC comt vs. VH comt, p<0.01). In some cases, expression levels of one gene within one brain region correlated significantly with expression levels of a different gene in a different brain region. For example, VH expression of csnk1e correlated significantly with mPFC expression of comt (p<0.001), and mPFC expression of csnk1e correlated significantly with VH expression of comt (p<0.001). This was particularly common with VH comt expression levels, which exhibited robust correlations with the NAC and mPFC expression of csnk1e (r's=0.50–0.55), grid2 (r's=0.57–0.59), and nrg1 (r's=0.58–0.66).
Table 1.
Correctional (r) matrix for gene expression and key behavioral phenotypes
|
csnkle
|
grid2
|
nrg1
|
comt
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAC | VH | mPFC | NAC | VH | mPFC | NAC | VH | mPFC | NAC | VH | mPFC | Blood | ||
| csnkle | NAC | – | ||||||||||||
| VH | 0.23 | – | ||||||||||||
| mPFC | 0.39* | 0.49** | – | |||||||||||
| grid2 | NAC | 0.94**** | 0.31 | 0.37* | – | |||||||||
| VH | 0.08 | 0.53** | 0.19 | 0.03 | – | |||||||||
| mPFC | 0.39* | 0.50** | 0.89**** | 0.42* | 0.25 | – | ||||||||
| nrg1 | NAC | 0.84**** | 0.30 | 0.33 | 0.88**** | 0.15 | 0.41 | – | ||||||
| VH | 0.14 | 0.82**** | 0.21 | 0.01 | 0.41* | 0.21 | 0.01 | – | ||||||
| mPFC | 0.36* | 0.61*** | 0.86**** | 0.43* | 0.23 | 0.83**** | 0.45** | 0.41* | – | |||||
| comt | NAC | 0.93**** | 0.17 | 0.30 | 0.93**** | 0.04 | 0.37* | 0.87**** | −0.17 | 0.31 | – | |||
| VH | 0.50** | 0.88**** | 0.55*** | 0.57*** | 0.47** | 0.59*** | 0.58*** | 0.53** | 0.66**** | 0.48** | – | |||
| mPFC | 0.40* | 0.60*** | 0.85**** | 0.42* | 0.15 | 0.81**** | 0.41* | 0.39* | 0.80**** | 0.36* | 0.66**** | – | ||
| Blood | 0.10 | 0.48** | 0.01 | 0.22 | 0.09 | 0.06 | 0.28 | 0.62 | 0.29 | 0.09 | 0.31 | 0.08 | – | |
| AMPH effect | ||||||||||||||
| 30 ms | 0.03 | −0.26 | 0.22 | −0.06 | −0.20 | 0.09 | −0.19 | −0.47** | −0.04 | 0.05 | −0.15 | 0.09 | −0.62**** | |
| 120 ms | −0.28 | −0.08 | 0.06 | −0.33 | 0.01 | −0.23 | −0.38* | −0.03 | −0.08 | −0.28 | −0.23 | 0.01 | −0.25 | |
| PA | −0.11 | 0.22 | −0.09 | −0.06 | 0.15 | −0.07 | 0.03 | 0.48** | 0.13 | −0.10 | 0.07 | 0.03 | 0.54** | |
p<0.05
p<0.01
p<0.001
p<0.0001
Fig. 3.
Simple regression plots for correlations among expression levels of csnk1e, grid2, nrg1, and comt in the NAC. These correlations typify the pattern also detected in the VH and mPFC, as described in the text, of highly correlated expression levels of the four genes within any given brain region
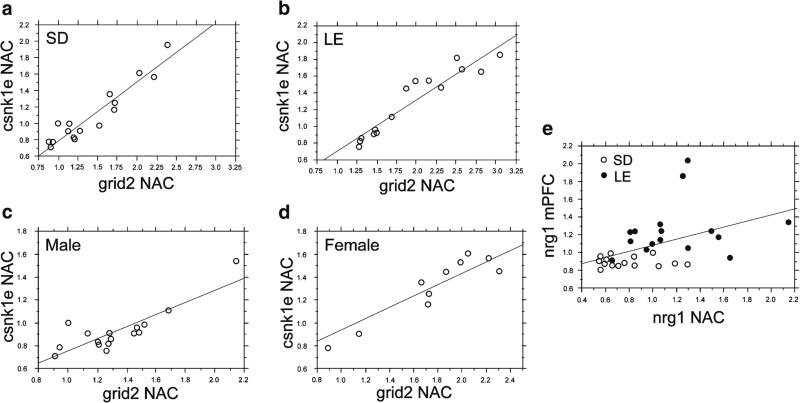
As noted above, the observed robust differences in gene expression levels generally favored LE over SD rats, and for the NAC, generally favored female over male rats. Thus, it is possible that some or all significant positive correlations among gene expression levels simply reflected the fact that, within the overall sample, there were subgroups of rats that always had low levels of gene expression, and other subgroups that always had high levels of gene expression. In other words, these correlations might reflect characteristics of this sample population (generally, LE>SD, female>male), rather than the characteristics of the genes per se. For the more robust correlations of gene expression within any given brain region, this did not seem to be the case. For example, within the NAC, levels of cnsk1e expression correlated significantly with levels of grid2 expression, not only for the overall group (r=0.94) but also for subgroups limited to SD rats (r=0.95) or LE rats (r=0.95), or male rats (r=0.85) or female rats (r=0.91) (Fig. 4a–d). However, correlated expression levels of specific genes across different brain regions did appear to reflect the distributional impact of the several different subgroups, rather than characteristics of the genes per se. For example, the relatively robust correlations of nrg1 expression levels across the NAC and mPFC for the overall population (r=0.45, p<0.05) were not reproduced in separate analyses limited to LE rats (r=0.20) or SD rats (r=−0.07) (Fig. 4e). Furthermore, in some cases, the admixture of both strains and sexes into a single regression analysis obscured strain- and sex-differences in these correlations. For example, the highly significant correlation of comt expression levels in the mPFC and NAC in the overall population (r=0.66, p<0.0001) reflected a robust relationship among LE rats (r=0.61) and particularly LE females (r=0.90) but not in SD rats (r=0.16), especially SD females (r=−0.08).
Fig. 4.
Correlations of expression levels of NAC csnk1e and NAC grid2, divided by strain and sex (a–d), showing that the robust correlation within one brain region across the entire sample (shown in Fig. 3) is maintained among each of these four subgroups. In contrast, in the few instances of significantly correlated expression levels across different brain regions (e.g., e, nrg1 expression in NAC vs. mPFC, r=0.45), these appeared appear reflect the distributional impact of the different subgroups and were not reproduced in separate analyses limited to LE rats (filled circles; r=0.20) or SD rats (open circles; r=−0.07)
Expression levels of comt in blood did not correlate significantly with expression of comt in any brain region but did correlate significantly with nrg1 expression in the VH (p<0.0001) and modestly with csnk1e expression in the VH (p<0.01).
Table 1 shows that gene expression across all genes and regions was remarkably inter-correlated. Most notable, comt expression, particularly in the mPFC and VH (see enclosed area in Table 1), appeared to serve as a “nexus” for all other expression levels. Given the relatively small sample size, a qualitative scheme for assessing “networks” of gene expression, across brain regions and genes, was pursued, based on detected bivariate “r” values (Fig. 5).
Fig. 5.
Summary representation of correlations among expression levels of the four different genes within each brain region (top) and of correlations of each gene across the three brain regions (bottom). Width of each line reflects the regression coefficient, with level of statistical significant indicated with asterisks. As described in the text, expression levels of different genes were highly correlated within any single brain region (top), while correlations of any specific gene across different brain regions were modest or not statistically significant (bottom)
Relationships among behavioral and gene expression data
Correlations between regional gene expression levels and AMPH effects on PPI at 30- and 120-ms intervals, and on startle magnitude, are seen in Table 1. AMPH effects on both PPI at 30 ms and on startle magnitude correlated significantly with nrg1 expression in the VH (r=−0.47, p<0.009 and r=0.48, p<0.007, respectively) and with comt expression in blood (r=−0.62, p < 0.0001 and r=0.54, p < 0.002, respectively). AMPH effects on PPI at 120 ms correlated significantly only with nrg1 expression in the NAC (r=−0.38, p<0.035). Baseline (vehicle) levels of PPI exhibited relatively weak and interval-dependent correlations with regional levels of gene expression (Table 1). However, averaged across the three brain regions, levels of comt expression correlated modestly with mean PPI levels (r=0.39, p<0.035), while levels of nrg1 expression correlated modestly with PPI at 60 (r=0.44, p<0.015) and 120-ms intervals (r=0.36, p<0.05).
We next used a two-step SEM procedure, whereby a measurement model is estimated first and then integrated with the structural model. Our a priori hypotheses (which served as the basis for selecting these structures for analysis) and preliminary bivariate correlations led to the prediction that the measured levels of gene expression would reflect latent factors specific to each brain region examined (NAC, mPFC, VH). Separate measurement models for the NAC (χ2(2)=5.75, p=0.06; CFI=00.98; SRMR=0.01) and mPFC (χ2 (2)=1.13, p=0.57; CFI=1.00; SRMR=0.01) showed excellent fit to the data with standardized factor loadings between 0.89 and 0.98 (Fig. 6); a similar model for gene expression levels in the VH did not converge on a valid model solution, likely due to sample size limitations. Subsequent models combined these two measurement models (both separately and together) with a simple structural model (regression paths on the outcomes of AMPH effect at 30 and 120 ms). The only significant impact of either latent variable on outcomes was found for the NAC factor on AMPH effect at 120 ms; in the final combined model with mPFC, this effect was significant (B=−0.38, p<0.05) even though model fit had weakened (χ2 (2)=53.2, p<0.001; CFI=0.92; SRMR=0.04).
Fig. 6.
Schematic diagram of the combined structural equation model; shown here are the four factor loadings for each latent construct and the association of the two latent variables to the 120 ms AMPH effect
Discussion
These data reproduce and extend the previously detected phenotypes of greater PPI-disruptive effects of DA agonists in SD vs. LE rats at relatively long prepulse intervals and greater PPI-enhancing effects of DA agonists at relatively shorter prepulse intervals. We previously reported this pattern with the direct DA agonist, apomorphine, in tests with male and female rats (Swerdlow et al. 2004a; Shilling et al. 2008), and with the indirect DA agonist, AMPH, in tests with female rats (Talledo et al. 2009). Here, the effect with AMPH was detected in a sample including males and females.
Using a microarray platform (Shilling et al. 2008), we reported significant LE>SD expression of nrg1 and comt in the NAC of male rats; in that study, we also detected but did not report moderate levels of grid2 expression in the NAC, and significantly greater NAC grid2 expression levels in LE vs. SD rats (FC=1.21, p<0.0007, unpublished data). These NAC patterns with nrg1, comt, and grid2 were replicated in the present experiment and extended to include expression levels of csnk1e, a gene known to be associated with sensitivity to AMPH effects in both rodents and humans (Palmer et al. 2005; Veenstra-VanderWeele et al. 2006). Strain differences (LE>SD) for csnk1e were also detected in the VH; for grid2, they were detected in the mPFC, and for nrg1 and comt, they were also detected in the VH and mPFC. In all cases where strain differences in gene expression were detected, levels in LE rats exceeded those in SD rats. Sex differences (F>M) in the expression of these four genes in the NAC, and of nrg1 (M>F) and comt (F>M) in the VH, were also detected. Strain and sex had independent (noninteracting) effects on the expression of these four genes across these three brain regions.
Expression levels in multiple genes across multiple brain regions were highly inter-related (Table 1). Using an uncorrected alpha level (p<0.05), statistical significance was reached in 45 out of the total 66 possible correlations of brain regional expression levels. Within any rat, the expression of different genes in the same brain region was more alike than was the expression of the same gene in different brain regions. Thus, expression levels of the four genes were highly correlated within each brain region (p's generally <0.0001), and these correlations were not a reflection of the admixture of different expression levels across groups differing in strain and/or sex. Perhaps the most parsimonious explanation for these highly correlated levels of expression is that expression of these genes is dependent on levels of cellular or metabolic activity within any given brain region. To the degree that this is true, one might predict that the expression of specific genes, particularly ones all related in some way to common sets of biological functions (e.g., DAergic or glutamatergic activity), might be strongly correlated based on their connection to a common determinant (e.g., level of activity in the NAC). In truth, however, each of these brain regions contains a large number of different cell types, each of which expresses different genes (and hence different proteins), and the overall “activity” of each structure reflects complex cellular interactions that would not necessarily be predicted to result in highly correlated activity among the many genes expressed by these cells. Thus, while “activity-dependent gene expression” might seem to be a parsimonious “first pass” explanation for the observed highly correlated levels of gene expression, it remains a hypothesis in search of supportive data. Interestingly, highly correlated levels of gene expression within one brain region (in this case, the PFC) have been reported in human post-mortem tissue (Colantuoni et al. 2011).
By contrast, relatively weaker correlations were detected for the expression of any single gene across brain regions (e.g., comt expression in the VH and mPFC) and between expression levels of one gene in one brain region and a second gene in a second brain region (e.g., VH expression of csnk1e vs. mPFC expression of comt, and mPFC expression of csnk1e vs. VH expression of comt). Even to the extent that such correlations achieved statistical significance, these weaker relationships appeared to largely reflect an “artifact” of the influence of the admixture of different sample subgroups.
Results from the structural equation model further elucidate the relations among levels of gene expression. Despite the small sample size, measurement models provided evidence for a latent factor in both the NAC and mPFC (a similar model could not be estimated for VH); in other words, the variance among the four observed gene expression levels within both the NAC and mPFC suggest they can each be accounted for by a higher-order unobserved latent variable. In the combined model, the latent factor of NAC (with factor loadings on the four gene levels measured in that region) was negatively related to the 120 ms AMPH effect (i.e., higher levels of NAC expression predicted lower levels of 120 ms AMPH effect), whereas the latent factor of mPFC did not predict this outcome. There was significant residual covariance between these two latent factors, suggesting higher levels of expression in NAC were associated with higher levels of expression in the mPFC; however, only levels of gene expression in the NAC were significantly related to the 120 ms AMPH effect.
While numerous previous reports have suggested that the NAC, mPFC, and other brain regions participate independently in the DAergic regulation of PPI (cf. Swerdlow et al. 2008), the present findings suggest that, in the intact rat—as it relates to the four genes and three regions studied here—only the levels of expression in the NAC are associated with the sensitivity of PPI to the disruptive effects of AMPH. Functional interactions between mPFC and NAC have been demonstrated previously using disconnection (e.g., Christakou et al. 2004) and other techniques; such an interaction was not detected in relationship to the expression of these four genes and PPI AMPH sensitivity. Notably, SEM also did not detect a contribution of the VH to this circuit, despite evidence that VH regulates PPI, at least in part via interactions with the mPFC (cf. Swerdlow et al. 2008). Clearly, many substrates contribute to the regulation of PPI that would not necessarily be expected to regulate PPI sensitivity to DA agonists.
Gene expression was not assessed in a “control” brain region, uninvolved in the regulation of PPI, or one not implicated in the pathophysiology of schizophrenia. Thus, we cannot conclude that the observed strain differences are specifically linked by anatomy to either laboratory or clinical phenotypes. In fact, peripheral (blood) comt expression levels also exhibited strain (LE>SD) and sex (male>female) differences, which did not correlate significantly with comt expression levels in any of the three brain regions. The sex pattern detected peripherally differed from that detected in the NAC and VH. The relationship of central vs. peripheral gene expression levels was assessed only with comt and thus does not preclude the possibility that expression levels of other genes in “accessible tissue” might be informative regarding the expression of these genes in brain tissue. However, based solely on these findings with comt, such extrapolation would not appear to be informative.
Specific issues raised by this data set will be addressed for each of the four genes assessed herein:
csnk1e
Casein kinase 1 epsilon is a component in the Darpp-32 (dopamine-and-cAMP-regulated-phosphoprotein-32 kDa) second messenger pathway (Cheong and Virshup 2010). Our finding of increased NAC csnk1e expression in LE vs. SD rats is not easily reconciled with a report (Palmer et al. 2005) of increased AMPH sensitivity in selectively bred mice with a tenfold greater expression of csnk1e in the NAC. However, in a more recent report, that group (Bryant et al. 2011) reported findings very consistent with the patterns detected herein: increased AMPH sensitivity in csnk1e knockout mice, and in mice treated acutely with the csnk1e inhibitor, PF-4800567. In our study, lower levels of NAC csnk1e expression were generally associated with greater AMPH sensitivity. For example, we detected greater AMPH-induced decreases in long-interval PPI in SD rats, which had lower levels of NAC csnk1e expression, compared with LE rats. Furthermore, female rats on average had significantly higher levels of NAC csnk1e expression, yet compared with male rats, tended to be less sensitive to AMPH-disrupted PPI at 120-ms intervals, AMPH-enhanced PPI at 30-ms intervals, and startle magnitude (male vs. female effect sizes, 0.40, 0.65, and 0.97, respectively). In humans, G/G variants of the rs135745 single-nucleotide polymorphism (SNP) of the CSNK1E gene are associated with reduced subjective response to d-AMPH (Veenstra-WanderWeele et al. 2006). We are presently testing the association of CSNK1E with subjective, neurocognitive, and PPI-altering effects of d-AMPH in humans.
grid2
Glutamate receptor delta-2 is an ionotropic glutamate receptor thought to be expressed in mice predominantly in cerebellar Purkinje cells (Doughty et al. 2000) but also detected elsewhere, including pineal glial cells (Yatsushiro et al. 2000), and its binding proteins are widely distributed in brain and peripheral tissues (Ly et al. 2002). Functionally, grid2 is thought to regulate apoptosis (Doughty et al. 2000), AMPA receptor function (Kohda et al. 2003), and potentially, hippocampal activity (Takatsuki et al. 2005), and d-serine is reported to act via glutamate delta-2 receptors to regulate long-term depression and motor coordination in mice (Kakegawa et al. 2011). In humans, GRID2 is significantly associated with performance on a number of schizophrenia-linked candidate endophenotypes, including PPI, event-related potential sensory gating, and neurocognitive measures; these associations were confirmed in bootstrap total significance test analyses (Greenwood et al. 2011) and replicated twice in separate samples of patients of European and African ancestry (Greenwood et al. 2012). In fact, in our studies, GRID2 displayed extensive pleiotropy, with significant associations to six endophenotypic measures (including PPI) in addition to the schizophrenia diagnosis.
While the distribution of delta-2 glutamate receptors in rat brain has not been studied extensively, at least moderate levels of grid2 expression in the rat NAC were detected both in our previous study (Shilling et al. 2008) and in the present sample. PPI is disrupted by NAC AMPA receptor activation, and blockade of NAC AMPA receptors prevents AMPH-induced PPI deficits (Wan and Swerdlow 1996). Clearly, we cannot discern levels of regional AMPA activity based on levels of grid2 expression; however, one might hypothesize that relatively reduced PPI AMPH sensitivity in LE vs. SD rats—and even downstream differences in NAC DA signaling (Qu et al. 2009) —reflects differences in NAC AMPA activity that are related, directly or indirectly, to greater NAC grid2 expression in LE rats.
nrg1
Expression levels of nrg1 in the NAC correlated significantly (negatively) with 120 ms PPI AMPH sensitivity, while levels of nrg1 in the VH correlated significantly with AMPH sensitivity for both 30 ms PPI (negatively) and startle magnitude (positively). The potential relationships between nrg1 expression in the NAC and VH to these two PPI phenotypes are particularly interesting, given the significant association of neuregulin and related genes with both schizophrenia and PPI (Stefansson et al. 2002; Greenwood et al. 2011, 2012). A recent report described reduced PPI in female, but not male, NRG1 hypomorphic Fisher rats at unspecified prepulse intervals (Taylor et al. 2011), suggesting sex-specific effects of type II nrg1 expression on basal PPI levels in rats.
comt
The present findings with comt expression in rats do not “translate” neatly to findings in humans. For example, averaging across all brain regions and prepulse intervals, we found a significant—albeit modest (p<0.035)—positive correlation between post-vehicle PPI levels and comt expression in the full sample of SD and LE rats while, in humans, an apparent negative relationship exists between PPI levels and COMT activity (Roussos et al. 2008). Possible explanations for this cross-species difference are discussed in the Electronic supplementary materials. However, these findings highlight a larger issue underlying this study: what relationship, if any, might patterns of gene expression in rat brain have to gene association findings in humans? Evidence that a particular phenotype is associated with higher or lower levels of comt (or other gene) expression does not necessarily help us understand an association of that phenotype with the COMT gene or a COMT SNP. Higher levels of gene expression are suggestive of higher levels of protein expression and activity; we are presently confirming this relationship with comt in SD and LE rats (studies in progress), but our past studies demonstrated not only higher NAC comt expression in LE vs. SD rats, but also higher levels of basal NAC and mPFC DA turnover in LE vs. SD rats (Swerdlow et al. 2005) (despite the posited limited role of comt in NAC DA metabolism). Similarly, particular SNPs (e.g., COMT Val158Met) are also associated with higher levels of protein activity.
Thus, with specific genes—comt being one of them—a particular phenotype resulting from a change in protein expression or activity might be generated by either one of two putatively independent processes: (1) an event that alters levels of gene expression, or (2) the presence of a particular SNP. Of course, the first of these two events might also include the presence of a particular SNP within a promoter sequence, thereby altering the regulation of gene expression. Interplay between specific SNPs and mRNA expression levels have been previously reported (e.g., Kao et al. 2010). Li et al. (2010) described brain region-specific differences in comt expression across inbred mouse strains that was associated with DA-linked neurochemical and behavioral phenotypes—some of which were sex-specific—reflecting the presence or absence of a single SINE element. Mice lacking this element produced mRNA with a long 3’ UTR and had lower levels of comt coding exons in HPC but not NAC or PFC, while its presence resulted in a premature polyadenylation site and the generation of a short isoform and higher HPC levels of comt coding exons. SD vs. LE differences in comt expression in the present study were not brain region-specific, nor was the “downstream” phenotype sex-specific; nonetheless, it is conceivable that the observed strain differences in comt expression might reflect downstream variants analogous to those observed by Li et al. (2010). Perhaps more importantly, our observation that comt expression was highly intercorrelated with the expression of the three other genes in this study is consistent with the known influence of this enzyme across multiple transmitter systems and on the expression of genes involved in a wide range of neurobiological processes (Li et al. 2010).
A more general issue to consider, as it relates to the current data, is what relationship, if any, exists between greater DA-mediated PPI “disruptability” in rats and reduced PPI in schizophrenia patients. The disruption of PPI by DA agonists is not an isomorphic or homologous “model” of schizophrenia; to the degree that this disruption is prevented by antipsychotics, it appears to be a predictive model for antipsychotic potency in schizophrenia (r=0.99; Swerdlow et al. 1994). Nonetheless, one might reasonably speculate (and there is some evidence to support this (Swerdlow et al. 1995a; Hutchison and Swift 1999; Hutchison et al. 1999; Bitsios et al. 2005, 2006; Golimbet et al. 2007; Talledo et al. 2009)) that PPI in humans fluctuates with levels of forebrain DA activity, and furthermore, that the degree to which increased forebrain DA activity disrupts PPI in any individual might: (1) be regulated by genetically programmed forebrain circuit functions, and (2) be a risk factor for the sustained loss of PPI under converging influences of stress and/or other “risk” genes. In this way, the molecular basis for PPI disruptability in response to exogenously stimulated DA activity in rats might “model” the molecular basis for vulnerability to an endogenous DA-mediated reduction in PPI in humans. To the degree that such vulnerability might contribute to the risk of developing psychopathology, we would predict that the mechanisms responsible for this risk might be tested using the present model.
In summary, rat strains differing in their sensitivity to the PPI-disruptive effects of DA agonists and in DA signaling mechanisms that regulate this sensitivity also differ in the expression levels of four PPI- and schizophrenia-linked genes, within three PPI- and schizophrenia-linked brain regions. Within each brain region, expression of the four genes was highly correlated. PPI AMPH sensitivity correlated significantly with levels of nrg1 expression in the NAC and VH. Structural equation modeling suggests that the latent factor of the NAC was significantly (and negatively) associated with PPI AMPH sensitivity at 120-ms intervals.
Supplementary Material
Acknowledgments
Expert technical assistance was provided by Ms. Samantha Hines and Mr. Steven Pham. Assistance in manuscript preparation was provided by Ms. Maria Bongiovanni. Gene expression measures were performed with the support of the Genomics Core at the UC San Diego Center for AIDS Research (AI36214), the VA San Diego Healthcare System, and the Veterans Medical Research Foundation. Supported by NIH grants: MH068366, MH059803, MH065571, and MH042228
Drs. Swerdlow's and Light's work has been funded by the NIH/NIMH. Dr. Swerdlow has been a paid consultant for participation in a Scientific Advisory Board for Neurocrine, Inc., for work unrelated to this project. Dr. Light has received compensation as a paid consultant for participation in a Scientific Advisory Board for Astellas for work unrelated to this project.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-012-2758-1) contains supplementary material, which is available to authorized users.
Conflicts of interest Drs. Shilling, Trim, and Saint Marie and Ms. Breier declare no potential conflict of interest.
Contributor Information
Neal R. Swerdlow, Department of Psychiatry, School of Medicine, University of California San Diego, La Jolla, CA 92093-0804, USA
Paul D. Shilling, Department of Psychiatry, School of Medicine, University of California San Diego, La Jolla, CA 92093-0804, USA
Michelle Breier, Department of Psychiatry, School of Medicine, University of California San Diego, La Jolla, CA 92093-0804, USA.
Ryan S. Trim, Department of Psychiatry, School of Medicine, University of California San Diego, La Jolla, CA 92093-0804, USA
Gregory A. Light, Department of Psychiatry, School of Medicine, University of California San Diego, La Jolla, CA 92093-0804, USA VA San Diego Healthcare System, San Diego, CA, USA; VISN 22, Mental Illness Research, Education and Clinical Center (MIRECC), San Diego, CA, USA.
Richard Saint Marie, Department of Psychiatry, School of Medicine, University of California San Diego, La Jolla, CA 92093-0804, USA.
References
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neurosci Lett. 2003;353:45–48. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Frangou S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. J Psychopharmacol. 2005;182:144–152. doi: 10.1007/s00213-005-0056-x. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Theou K, Frangou S. Increased prepulse inhibition of the acoustic startle response is associated with better strategy formation and execution times in healthy males. Neuropsychologia. 2006;44:2494–2499. doi: 10.1016/j.neuropsychologia.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. Wiley; New Jersey: 1989. [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Parker CC, Zhou L, Olker C, Chandrasekaran RY, Wager TT, Bolivar VJ, Loudon AS, Vitaterna MH, Turek FW, Palmer AA. Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids. Neuropsychopharmacol. 2011;37:1026–1035. doi: 10.1038/npp.2011.287. doi:10.1038/npp.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JK, Virshup DM. Casein kinase 1: complexity in the family. Int J Biochem Cell Biol. 2010;43:465–469. doi: 10.1016/j.biocel.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, Kleinman JE. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nat. 2011;478:519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty ML, De Jager PL, Korsmeyer SJ, Heintz N. Neurodegeneration in Lurcher mice occurs via multiple cell death pathways. J Neurosci. 2000;20:3687–3694. doi: 10.1523/JNEUROSCI.20-10-03687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Golimbet VE, Alfimova MV, Gritsenko IK, Ebstein RP. Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci Behav Physiol. 2007;37:601–606. doi: 10.1007/s11055-007-0058-8. [DOI] [PubMed] [Google Scholar]
- Graham F. The more or less startling effects of weak prestimuli. Psychophysiol. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and twelve endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS Genet. 2012;7:e1002134. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- Hutchison KE, Swift R. Effect of d-amphetamine on prepulse inhibition of the startle reflex in humans. Psychopharmacol. 1999;143:394–400. doi: 10.1007/s002130050964. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, Emi K, Motohashi J, Konno R, Zaitsu K, Yuzaki M. d-Serine regulates cerebellar LTD and motor coordination through the δ2 glutamate receptor. Nat Neurosci. 2011;14:603–611. doi: 10.1038/nn.2791. [DOI] [PubMed] [Google Scholar]
- Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, Law AJ. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc Natl Acad Sci U S A. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K, Kamiya Y, Matsuda S, Kato K, Umemori H, Yuzaki M. Heteromer formation of delta2 glutamate receptors with AMPA or kainate receptors. Brain Res Mol Brain Res. 2003;110:27–37. doi: 10.1016/s0169-328x(02)00561-2. [DOI] [PubMed] [Google Scholar]
- Li Z, Mulligan MK, Wang X, Miles MF, Lu L, Williams RW. A transposon in Comt generates mRNA variants and causes widespread expression and behavioral differences among mice. PLoS One. 2010;5:e12181. doi: 10.1371/journal.pone.0012181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly CD, Roche KW, Lee HK, Wenthold RJ. Identification of rat EMAP, a delta-glutamate receptor binding protein. Biochem Biophys Res Commun. 2002;291:85–90. doi: 10.1006/bbrc.2002.6413. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Sixth Edition Author; Los Angeles: 1998-2010. [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Qu Y, Saint Marie RL, Breier MR, Ko D, Stouffer D, Parsons LH, Swerdlow NR. Neural basis for a heritable phenotype: Differences in the effects of apomorphine on startle gating and ventral pallidal GABA efflux in male Sprague Dawley and Long Evans rats. Psychopharmacol. 2009;207:271–280. doi: 10.1007/s00213-009-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Mössner R, Maier W, Kühn KU. Sensorimotor gating of schizophrenia patients depends on catechol O-methyltransferase Val158Met polymorphism. Schizophr Bull. 2010;36:341–346. doi: 10.1093/schbul/sbn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychol Med. 2008;38:1651–1658. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Saint Marie RL, Shoemaker JM, Swerdlow NR. Strain differences in the gating-disruptive effects of apomorphine: relationship to gene expression in nucleus accumbens signaling pathways. Biol Psychiatry. 2008;63:748–758. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartson H, Geyer MA, Braff DL. Men are more inhibited than women by weak pre-pulses. Biol Psychiatry. 1993;34:253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA, Braff DL. “Normal” personality correlates of sensorimotor, cognitive and visuospatial gating. Biol Psychiatry. 1995a;37:286–299. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. J Neurol Neurosurg Psychiatry. 1995b;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001a;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Platten A, Kim YK, Gaudet I, Shoemaker J, Pitcher L, Auerbach P. Sensitivity to the dopaminergic regulation of prepulse inhibition in rats: evidence for genetic, but not environmental determinants. Pharmacol Biochem Behav. 2001b;70:219–226. doi: 10.1016/s0091-3057(01)00598-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Pitcher L, Platten A, Kuczenski P, Eleey CC, Auerbach P. Genetic differences in startle gating-disruptive effects of apomorphine: evidence for central mediation. Behav Neurosci. 2002;116:682–690. doi: 10.1037//0735-7044.116.4.682. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Platten A, Pitcher L, Goins J, Crain S. Heritable differences in the effects of amphetamine but not DOI on startle gating in albino and hooded outbred rat strains. Pharmacol Biochem Behav. 2003a;75:191–197. doi: 10.1016/s0091-3057(03)00078-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP. Amphetamine effects on prepulse inhibition across species: replication and parametric extension. Neuropsychopharmacol. 2003b;28:640–650. doi: 10.1038/sj.npp.1300086. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Auerbach PP, Pitcher L, Goins J, Platten A. Heritable differences in the dopaminergic regulation of sensorimotor gating. II. Temporal, pharmacologic and generational analyses of apomorphine effects on prepulse inhibition. Psychopharmacol. 2004a;174:452–462. doi: 10.1007/s00213-003-1480-4. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacol Biochem Behav. 2004b;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Platten A, Pitcher L, Goins J, Auerbach PP. Heritable differences in the dopaminergic regulation of sensorimotor gating. I. Apomorphine effects on startle gating in albino and hooded outbred rat strains and their F1 and N2 progeny. Psychopharmacol. 2004c;174:441–451. doi: 10.1007/s00213-003-1481-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Kuczenski R, Goins JC, Crain SK, Ma LT, Bongiovanni MJ, Shoemaker JM. Neurochemical analysis of rat strain differences in the startle gating-disruptive effects of dopamine agonists. Pharmacol Biochem Behav. 2005;80:203–211. doi: 10.1016/j.pbb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Krupin AS, Bongiovanni MJ, Shoemaker JM, Goins JC, Hammer RP., Jr Heritable differences in the dopaminergic regulation of behavior in rats: relationship to D2-like receptor G-protein function. Neuropsychopharmacol. 2006;31:721–729. doi: 10.1038/sj.npp.1300877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Bongiovanni MJ, Neary AC, Tochen LS, Saint Marie RL. Strain differences in the disruption of prepulse inhibition of startle after systemic and intra-accumbens amphetamine administration. Pharmacol Biochem Behav. 2007;87:1–10. doi: 10.1016/j.pbb.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacol. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Breier MR, Saint Marie RL. Probing the molecular basis for an inherited sensitivity to the startle-gating disruptive effects of apomorphine in rats. Psychopharmacol. 2011;216:401–410. doi: 10.1007/s00213-011-2228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki K, Kawahara S, Mishina M, Kirino Y. Characterization of hippocampal theta rhythm in wild-type mice and glutamate receptor subunit delta2 mutant mice during eyeblink conditioning with a short trace interval. Brain Res. 2005;1063:159–167. doi: 10.1016/j.brainres.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Talledo J, Sutherland Owens A, Schortinghuis T, Swerdlow NR. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacol. 2009;204:165–175. doi: 10.1007/s00213-008-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Markham JA, Taylor AR, Kanaskie BZ, Koenig JI. Sex-specific neuroendocrine and behavioral phenotypes in hypomorphic type II neuregulin 1 rats. Behav Brain Res. 2011;224:223–232. doi: 10.1016/j.bbr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH, Jr, de Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to d-amphetamine. Neuropsychopharmacol. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine–glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res. 1996;722:168–176. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]
- Yatsushiro S, Hayashi M, Morita M, Yamamoto A, Moriyama Y. Glutamate receptor subunit delta2 is highly expressed in a novel population of glial-like cells in rat pineal glands in culture. J Neurochem. 2000;75:1115–1122. doi: 10.1046/j.1471-4159.2000.0751115.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.