Abstract
Rationale: The 6-minute-walk distance (6MWD) test predicts mortality in chronic obstructive pulmonary disease (COPD). Whether variability in study type (observational vs. interventional) or region performed limits use of the test as a stratification tool or outcome measure for therapeutic trials is unclear.
Objectives: To analyze the original data from several large observational studies and from randomized clinical trials with bronchodilators to support the qualification of the 6MWD test as a drug development tool in COPD.
Methods: Original data from 14,497 patients with COPD from six observational (n = 9,641) and five interventional (n = 4,856) studies larger than 100 patients and longer than 6 months in duration were included. The geographical, anthropometrics, FEV1, dyspnea, comorbidities, and health status scores were measured. Associations between 6MWD and mortality, hospitalizations, and exacerbations adjusted by study type, age, and sex were evaluated. Thresholds for outcome prediction were calculated using receiver operating curves. The change in 6MWD after inhaled bronchodilator treatment and surgical lung volume reduction were analyzed to evaluate the responsiveness of the test as an outcome measure.
Measurements and Main Results: The 6MWD was significantly lower in nonsurvivors, those hospitalized, or who exacerbated compared with those without events at 6, 12, and greater than 12 months. At these time points, the 6MWD receiver operating characteristic curve–area under the curve to predict mortality was 0.71, 0.70, and 0.68 and for hospitalizations was 0.61, 0.60, and 0.59, respectively. After treatment, the 6MWD was not different between placebo and bronchodilators but increased after surgical lung volume reduction compared with medical therapy. Variation across study types (observational or therapeutic) or regions did not confound the ability of 6MWD to predict outcome.
Conclusions: The 6MWD test can be used to stratify patients with COPD for clinical trials and interventions aimed at modifying exacerbations, hospitalizations, or death.
Keywords: chronic obstructive pulmonary disease, 6-minute-walk distance, outcomes
At a Glance Commentary
Scientific Knowledge on the Subject
The 6-minute-walk distance (6MWD) test is used in the evaluation of patients with chronic obstructive pulmonary disease. However, its practical value in trials has been questioned because of potential variability in how the test is performed and possible lack of reproducibility of results when patients with chronic obstructive pulmonary disease are tested under different conditions (observational vs. interventional studies). Whether the test can help stratify patients for interventions and its responsiveness to pharmacologic agents remains poorly studied.
What This Study Adds to the Field
This analysis of a large number of patients with chronic obstructive pulmonary disease recruited into observational and interventional studies shows that the 6MWD test can be conducted in different settings with similar accuracy and outcomes. The 6MWD test can be used to stratify patients to be included in studies aimed at modifying those outcomes. The 6MWD is a responsive outcome measure for lung volume reduction but not inhaled bronchodilators.
Chronic obstructive pulmonary disease (COPD) is a leading cause of disability and mortality in the world (1). Identification of biomarkers and clinical measures that can determine the degree of overall impairment, help prognosticate outcome, and reflect disease progression or regression in COPD is highly desirable. The regulatory qualification of drug development tools to help in the stratification of patients for potential inclusion in clinical trials is a major goal of the COPD biomarker qualification consortium (CBQC), which achieved its first successful qualification of plasma fibrinogen in 2015 (2).
In the general population, exercise capacity is an excellent predictor of the risk for all-cause mortality (3, 4) and therefore fulfills the definition of a biomarker (5). Determination of exercise capacity is even more informative in noncommunicable chronic diseases (6–8), including COPD (9–11). The capacity to exercise is usually determined either in a laboratory using a formal cardiopulmonary test (12) or in the field using simpler tests, including the distance walked over time. The 6-minute-walk distance (6MWD) has proven useful in assessing the functional status of patients with COPD (13–20) because it is easy to perform, inexpensive, and amenable to standardization (21). Several reference equations have been developed for healthy adults to determine normal values for this test (22, 23). However, the distance walked in meters has similar capacity to predict mortality as the values corrected by using reference equations (13, 19, 24), so the 6MWD is usually reported in meters.
Two recent American Thoracic Society (ATS) and European Respiratory Society (ERS) reviews on exercise testing support the concept that for patients with COPD, the 6MWD is a potentially useful biomarker because it is associated with poor health-related quality of life (19, 25), impaired lung function (19, 26), decreased activity (27), and survival (9, 13, 19, 24) and may respond to certain interventions (19, 20). In addition, the impaired functional status affecting patients with COPD is likely multifactorial: influenced not only by the severity of lung compromise, but also by extrapulmonary manifestations, such as muscle weakness, pulmonary vascular disease, comorbidities, and depression (28). An improved 6MWD has been reported following pulmonary rehabilitation (29–31), lung volume reduction surgery (LVRS), and lung transplantation (7). Finally, several studies have validated a minimal clinically importance distance of around 30 m in COPD and other conditions (8, 13, 20, 24), so a response of this magnitude could serve as an improvement in outcome. Yet, the responsiveness of 6MWD to pharmacologic interventions in COPD remains poorly studied and the value of the 6MWD as an outcome in clinical trials has been questioned because of potential variability in its results (32).
This study analyzed the original data from several large observational studies and from randomized clinical trials with bronchodilators to support the qualification of the 6-minute-walk test as a drug development tool in COPD. We tested the following hypotheses. First, that the 6MWD is a valid biomarker to stratify patients for risk of death, hospitalizations, and exacerbations in a wide population of patients with COPD including those participating in observational and clinical therapeutic trials in different settings. Second, there are simple threshold values of distance walked that could help enrich studies aiming to test interventions effective for outcomes, such as survival or hospitalizations for COPD exacerbations. Third, the change in 6MWD is a potential patient-centered outcome measure for therapies aimed at improving functional capacity in COPD.
Methods
All available studies that included more than 100 subjects and a minimum follow-up period of 6 months were included in the analysis. To ensure availability of original data and inclusion of data generated following the approval of current classes of bronchodilators, studies completed between 1991 and 2012 were selected. Every effort was made to retrieve the original data from all published trials available in the literature, but in some cases, either the authors were not able to provide it or the authors were no longer active. Therefore, the analysis integrated previously collected data from five prospective, randomized, controlled clinical trials (AZD589960003, BI205.247, GSK207499, Novartis QAB146832334, and QAB1491323) and six observational studies (13, 26, 33–36) of patients with COPD where the 6MWD was included as part of the study protocol (see Table E1 in the online supplement for details).
In all subjects, the original anthropometrics, regional residence, sex, spirometry, and comorbidity were retrieved. All-cause mortality was documented in all studies reviewed. Hospitalizations were documented as being due to COPD, whereas exacerbations included those requiring a change in medication in the clinical trials or a documentation of exacerbations in the observational studies. The data obtained from the original source were organized into those corresponding to observational trials and those belonging to intervention with bronchodilators. Because of the similarity in the clinical and physiologic characteristics in both groups, a single dataset was generated to evaluate the relationship between 6MWD and outcomes. Analyses were not conducted on individual studies, unless otherwise noted (e.g., National Emphysema Treatment Trial [NETT], which examined the effect of LVRS). The data integration was conducted by INC Research (Bethesda, MD) and statistical analyses were completed by Evidera (Bethesda, MD), independent contract firms with expertise in clinical trials data management and outcomes research. All studies included were reviewed by institutional review boards and all patients had to sign informed consent forms.
Test Performance
Details of the test performance for each of the trials are provided in Table E1. In brief, the 6MWD was performed in all studies under the direction of a trained technician. Most studies (8 of 11) followed the ATS guidelines for the 6MWD. All of the observational studies except the ECLIPSE (Evaluation of COPD Longitudinally to Predict Significant Endpoints) study included two walks and the longer one was used for the analyses. ECLIPSE only performed one single 6MWD test. The value recorded in the pharmacologic or LVRS trial original record was used for those trials. Oxygen was supplemented for patients who developed hypoxemia in the observational trials, but no patient on long-term oxygen therapy was included in any of the clinical trials.
Data Management
The contracted company Evidera reconciled all variables from each study so that they could be analyzed as a single dataset. This involved review of each variable in each study in the context of how the variable was operationalized, and then manipulation of the variable so that studies could subsequently be analyzed together in the full database. Evidera created an iterative quality control process applied to each dataset individually that included the following steps: logic checks to ensure that these key variables were within the acceptable range, and that the values appeared reasonable based on knowledge of the COPD population and the nature of each study. Values in these descriptive tables were compared with those from a primary publication and any discrepancies were resolved. Individual study sponsors and authors were contacted to review the programmed descriptive tables to ensure the data were consistent with their expectations for each individual study, and to respond to any data-related queries associated with previously identified issues or discrepancies. All outstanding issues were resolved with assistance from the sponsors, the data integration company (INC Research), or CBQC Working Group members, as necessary.
Data Analysis
For this report the relationships between baseline 6MWD and demographic and clinical characteristics of the patients included were established. The strength of the association between 6MWD and the three outcomes of interest (all-cause mortality, COPD hospitalizations, and exacerbations) was determined. Because of its known ceiling effect, calculations were made to determine the 6MWD inflection threshold below which the test increased the power to identify a high-risk population for an outcome of interest and established the enriching power of different thresholds to predict the desired outcome. We also evaluated the effect of therapy on the change of 6MWD over time. For this analysis only clinical trials including a placebo arm and the NETT studies were used. Bronchodilator therapy included long-acting β-agonist and long-acting muscarinic antagonist (anticholinergic) bronchodilators or bronchodilator plus inhaled corticosteroid.
Power analyses were conducted to assess the effect of selected 6MWD thresholds of 250, 300, 350, 400, and 450 m on the sample size of COPD subjects who would need to be enrolled in a clinical trial assessing hospitalized COPD exacerbations or death.
Statistical methods
Analyses were not conducted separately for individual studies unless otherwise noted. Individual treatments were not recorded and analyzed, but rather treatment classes (i.e., placebo, bronchodilator therapy) were used as an analysis variable where appropriate. All statistical tests were two-sided and used a significance level of 0.05 unless otherwise noted. All descriptive analyses and regression models were considered exploratory, and for the more complicated longitudinal models, an iterative process was used to generate models and guide subsequent analyses. Thus, no adjustments were made to account for multiplicity. SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC) was used to conduct the analyses.
Results
Study Groups
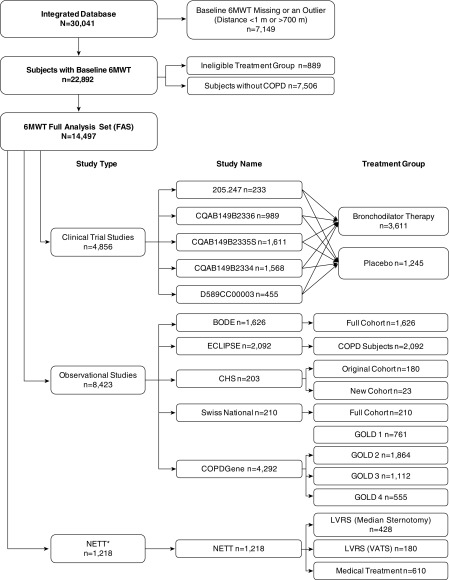
A flow diagram of the studies included in this analysis is shown in Figure 1 and the details of each study design and 6MWD test methodology are included in Table E1. From the initial pool of 30,041 potential subjects, a total of 14,497 met the study criteria; 9,641 belonged to the six observational studies, which followed patients for a period of 2–5 years; and 4,856 corresponded to patients enrolled in five interventional studies, which followed patients for 6–12 months. The NETT trial followed patients for up to 5 years.
Figure 1.
Flow diagram of the patients included in the analyses. For details on each of the studies, see Table E1. *NETT evaluated effectiveness of LVRS and will be analyzed separately in selected analyses. 6MWT = 6-minute-walk distance test; BODE = Body mass index, Obstruction, Dyspnea, and Exercise index; CHS = Cardiovascular Health Study; COPD = chronic obstructive pulmonary disease; ECLIPSE = Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; GOLD = Global Initiative for Chronic Obstructive Lung Disease; LVRS = lung volume reduction surgery; NETT = National Emphysema Therapy Trial; VATS = video-assisted thoracoscopic surgery.
Subject Characteristics
The baseline characteristics of all included patients and groupings according to the type of study are shown in Table 1. Patients recruited for clinical trials were similar in all clinical characteristics (including the 6MWD) to those in observational studies. Subjects were on average 64 years of age and close to 70% were men; more than 3,500 of the subjects (24%) included were women. Most subjects had never reported an exacerbation in the year before enrollment. Because of the nature of the trial, patients included in the NETT were older, had a narrower distribution of COPD severity, all had emphysema, and all came from the United States. The distribution of the 6MWD at baseline for observational and pharmacologic trials is shown in Figure 2 and for each group of trials in Figure E1. The mean distance walked in 6 minutes was around 360 m in both groups and the distribution was very similar in the observational and therapeutic trials.
Table 1.
Baseline Anthropometrics, Clinical, and Physiologic Characteristics of the Patients Included in the Analysis
| Total (n = 14,497) | Observational (n = 8,423) | Pharmacologic (n = 4,856) | NETT (n = 1,218) | |
|---|---|---|---|---|
| Age, yr, mean (SD)* | 64 (9) | 64 (9) | 64 (9) | 67 (5) |
| Sex, females, n (%) | 4,867 (34) | 2,979 (35) | 1,416 (29) | 472 (39) |
| Race, nonwhite, n (%)* | 1,854 (13) | 1,070 (13) | 722 (15) | 62 (5) |
| Region of the world, n (%) | ||||
| Americas | 9,066 (62.5) | 6,157 (73.1) | 1,691 (34.8) | 1,218 (100.0) |
| European | 4,933 (34.0) | 2,230 (26.5) | 2,703 (55.7) | 0 (0.0) |
| Southeast Asia | 254 (1.8) | 0 (0.0) | 254 (5.2) | 0 (0.0) |
| Western Pacific | 165 (1.1) | 36 (0.4) | 129 (2.7) | 0 (0.0) |
| African | 79 (0.5) | 0 (0.0) | 79 (1.6) | 0 (0.0) |
| GOLD COPD spirometric severity, n (%)* | ||||
| 0 | 12 (0.1) | 12 (0.1) | 0 (0.0) | 0 (0.0) |
| 1 | 1,164 (8.2) | 1,051 (12.9) | 113 (2.4) | 0 (0.0) |
| 2 | 5,618 (39.7) | 3,204 (39.4) | 2,411 (50.3) | 3 (0.2) |
| 3 | 5,200 (36.7) | 2,714 (33.3) | 2,084 (43.5) | 402 (33.0) |
| 4 | 2,158 (15.2) | 1,161 (14.3) | 184 (3.8) | 813 (66.7) |
| Missing/unclassified | 345 (2.4) | 281 (3.3) | 64 (1.3) | 0 (0.0) |
| History of exacerbations in previous 12 mo, n (%)* | ||||
| 0 | 7,851 (71.3) | 3,937 (61.7) | 3,914 (84.7) | — |
| 1 | 1,819 (16.5) | 1,328 (20.8) | 491 (10.6) | — |
| 2 | 709 (6.4) | 605 (9.5) | 104 (2.2) | — |
| 3 or more | 628 (5.7) | 514 (8.15) | 114 (2.5) | — |
| Missing | 3,490 (24.1) | 2,039 (24.2) | 233 (4.8) | 1,218 (100) |
| Comorbidities, yes, n (%) | ||||
| Hypertension | 5,833 (45.8) | 3,872 (47.7) | 1,961 (42.4) | — |
| Cardiovascular | 2,483 (30.0) | 2,483 (30.0) | — | — |
| Diabetes | 1,469 (12.7) | 1,136 (13.6) | 333 (10.5) | — |
| 6MWD, m, mean (SD) | 366.5 (119.59) | 369.9 (123.64) | 360.1 (117.64) | 368.4 (95.45) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; NETT = National Emphysema Therapy Trial.
Some values were statistically significantly different but of limited clinical importance.
There were statistical differences among all groups (P < 0.05).
Figure 2.
The distribution of baseline 6-minute-walk distance (6MWD) was similar in the observational and interventional studies as shown in A and B, respectively. (A) Histogram of the 6MWD distribution in observational studies (n = 8,423). (B) Histogram of the 6MWD distribution in interventional studies (n = 4,856).
Association between 6MWD and Outcomes
The 6MWD was shorter in patients who died during the follow-up period whether assessed over a follow-up interval of 6 months, 12 months, or more than a year as shown in Table 2. The same was true for hospitalizations and exacerbations. The 6MWD was shorter in older patients, it was also lower in women than men and varied with ethnicity as can be seen in Table E4. As the 6MWD decreases there is a significant increase in the relative proportion of subjects who died (Figure 3). Below the threshold of 6MWD of 350 m, more than half of the subjects at risk died over a 12-month period, whereas at or above that threshold, more than half of those at risk survived.
Table 2.
Baseline 6MWD by Endpoint Status for Acute Health Events in the 6-, 12-, and >12-Month Datasets
| 6MWD (m) |
|||||
|---|---|---|---|---|---|
| N | Mean (SD) | Median | Q1, Q3 | Min–Max | |
| Baseline to 6 mo | |||||
| Death | |||||
| Survivor | 10,045 | 364.4 (117.68) | 370.0 | 290.0, 443.0 | 1.5–700.0 |
| Nonsurvivor | 160 | 289.3 (121.62) | 309.4 | 195.0, 366.5 | 24.0–581.6 |
| COPD hospitalization | |||||
| No hospitalization for COPD exacerbation | 6,644 | 363.5 (116.85) | 366.0 | 290.0, 440.0 | 1.5–700.0 |
| Had ≥1 hospitalization for COPD exacerbation | 304 | 320.2 (114.00) | 315.0 | 240.0, 401.4 | 30.0–628.0 |
| COPD exacerbation | |||||
| No COPD exacerbation | 5,381 | 366.7 (117.82) | 370.0 | 296.0, 446.0 | 1.5–700.0 |
| Had ≥1 COPD exacerbation | 1,567 | 344.3 (112.72) | 355.0 | 269.0, 420.0 | 20.0–700.0 |
| Baseline to 12 mo | |||||
| Death | |||||
| Survivor | 7,037 | 368.7 (115.41) | 375.0 | 300.0, 447.4 | 6.1–700.0 |
| Nonsurvivor | 335 | 299.5 (123.55) | 305.0 | 212.1, 380.0 | 1.5–690.0 |
| COPD hospitalization | |||||
| No hospitalization for COPD exacerbation | 3,689 | 368.7 (113.88) | 375.0 | 300.0, 445.0 | 1.5–700.0 |
| Had ≥1 hospitalization for COPD exacerbation | 426 | 331.1 (107.18) | 332.0 | 255.0, 405.0 | 60.0–628.0 |
| COPD exacerbation | |||||
| No COPD exacerbation | 2,377 | 374.7 (114.40) | 380.0 | 300.0, 450.0 | 1.5–700.0 |
| Had ≥1 COPD exacerbation | 1,738 | 351.3 (111.51) | 360.0 | 275.0, 426.0 | 20.0–700.0 |
| Baseline to >12 mo | |||||
| Death | |||||
| Survivor | 4,721 | 374.2 (114.69) | 379.5 | 302.0, 450.0 | 6.1–700.0 |
| Nonsurvivor | 628 | 304.4 (127.90) | 302.0 | 212.9, 395.0 | 1.5–690.0 |
| COPD hospitalization | |||||
| No hospitalization for COPD exacerbation | 1,591 | 376.2 (114.60) | 379.6 | 305.0, 450.0 | 1.5–700.0 |
| Had ≥1 hospitalization for COPD exacerbation | 501 | 330.6 (112.10) | 330.0 | 252.0, 410.0 | 20.0–628.0 |
| COPD exacerbation | |||||
| No COPD exacerbation | 697 | 377.8 (119.01) | 375.0 | 306.0, 450.0 | 1.5–700.0 |
| Had ≥1 COPD exacerbation | 1,395 | 359.0 (113.43) | 364.0 | 287.7, 433.0 | 20.0–690.0 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; COPD = chronic obstructive pulmonary disease; Max = maximum; Min = minimum; Q1 = first quartile; Q3 = third quartile.
For all outcomes, the differences between 6MWD were statistically significant (P < 0.001).
Figure 3.
Proportion of subjects who did (dark bars) or did not (light bars) have the outcome of interest for each category of 6-minute-walk distance (6MWD) at 12 months. Vertical axis: Proportion of total subjects who did or did not have the outcome of interest in each 6MWD category. (A–C) Distribution of baseline 6MWD by mortality (A), hospitalization (B), and exacerbation (C) at 12 months. (A) The proportion of subjects who died (light bars) was larger than that of survivors for shorter baseline 6MWDs. The same was true for hospitalizations (B) and exacerbations (C). Above the threshold of 350 m, the proportion of patients who had the outcome of interest was less than those who did not. The numbers of subjects are given inside the bars.
The association between 6MWD and outcomes was stronger for the risk of death or hospitalizations than with exacerbations (primarily those treated as an outpatient) at all time points as shown in Table 3 and for 12 months mortality, hospitalizations, and exacerbations in Figure E2. This was true for both genders. The strength of association remained statistically significant after the 12 months of follow-up.
Table 3.
Area Under the Curve from the Receiver Operating Characteristic Curve Analysis Based on the (Sensitivity and the 1 − Specificity of the) 6MWD for Assessing Outcomes in Patients with a 6-, 12-, or Longer than 12-Month Follow-up
| 6-Month Dataset* |
12-Month Dataset† |
>12-Month Dataset‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | |
| Mortality | 0.725 | 0.731 | 0.733 | 0.684 | 0.687 | 0.694 | 0.676 | 0.678 | 0.686 |
| Hospitalization | 0.610 | 0.610 | 0.595 | 0.598 | 0.599 | 0.598 | 0.594 | 0.581 | 0.681 |
| Exacerbations | 0.555 | 0.546 | 0.554 | 0.558 | 0.550 | 0.554 | 0.530 | 0.527 | 0.515 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; 6MWT = 6-minute-walk distance test; NETT = National Emphysema Therapy Trial.
All values were statistically significant.
Pooled 6-month dataset includes all 11 6MWT studies (five clinical trials, five observational studies, and NETT medical treatment arm) combined.
The 12-month dataset includes eight 6MWT studies (two clinical trials, five observational studies, and NETT medical treatment arm) combined.
The >12-month dataset includes six 6MWT studies (five observational studies and NETT medical treatment arm) combined.
Results of the power analysis show that the number of patients needed to be recruited to detect an effect on death or hospitalizations for COPD exacerbations decreases as the baseline distance walked is shorter (Table 4).
Table 4.
Estimated Sample Sizes by Baseline 6MWD Level Based on Hospitalized COPD Exacerbations or Death up to 12 Months of Follow-up
| 6MWD, m | Group Sample Size by Hazard Ratio for Hospitalization |
Group Sample Size by Hazard Ratio for Death |
||||
|---|---|---|---|---|---|---|
| 0.6 | 0.7 | 0.8 | 0.6 | 0.7 | 0.8 | |
| <250 | 318 | 612 | 1,478 | 500 | 952 | 2,288 |
| <300 | 348 | 666 | 1,606 | 618 | 1,176 | 2,820 |
| <350 | 386 | 738 | 1,780 | 718 | 1,364 | 3,268 |
| <400 | 428 | 818 | 1,968 | 980 | 1,860 | 4,450 |
| <450 | 474 | 904 | 2,174 | 1,066 | 2,022 | 4,836 |
| No threshold | 520 | 992 | 2,386 | 1,226 | 2,324 | 5,552 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; COPD = chronic obstructive pulmonary disease.
Effect of Therapy on the 6MWD
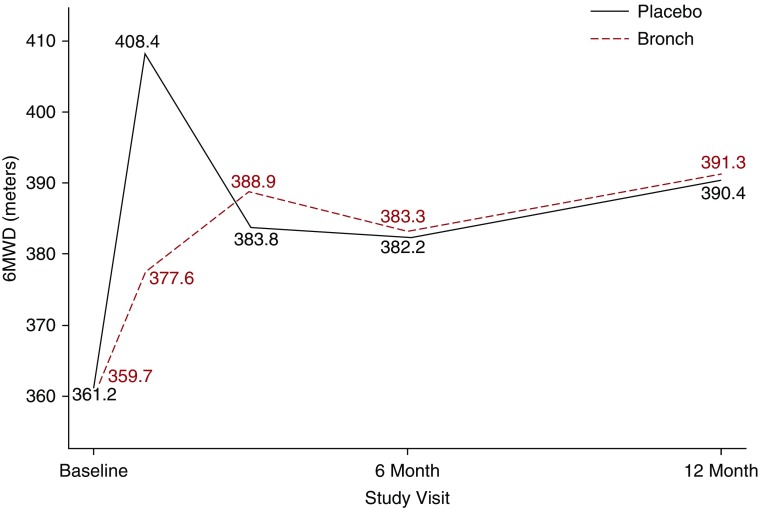
The analysis of the response to therapy differed depending on the intervention and is presented in Figure 4. In the inhaled bronchodilator interventions, neither placebo nor active agents had any benefit on the 6MWD at 6 months or 1 year. In contrast, as shown in Figure 5, LVRS resulted in a significant increase in the 6MWD that paralleled the increase in the FEV1 observed after LVRS.
Figure 4.
Response in the 6-minute-walk distance (6MWD) to pharmacologic therapy (bronchodilator [Bronch]) or placebo in the randomized controlled clinical trials. The change in the 6MWD in interventional studies after the administration of either placebo (solid dark line) or active treatment (dashed red line) was similar at 6 and 12 months, which were the end times for the trials included in this analysis.
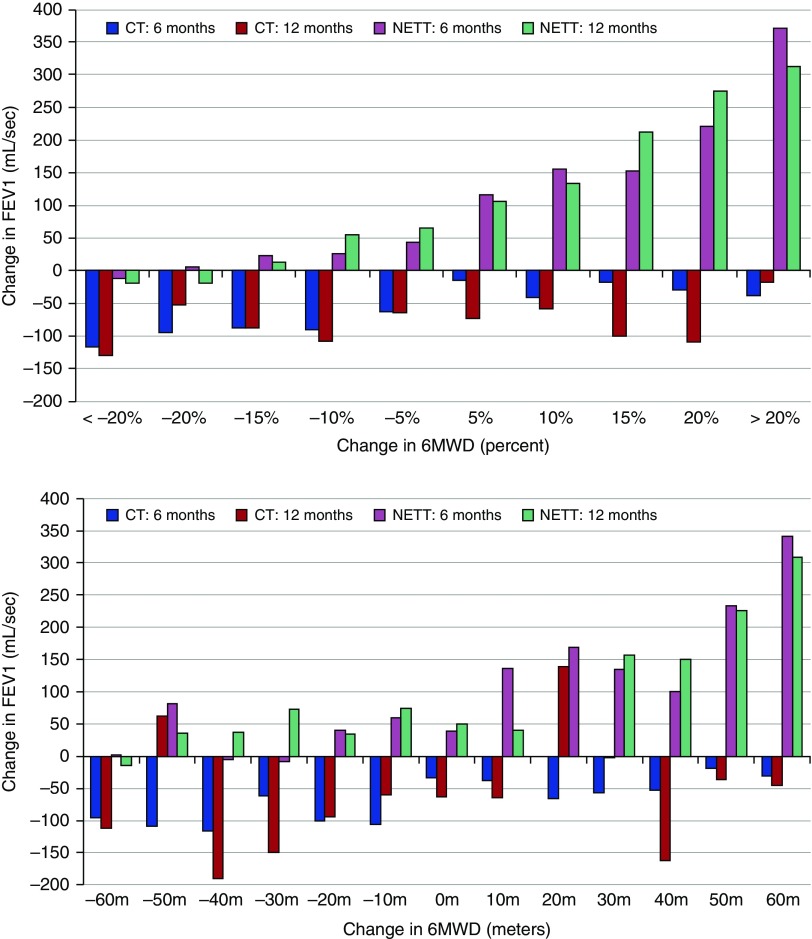
Figure 5.
Effect of controlled trials of inhaled pharmacotherapy (CT) and lung volume reduction (NETT) on the 6-minute-walk distance (6MWD) and FEV1. The 6MWD, expressed on the horizontal axis as percentage change from baseline (top) or as absolute distance in meters (bottom), increased proportionally to the improvement in FEV1 (vertical axis) in patients included in the National Emphysema Therapy Trial (NETT). The decrease in FEV1 shown in the lower portion of the graphs shows that overall, there was a decrease in lung function and there was no relation between change in 6MWD and lung function in the pharmacologic trials (CT).
Discussion
This study of 14,497 patients with COPD, comprising 9,641 belonging to six observational studies, which followed patients for a period of 2–5 years, and 4,856 patients enrolled in five interventional studies, which followed patients for 6–12 months, had the following findings. First, on average the 6MWD of patients recruited into studies was found to be similar independent of the nature of the study (observational or interventional clinical trial). Second, a 6MWD of 350 m selects a threshold below which there is a significant increase in the risk for the following outcomes: mortality, hospitalizations, and exacerbations. Third, the magnitude of the increase in 6MWD after LVRS is proportional to the improvement in lung function after the surgery, a finding that was not observed in the inhaled bronchodilator trials.
It has been assumed that the process of recruitment for pharmacologic intervention trials selects patients that do not represent patients at large and that they differ from those recruited in observational studies and also that the 6MWD has great variability, depending largely on where and how it is completed. A recent systematic review by the ATS/ERS working group on walking tests concluded that several methodologic factors affect the 6MWD (19). These included track length, encouragement, supplemental oxygen, and walking aids. In the trials reviewed by the CBQC, 8 of the 11 followed the ATS/ERS recommendations using corridors of 30 m. Three interventional trials used corridors of 10 to 30 m long and none allowed the use of walking aids.
The results from our analysis of more than 14,000 patients included in observational and clinical studies suggests otherwise. On average patients recruited into clinical trials or observational cohorts not only had similar age and sex, but also showed no important clinical differences in airflow obstruction severity, comorbidity, or in the median 6MWD and its distribution (Table 1 and Figure 2). In addition, the prevalence of three comorbidities that can affect the 6MWD (coronary artery disease, hypertension, and diabetes) was similar in both groups. Although the 6MWD was lower in women and differed by region, adjustment of the analysis by these variables failed to detect any difference in the findings. This similarity across populations provides confidence that the test is applicable in different conditions. The fact that most studies followed the ATS/ERS recommendations, used similar encouragement commands, and did not allow for the use of walking aids is in line with the findings from the ERS/ATS working group (19) and may have helped minimize differences between settings for the test.
The 6MWD proved useful to stratify patients with COPD for the risk of mortality and hospitalizations due to COPD in the overall cohort. Although significant, the receiver operating characteristic curve value for exacerbations in general (i.e., including those treated as an outpatient) is lower than that seen for hospitalizations and death, suggesting that it may not be as good a tool for routine exacerbations studies. The consistent decrease at all time points of the area under the curve moving from death through hospitalization to exacerbation (Table 3) has led us to generate the concept that 6MWD, and likely other markers of frailty, measures survivability and that the current definition of exacerbations using primarily symptoms is not tightly related to functional capacity. Although the receiver operating characteristic curve values were rather modest, they are close to those offered by accepted predictive biomarkers of cardiovascular mortality, such as cholesterol and high blood pressure (37). The systematic analysis that was applied to different thresholds of 6MWD included differential assessment at increments of 10 m between 300 and 400 m. Using this approach, a value of 350 m seems to provide that inflection point below which the risk of death and hospitalizations increases quasilinearly as the walking distance decreases (Figure 3). This was true independent of type of study, sex, and region as seen in Table E1. This finding is consistent with previous reports from the BODE (Body mass index, Obstruction, Dyspnea and Exercise study) (13, 38) and the ECLIPSE (24, 39) observational cohorts, although we caution that both of these were included in this analysis.
The current analysis expands on the findings of the ERS/ATS work force and the ERS task force on the use of exercise testing to evaluate interventional efficacy (19, 20). The first of the reports concentrated on the properties of different walking tests (including the 6MWD) in different respiratory conditions (including COPD, interstitial lung disease, pulmonary arterial hypertension, and other chronic respiratory diseases), but devoted a relatively small portion of their analysis to the quantification of the relationship between the 6MWD and the risk of death in COPD (19). Given the nature and extension of the review, even less was done on the relationship between 6MWD and hospitalizations and exacerbations, a topic that is at the center of this report. The ATS/ERS group could not perform a detailed metaanalysis and relied on the defined P value of 0.05 in each published study to determine an association between decreased 6MWD and increased risk of death or hospitalizations. The second report (20) comprehensively expands on the responsiveness of different laboratory and field exercise test to interventions but does not address the relationship between 6MWD and hospitalizations or mortality.
In the current study, we explored in depth those associations with the aim of helping enrich clinical trials. We observed that different risks provided by values of 6MWD less than 350 m can help enrich studies designed to improve outcomes that are of public health importance. This was tested in the cohort as a whole and shown in Table 4. The results indicate that using the 6MWD as a screening tool in COPD can help decrease the number of patients needed to assess a given outcome. However, the total number of subjects needed to assess clinically relevant improvements in survival over 12 months remains relatively high, because COPD is a slowly progressing disease with a relatively low yearly mortality rate. Smaller numbers would be required for studies of duration longer than 12 months.
The similar response to placebo and bronchodilator agents seen in the pooled analysis of the five pharmacologic trials supports the concept that the 6MWD is not a useful test to evaluate a treatment response to these agents, a finding that is in agreement with the recent ERS task force report (20). However, the excellent relationship between improvement in 6MWD and lung function improvement in the NETT trial (Figure 5) supports its value as an outcome measurement in other types of interventions, such as pulmonary rehabilitation, where improvements in 6MWD are consistently reported (10, 29, 40–47). Unfortunately, data from studies investigating the value of pulmonary rehabilitation were not available for this analysis but were part of the report from the ERS/ATS working group, that document a response of the 6MWD to pulmonary rehabilitation trials (19).
There were some limitations to this study. The data were analyzed retrospectively and three studies included only one walk test. However, the collection of the data was planned prospectively as a primary variable. Whether one or two walks were performed did not alter the results of the analyses. However, in interventions where the 6MWD is to be used as an outcome measurement, the difference of 26 m reported by the ATS/ERS working group is significant enough to support its inclusion in any intervention trial. A second limitation relates to the analysis of the data, which may not have followed the methodology proposed by some authors (48, 49). However, the primary data were retrieved and queried and conventional statistical techniques used and the results are not only logical, but clinically and biologically plausible. Finally, the patients included in this report may have participated in pulmonary rehabilitation before enrollment in the observational and NETT studies. No data were available that could help analyze the potential impact of rehabilitation on the results here presented. However, the value of the 6MWD to predict outcome remained significant in the NETT study, where rehabilitation was universal.
Conclusions
This analysis of patients with COPD recruited into observational and interventional studies shows that the 6MWD can be conducted in different settings with similar accuracy and predictive outcomes. A 6MWD less than 350 m progressively increases the risk for death and hospitalizations and can be used to stratify patients to be included in studies aimed at modifying those outcomes. The 6MWD is a responsive outcome measure for lung volume reduction but not inhaled bronchodilators. The data here reported do not permit speculation regarding its value as an outcome tool for pharmacologic therapies that might work through mechanisms other than bronchodilatation.
Footnotes
This study was funded by the COPD biomarker qualification consortium using funds contributed by four industry members (AstraZeneca, Boehringher Ingleheim, GlaxoSmithKline, and Novartis) and managed by the nonprofit COPD Foundation. The role of the funding source was to provide the funds needed for the analysis and for the meetings needed to complete the project. M.I.P.’s contribution to this manuscript was supported by the National Institute for Health Research (NIHR) Respiratory Biomedical Research Unit at the Royal Brompton and Harefield National Health Service Foundation Trust and Imperial College, London UK. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health. R.C. occupies the Grancell/Burns Chair in the Rehabilitative Sciences.
Author Contributions: Conception and design of the study, and analysis and interpretation: B.C., G.C., M.I.P., R.C., K.T., R.T.-S., F.S., and S.R. Drafting the manuscript for important intellectual content: B.C., G.C., R.C., K.T., R.T.-S., S.R., D.M., F.S., and A.K.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201508-1653OC on June 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.Miller BE, Tal-Singer R, Rennard SI, Furtwaengler A, Leidy N, Lowings M, Martin UJ, Martin TR, Merrill DD, Snyder J, et al. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease: perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am J Respir Crit Care Med. 2016;193:607–613. doi: 10.1164/rccm.201509-1722PP. [DOI] [PubMed] [Google Scholar]
- 3.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation. 1998;98:2836–2841. doi: 10.1161/01.cir.98.25.2836. [DOI] [PubMed] [Google Scholar]
- 5.Casaburi R, Celli B, Crapo J, Criner G, Croxton T, Gaw A, Jones P, Kline-Leidy N, Lomas DA, Merrill D, et al. The COPD biomarker qualification consortium (CBQC) COPD. 2013;10:367–377. doi: 10.3109/15412555.2012.752807. [DOI] [PubMed] [Google Scholar]
- 6.Arslan S, Erol MK, Gundogdu F, Sevimli S, Aksakal E, Senocak H, Alp N. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34:166–169. [PMC free article] [PubMed] [Google Scholar]
- 7.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–664. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 9.Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23:28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 10.Bowen JB, Votto JJ, Thrall RS, Haggerty MC, Stockdale-Woolley R, Bandyopadhyay T, ZuWallack RL. Functional status and survival following pulmonary rehabilitation. Chest. 2000;118:697–703. doi: 10.1378/chest.118.3.697. [DOI] [PubMed] [Google Scholar]
- 11.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167:544–549. doi: 10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 12.Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, Gosselink R, O’Donnell DE, Puente-Maestu L, Schols AM, Singh S, et al. ERS Task Force. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29:185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 13.Cote CG, Casanova C, Marín JM, Lopez MV, Pinto-Plata V, de Oca MM, Dordelly LJ, Nekach H, Celli BR. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31:571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 14.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32:637–643. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 15.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Townsend M, Keller J, Singer J, Nogradi S. Measuring functional status in chronic lung disease: conclusions from a randomized control trial. Respir Med. 1989;83:293–297. doi: 10.1016/s0954-6111(89)80199-4. [DOI] [PubMed] [Google Scholar]
- 17.Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, Thistlethwaite PA, Tuder RM, Erzurum SC, Geraci MW, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:1153–1160. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 19.Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 20.Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, Neder JA, O’Donnell DE, Onorati P, Porszasz J, Rabinovich R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47:429–460. doi: 10.1183/13993003.00745-2015. [DOI] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, Jardim J, Lopez MV, Marin JM, Montes de Oca M, et al. Six Minute Walk Distance Project (ALAT) The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 23.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 24.Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, Calverley PM, Tal-Singer R, Agustí A, Bakke PS, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187:382–386. doi: 10.1164/rccm.201209-1596OC. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 26.Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. [Google Scholar]
- 27.Pitta F, Troosters T, Spruit MA, Decramer M, Gosselink R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2005;86:1979–1985. doi: 10.1016/j.apmr.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Spruit MA, Polkey MI, Celli B, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, Calverley PM, Tal-Singer R, Agusti A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13:291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26:630–636. doi: 10.1183/09031936.05.00045505. [DOI] [PubMed] [Google Scholar]
- 30.Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F National Emphysema Treatment Trial (NETT) Research Group. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37:784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SJ, Sodergren SC, Hyland ME, Williams J, Morgan MD. A comparison of three disease-specific and two generic health-status measures to evaluate the outcome of pulmonary rehabilitation in COPD. Respir Med. 2001;95:71–77. doi: 10.1053/rmed.2000.0976. [DOI] [PubMed] [Google Scholar]
- 32.Pepin V, Saey D, Whittom F, LeBlanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1517–1522. doi: 10.1164/rccm.200507-1037OC. [DOI] [PubMed] [Google Scholar]
- 33.Sciurba F, Criner GJ, Lee SM, Mohsenifar Z, Shade D, Slivka W, Wise RA National Emphysema Treatment Trial Research Group. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167:1522–1527. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 34.Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT): Part I: lessons learned about emphysema. Am J Respir Crit Care Med. 2011;184:763–770. doi: 10.1164/rccm.201103-0454CI. [DOI] [PubMed] [Google Scholar]
- 35.Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Antó JM, Agustí AG, Gómez FP, Rodríguez-Roisín R, Moons KG, Kessels AG, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
- 36.Kohli P, Pinto-Plata V, Divo M, Malhotra A, Harris RS, Lazaar A, Flynn A, Tal-Singer R, Panettieri RA, Jr, Celli B. Functional capacity, health status, and inflammatory biomarker profile in a cohort of patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2015;35:348–355. doi: 10.1097/HCR.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlöv J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 38.Casanova C, Cote CG, Marin JM, de Torres JP, Aguirre-Jaime A, Mendez R, Dordelly L, Celli BR. The 6-min walking distance: long-term follow up in patients with COPD. Eur Respir J. 2007;29:535–540. doi: 10.1183/09031936.00071506. [DOI] [PubMed] [Google Scholar]
- 39.Spruit MA, Watkins ML, Edwards LD, Vestbo J, Calverley PM, Pinto-Plata V, Celli BR, Tal-Singer R, Wouters EF Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104:849–857. doi: 10.1016/j.rmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:1329–1335. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 41.Crisafulli E, Costi S, Luppi F, Cirelli G, Cilione C, Coletti O, Fabbri LM, Clini EM. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008;63:487–492. doi: 10.1136/thx.2007.086371. [DOI] [PubMed] [Google Scholar]
- 42.de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, Celli B. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121:1092–1098. doi: 10.1378/chest.121.4.1092. [DOI] [PubMed] [Google Scholar]
- 43.Foglio K, Bianchi L, Bruletti G, Porta R, Vitacca M, Balbi B, Ambrosino N. Seven-year time course of lung function, symptoms, health-related quality of life, and exercise tolerance in COPD patients undergoing pulmonary rehabilitation programs. Respir Med. 2007;101:1961–1970. doi: 10.1016/j.rmed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Lacasse Y, Martin S, Lasserson TJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura Medicophys. 2007;43:475–485. [PubMed] [Google Scholar]
- 45.Puhan M, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;(1):CD005305. doi: 10.1002/14651858.CD005305.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 47.Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109:207–212. doi: 10.1016/s0002-9343(00)00472-1. [DOI] [PubMed] [Google Scholar]
- 48.Abo-Zaid G, Sauerbrei W, Riley RD. Individual participant data meta-analysis of prognostic factor studies: state of the art? BMC Med Res Methodol. 2012;12:56. doi: 10.1186/1471-2288-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgess S, White IR, Resche-Rigon M, Wood AM. Combining multiple imputation and meta-analysis with individual participant data. Stat Med. 2013;32:4499–4514. doi: 10.1002/sim.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]