Abstract
Background
Most large, prospective studies of the effects of diabetes on mortality have focused on high-income countries where patients have access to reasonably good medical care and can receive treatments to establish and maintain good glycemic control. In those countries, diabetes less than doubles the rate of death from any cause. Few large, prospective studies have been conducted in middle-income countries where obesity and diabetes have become common and glycemic control may be poor.
Methods
From 1998 through 2004, we recruited approximately 50,000 men and 100,000 women 35 years of age or older into a prospective study in Mexico City, Mexico. We recorded the presence or absence of previously diagnosed diabetes, obtained and stored blood samples, and tracked 12-year disease-specific deaths through January 1, 2014. We accepted diabetes as the underlying cause of death only for deaths that were due to acute diabetic crises. We estimated rate ratios for death among participants who had diabetes at recruitment versus those who did not have diabetes at recruitment; data from participants who had chronic diseases other than diabetes were excluded from the main analysis.
Results
At the time of recruitment, obesity was common and the prevalence of diabetes rose steeply with age (3% at 35 to 39 years of age and >20% by 60 years of age). Participants who had diabetes had poor glycemic control (mean [±SD] glycated hemoglobin level, 9.0±2.4%), and the rates of use of other vasoprotective medications were low (e.g., 30% of participants with diabetes were receiving antihypertensive medication at recruitment and 1% were receiving lipid-lowering medication). Previously diagnosed diabetes was associated with rate ratios for death from any cause of 5.4 (95% confidence interval [CI], 5.0 to 6.0) at 35 to 59 years of age, 3.1 (95% CI, 2.9 to 3.3) at 60 to 74 years of age, and 1.9 (95% CI, 1.8 to 2.1) at 75 to 84 years of age. Between 35 and 74 years of age, the excess mortality associated with previously diagnosed diabetes accounted for one third of all deaths; the largest absolute excess risks of death were from renal disease (rate ratio, 20.1; 95% CI, 17.2 to 23.4), cardiac disease (rate ratio, 3.7; 95% CI, 3.2 to 4.2), infection (rate ratio, 4.7; 95% CI, 4.0 to 5.5), acute diabetic crises (8% of all deaths among participants who had previously diagnosed diabetes), and other vascular disease (mainly stroke). Little association was observed between diabetes and mortality from cirrhosis, cancer, or chronic obstructive pulmonary disease.
Conclusions
In this study in Mexico, a middle-income country with high levels of obesity, diabetes was common, glycemic control was poor, and diabetes was associated with a far worse prognosis than that seen in high-income countries; it accounted for at least one third of all deaths between 35 and 74 years of age. (Funded by the Wellcome Trust and others.)
Diabetes is increasingly common in many countries1,2 and has been found to increase the risk of death from a wide range of diseases.3 However, most large studies of diabetes have been conducted in high-income countries where patients have access to good medical care and can receive treatments to establish and maintain good glycemic control. In a meta-analysis of 97 prospective studies, which were conducted mainly in high-income countries, self-reported diabetes less than doubled the rate of death from any cause.3,4 In contrast, in middle-income and low-income countries, where resources to manage diabetes may be more limited and vascular-protective medications may be underused,5 the effects of diabetes on mortality from other diseases could be substantially larger. In many such countries, the prevalence of diabetes has increased considerably over the past few decades.1,2
Mexico is a middle-income country6 that has among the highest prevalences of obesity and diabetes in the world,1,7 and among persons with diabetes in Mexico, glycemic control is often poor8 and is often not combined with treatment to control other risk factors (e.g., blood pressure and cholesterol). We report the findings from a prospective study of the effect of diabetes on cause-specific mortality in approximately 150,000 Mexican adults who were followed for an average of 12 years.
Methods
Recruitment and Study Oversight
From 1998 through 2004, we visited households within two districts of Mexico City and invited all residents 35 years of age or older to participate in a prospective study.9 We recorded age, sex, socioeconomic status, lifestyle factors (e.g., alcohol intake, smoking status, and physical activity), current medications, and medical history (including previously diagnosed diabetes). We measured height, weight, and waist and hip circumferences and measured blood pressure while the participant was sitting. A 10-ml blood sample was obtained from each participant, transported at 4 to 10°C in insulated boxes that contained ice packs, refrigerated overnight at 4°C, and then separated the next morning. Plasma and buffy-coat samples were stored locally at −80°C, then transported on dry ice to Oxford (United Kingdom) for long-term storage over liquid nitrogen.
Research ethics approval was obtained from the Mexican Ministry of Health, the Mexican National Council for Science and Technology, and the University of Oxford, United Kingdom. All the study participants provided written informed consent. The authors vouch for the accuracy and completeness of the data.
Glycated Hemoglobin Measurements
Assays of glycated hemoglobin can be performed reliably10 from buffy-coat samples. We performed these assays in the International Organization for Standardization (ISO) 17025–accredited Wolfson Laboratories of the Clinical Trial Service Unit and Epidemiological Studies Unit, by means of validated high-performance liquid chromatography with the use of HA-8180 analyzers (Arkray) with calibrators traceable to standards specified by the International Federation of Clinical Chemistry (IFCC). The resulting values were expressed in IFCC units of millimoles per mole; we then converted the values to Diabetes Control and Complications Trial (DCCT) glycated hemoglobin percentages by multiplying the number of millimoles per mole by 0.0915 and adding 2.15.11
Mortality Follow-up
Death registration in Mexico City is reliable and complete, with almost all adult deaths certified medically and with few attributed to unknown causes.12 Deaths were tracked up to January 1, 2014, through electronic linkage to the death registry. Deaths of study participants that were recorded in the death registry were confirmed by subsequent visits to the participants’ homes. All diseases noted on the death certificate are coded in the registry according to criteria specified in the International Classification of Diseases, 10th Revision (ICD-10).13 Study clinicians reviewed death certificates and accepted diabetes as the underlying cause only for deaths that were considered to be due to acute diabetic crises. For all other deaths with any mention of diabetes as an immediate or antecedent cause of death (i.e., in Part I of the death certificate), the study clinicians selected an appropriate underlying cause other than diabetes.
Statistical Analysis
For the main analyses in this study, the presence of diabetes was defined as a (self-reported) previous medical diagnosis of diabetes, the use of antidiabetic medication, or both. We also performed sensitivity analyses in which the definition of diabetes was expanded to include a baseline glycated hemoglobin level of at least 6.5%. Participants who had received a diagnosis of diabetes before 35 years of age and who required insulin at the time of recruitment were considered likely to have type 1 diabetes.
The relationship between the presence of diabetes at the time of recruitment and the mortality rate was evaluated with the use of a Cox regression model that excluded data from participants who had certain other chronic diseases (i.e., chronic kidney disease, ischemic heart disease, stroke, cirrhosis, cancer, or emphysema) and from participants for whom any covariates were missing. These rate ratios for death were adjusted for the following covariates: age at risk (up to ten 5-year age groups), location (two districts), educational level (four groups), smoking status (never, former, light, moderate, or heavy), and anthropometric characteristics (height, weight, and waist and hip measurements). Rate ratios for death were not adjusted for either blood pressure or lipid levels, since these factors could themselves be affected by diabetes and adjustment for them would make little difference.3 Most age-specific analyses combined the rate ratios for men and women (which were generally similar).
In all analyses, we standardized death rates to a uniform age distribution in men and women combined by averaging all the age-specific rates in the age range of 35 to 74 years. We anticipated that mortality rates among study participants could differ substantially from those among other Mexicans. Therefore, to extrapolate our data to Mexico as a whole, we estimated absolute disease-specific mortality rates by multiplying all our standardized death rates by a common factor so that their total matched the Mexican national death rate for the year 2012.14 For each disease category, we used the rate ratio at 35 to 74 years of age to calculate the mean mortality rate among the proportions of persons with (p) and without (1 − p) diabetes as rate ratio× A and A, respectively, with A calculated such that (p × rate ratio × A) + (1 −p) × A equaled the estimated disease-specific national mortality rate. Analyses were performed with the use of SAS software, version 9.3 (SAS Institute) and the R statistical package, version 2.11.1 (www.r-project.org/).
Results
Recruitment
Of 112,333 eligible households visited, 106,059 (94%) yielded a total of 159,755 potential study participants. Of these potential participants, 8135 had a history of ischemic heart disease, stroke, cancer, cirrhosis, chronic obstructive pulmonary disease, or chronic kidney disease and the data from these participants were therefore excluded from our analyses of rate ratios; the data from an additional 5574 participants were excluded from our analyses either because data were missing or because the participants were 85 years of age or older at the time of recruitment. Table 1 shows the characteristics of the remaining 146,046 participants at the time of recruitment.
Table 1. Baseline Characteristics of Participants 35 to 84 Years of Age, According to Sex and Diabetes Status at Recruitment.*.
| Variable | Men (N = 47,887) | Women (N = 98,159) | ||
|---|---|---|---|---|
| Previously Diagnosed Diabetes (N = 6,229) | No Previously Diagnosed Diabetes (N = 41,658) | Previously Diagnosed Diabetes (N = 12,839) | No Previously Diagnosed Diabetes (N = 85,320) | |
| Age — yr | 59±11 | 52±12 | 59±11 | 50±12 |
| Duration of diabetes — yr† | 9±7 | NA | 9±7 | NA |
| Age of onset of diabetes <35 yr and insulin use at baseline — no. (%)‡ | 73 (1) | NA | 142 (1) | NA |
| Glycated hemoglobin level | ||||
| No. of participants with data§ | 6,085 | 40,352 | 12,587 | 83,077 |
| Mean level — % | 8.9±2.5 | 5.6±1.0 | 9.0±2.4 | 5.6±0.9 |
| Median level (IQR) — % | 8.7 (6.7–10.8) | 5.4 (5.3–5.7) | 8.8 (7.0–10.9) | 5.4 (5.3–5.7) |
| Level ≥6.5% — no. (%)¶ | 4,818 (79) | 2,412 (6.0) | 10,342 (82) | 4,706 (5.7) |
| Level >9.0% — no. (%)¶ | 2,866 (47) | 892 (2.2) | 6,137 (49) | 1,584 (1.9) |
| Level >10.0% — no. (%)‖ | 2,093 (34) | 642 (1.6) | 4,564 (36) | 1,250 (1.5) |
| Residence — no. (%) | ||||
| Coyoacán district | 2,316 (37) | 18,323 (44) | 4,158 (32) | 33,273 (39) |
| Iztapalapa district** | 3,913 (63) | 23,335 (56) | 8,681 (68) | 52,047 (61) |
| Graduated from university or college | 932 (15) | 10,598 (25) | 498 (4) | 11,032 (13) |
| Current smoker | 2,430 (39) | 18,788 (45) | 1,804 (14) | 17,935 (21) |
| Anthropometric and blood pressure measurements†† | ||||
| Height — cm | 163±7 | 163±8 | 150±7 | 150±9 |
| Weight — kg | 73±13 | 74±15 | 66±13 | 66±17 |
| Body-mass index‡‡ | 27.2±5.1 | 27.6±5.8 | 29.3±5.2 | 29.2±6.7 |
| Waist circumference — cm | 96±12 | 97±13 | 96±12 | 94±16 |
| Hip circumference — cm | 99±11 | 101±12 | 106±11 | 106±14 |
| Waist-to-hip ratio | 0.7±0.07 | 0.96±0.08 | 0.91±0.07 | 0.89±0.09 |
| Blood pressure — mm Hg | ||||
| Systolic | 132±16 | 131±18 | 134±16 | 129±21 |
| Diastolic | 85±10 | 85±12 | 84±10 | 83±13 |
| Antidiabetic medication — no. (%) | ||||
| Insulin | 358 (6) | NA | 993 (8) | NA |
| Biguanide, such as metformin | 1,043 (17) | NA | 2,394 (19) | NA |
| Sulfonylurea | 4,235 (68) | NA | 8,847 (69) | NA |
| Other antidiabetic medication | 86 (1) | NA | 188 (1) | NA |
| Other long-term medication — no. (%) | ||||
| Antihypertensive medication | ||||
| Any§§ | 1,281 (21) | 3,567 (9) | 4,359 (34) | 12,006 (14) |
| Renin–angiotensin system inhibitor | 1,012 (16) | 2,626 (6) | 3,425 (27) | 8,756 (10) |
| Other | 353 (6) | 1,332 (3) | 1,190 (9) | 4,251 (5) |
| Any antithrombotic medication | 138 (2) | 911 (2) | 298 (2) | 2,277 (3) |
| Any lipid-lowering medication | 92 (1) | 167 (<0.5) | 131 (1) | 352 (<0.5) |
Plus–minus values are means ±SD. Differences between men with diabetes and men without diabetes were significant (P<0.05) for all characteristics except waist circumference, diastolic blood pressure, and the use of antithrombotic medication. All differences between women with diabetes and women without diabetes were significant except for body-mass index and hip circumference. Analyses excluded data from participants with previously diagnosed chronic disease (chronic kidney disease, ischemic heart disease, stroke, cirrhosis, cancer, or emphysema). IQR denotes interquartile range, and NA not applicable.
At recruitment, we recorded only the decade of diagnosis of diabetes; mean duration of diabetes was estimated by assuming each diagnosis date to be in the middle of its possible range.
These factors were considered to be indicative of type 1 diabetes.
Glycated hemoglobin assays are available for 97% of participants.
Glycated hemoglobin values that indicate no diabetes are nonnormally distributed, with many being several standard deviations above the median. Values of at least 6.5% indicate undiagnosed diabetes; values greater than 9.0% reflect poor glycemic control. (Mean glycated hemoglobin was 8.4%, 9.1%, and 9.5% among participants who reported taking 0, 1, or at least 2 antidiabetic medications, respectively, but adherence to medication is unknown.)
Among participants with previously diagnosed diabetes, the percentage with a glycated hemoglobin level of greater than 10.0% was 44% among persons 35 to 44 years of age, 44% among persons 45 to 54 years of age, 37% among persons 55 to 64 years of age, 27% among persons 65 to 74 years of age, and 19% among persons 75 to 84 years of age.
Iztapalapa is a poorer district than Coyoacán.
Values were standardized to the age distribution of all men and women without chronic disease (other than diabetes) at recruitment.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Some participants were receiving more than one antihypertensive medication.
Self-Reported Diabetes Status and Glycated Hemoglobin at Recruitment
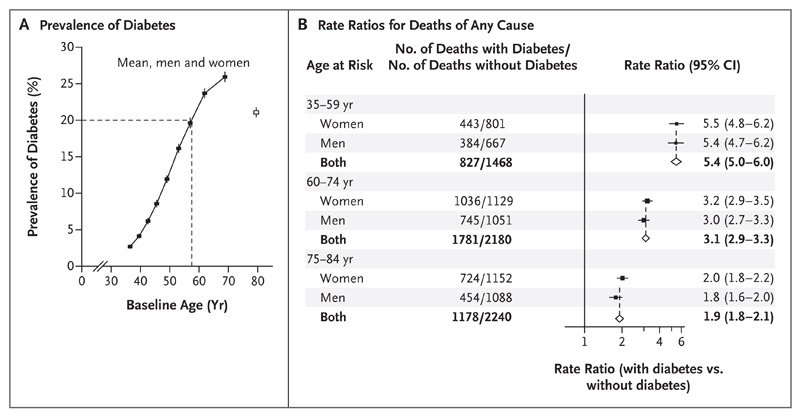
The prevalence of previously diagnosed diabetes among men and women combined increased steeply with age, from 3% at 35 to 39 years of age to greater than 20% by 60 years of age (Fig. 1). The mean (±SD) time from the diagnosis of diabetes to the time of recruitment was 7±6 years among persons 35 to 59 years of age, 11±8 years among persons 60 to 74 years of age, and 13±9 years among persons 75 to 84 years of age (Table 1). Most participants with diabetes had type 2 diabetes, because only 1% had received a diagnosis of diabetes before 35 years of age and required insulin. Among all participants with previously diagnosed diabetes, approximately two thirds reportedly used a sulfonylurea, approximately one fifth used a biguanide, and approximately 80% used at least some antidiabetic medication.
Figure 1. Prevalence of Previously Diagnosed Diabetes and Its Relevance to Rates of Death from Any Cause during 12-Year Follow-up.
Panel A shows the prevalence of previously diagnosed diabetes among all study participants. I bars represent 95% confidence intervals. The dashed lines indicate the age at which the prevalence of diabetes was 20%. Panel B shows the rate ratios for death from any cause among participants with and participants without previously diagnosed diabetes at recruitment. The rate ratios for death exclude data from any participants who had previously diagnosed chronic disease other than diabetes (chronic kidney disease, ischemic heart disease, stroke, cirrhosis, cancer, or emphysema) and are adjusted for standard features (age, smoking status, district, educational level, height, weight, and waist and hip circumferences). The size of each square is proportional to the amount of data available, and unshaded diamonds represent the values for men and women combined. Horizontal lines represent 95% confidence intervals.
Baseline glycated hemoglobin levels were available for 142,101 participants (97%) (Table 1). Among those with previously diagnosed diabetes at the time of recruitment, 36% of participants had a baseline glycated hemoglobin level of greater than 10.0%, and the mean glycated hemoglobin level was 9.0% (and was even higher among younger participants) (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). By contrast, the mean baseline glycated hemoglobin level among participants without previously diagnosed diabetes was 5.6%, with only 5.8% of the participants having a level of at least 6.5% (which would indicate undiagnosed diabetes) and 1.5% having a level of greater than 10.0%.
There was little difference in body-mass index between participants with and participants without previously diagnosed diabetes (Table 1). However, among participants without diabetes, body-mass index at the time of recruitment was strongly predictive of incident diabetes during follow-up, as indicated by its strong relationship with the prevalence of diabetes in a subgroup of survivors who were resurveyed from 2015 through 2016 (Fig. S1 in the Supplementary Appendix).
Diabetes and Rates of Death from Any Cause
Over the course of approximately 12 years of follow-up, 9674 deaths from all causes occurred between 35 and 84 years of age among participants who had no known disease other than diabetes before recruitment. Figure 1 shows the rate ratios for death from any cause among participants with versus participants without previously diagnosed diabetes, according to sex and age group. These rate ratios for death, which were similar for men and women, were 5.4 (95% confidence interval [CI], 5.0 to 6.0) at 35 to 59 years of age, 3.1 (95% CI, 2.9 to 3.3) at 60 to 74 years of age, and 1.9 (95% CI, 1.8 to 2.1) at 75 to 84 years of age. The age-specific rate ratios were similar in the two study districts (data not shown).
Table 2 shows the excess number of deaths before 75 years of age associated with diabetes that had been diagnosed before recruitment. Although previously diagnosed diabetes represents a crude measure of exposure to diabetes and does not include the effects of diabetes with onset during follow-up, the excess risk associated with previously diagnosed diabetes still accounted for 30% of all deaths. When the definition of diabetes at the time of recruitment was broadened to include all participants who had a glycated hemoglobin level of at least 6.5% (i.e., to include participants who had diabetes that was undiagnosed), the percentage of all deaths accounted for by the excess risk associated with diabetes increased from 30% to 35% (Table S1 in the Supplementary Appendix).
Table 2. Excess Risk of Death Associated with Previously Diagnosed Diabetes at Recruitment.*.
| Age at Recruitment | No. of Participants | Mean Years of Follow-up per Survivor | No. of Deaths during Follow-up (and before 75 Years of Age) | Rate Ratio for Death from Any Cause (95% CI)† | Excess Deaths before 75 Years of Age Associated with Previously Diagnosed Diabetes | |||
|---|---|---|---|---|---|---|---|---|
| With Diabetes | Without Diabetes | With Diabetes | Without Diabetes | No. of Deaths | % of Total | |||
| 35–44 yr | 2,184 | 50,568 | 11.9 | 232 | 672 | 7.5 (6.5–8.7) | 201 | 22±0.3 |
| 45–54 yr | 5,043 | 35,838 | 11.9 | 671 | 966 | 4.8 (4.4–5.3) | 532 | 32±0.4 |
| 55–64 yr | 5,753 | 21,230 | 11.7 | 1,128 | 1,348 | 3.3 (3.0–3.5) | 781 | 32±0.6 |
| 65–74 yr | 4,431 | 13,179 | 5.3 | 577 | 662 | 2.7 (2.4–3.1) | 366 | 30±1.0 |
| Total | 17,411 | 120,815 | 2,608 | 3,648 | 1,880 | 30‡ | ||
Plus–minus values are means ±SE.
Estimates of age-specific rate ratios for death among participants with diabetes at recruitment versus participants without diabetes at recruitment were adjusted for age, sex, district, educational level, smoking status, height, weight, and waist and hip circumferences. Analyses excluded data from participants with a previous diagnosis of chronic kidney disease or ischemic heart disease, stroke, cirrhosis, cancer, or emphysema.
When the definition of diabetes was expanded to include participants who had undiagnosed diabetes (glycated hemoglobin level ≥6.5%) at recruitment, the percentage of all deaths associated with diabetes increased to 35% (Table S1 in the Supplementary Appendix). The attributable fraction is approximately one third for both deaths from vascular disease and the aggregate of all other deaths. (This value still excludes any deaths due to diabetes with an onset after recruitment among persons with a glycated hemoglobin level <6.5% at recruitment.)
Diabetes and Disease-Specific Mortality
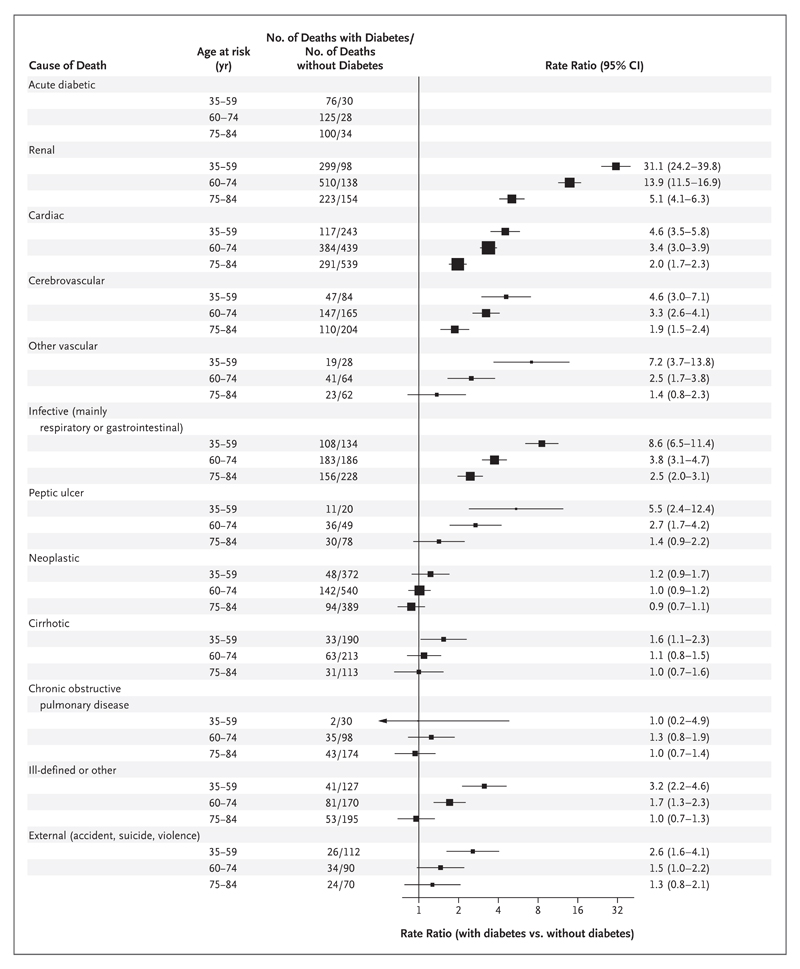
Figure 2 lists the rate ratios for several underlying causes of death between 35 and 84 years of age (for additional details on the specific causes of death, see Figs. S2 through S7 in the Supplementary Appendix). Among participants in whom diabetes was diagnosed before recruitment, 301 of the 3786 deaths (8%) were from acute diabetic crises. In addition, 92 deaths due to acute diabetic crises occurred among participants who did not have previously diagnosed diabetes (median baseline glycated hemoglobin among these participants, 6.1%). Thus, approximately three quarters of the deaths that were attributed to acute diabetic crises during the 12-year follow-up period occurred among participants in whom diabetes had been diagnosed before recruitment.
Figure 2. Relevance of Previously Diagnosed Diabetes to Cause-Specific Mortality during 12-Year Follow-up.
Shown are the numbers of deaths and disease-specific rate ratios for death among participants with versus participants without previously diagnosed diabetes at recruitment, according to age group and to the disease to which the participant’s death was attributed. The rate ratios for death exclude data from any participants who had previously diagnosed chronic disease other than diabetes (chronic kidney disease, ischemic heart disease, stroke, cirrhosis, cancer, or emphysema) and are adjusted for standard features (age, smoking status, district, educational level, height, weight, and waist and hip circumferences). Rate ratios are not shown for deaths attributed to acute diabetic crises because all such deaths were due to diabetes, irrespective of whether diabetes was diagnosed before recruitment. The size of each square is proportional to the amount of data available. Horizontal lines represent 95% confidence intervals. Of the 393 participants who died from an acute diabetic crisis, baseline glycated hemoglobin levels were available for 389 participants, of whom 332 (85%) either had diabetes diagnosed before recruitment or had a baseline glycated hemoglobin level of at least 6.5% and 57 (15%) had no diagnosis of diabetes before recruitment and had a baseline glycated hemoglobin level of less than 6.5%. Numerical values for the rate ratios may vary slightly from the position of the squares because of rounding.
Likewise, with respect to mortality from the aggregate of all renal disease, 1032 deaths occurred among participants with previously diagnosed diabetes and 390 deaths occurred among those without previously diagnosed diabetes; thus, approximately three quarters of the deaths from renal disease during the 12-year follow-up period occurred among persons who had a diagnosis of diabetes before recruitment. The rate ratios for death from renal disease among patients with previously diagnosed diabetes were 31.1 (95% CI, 24.2 to 39.8) at 35 to 59 years of age, 13.9 (95% CI, 11.5 to 16.9) at 60 to 74 years of age, and 5.1 (95% CI, 4.1 to 6.3) at 75 to 84 years of age.
Apart from acute diabetic crises and renal disease, the conditions associated with the largest rate ratios for death were cardiac disease, cerebrovascular and other vascular disease, and infection. All involved similar age-specific rate ratios, which were more extreme at younger ages (and virtually identical for men and women; data not shown).
Diabetes was also strongly associated with mortality from peptic ulcer disease but was not associated with mortality from cirrhosis (which in most cases was alcoholic cirrhosis). There was little association between diabetes and death from chronic obstructive pulmonary disease, death from cancer as a whole, or death from particular types of cancer (Fig. S6 in the Supplementary Appendix), although with respect to death from particular types of cancer, the finding should be interpreted with caution, given the limited number of deaths from cancer in this study.
Overall, between 35 and 74 years of age, the excess risk of death associated with diabetes accounted for approximately one third of all deaths from vascular causes and one third of all other deaths. The analyses of mortality were not affected materially when they were repeated without adjustment for smoking status, level of education, and anthropometric characteristics; when they were repeated with additional adjustment for alcohol intake, physical activity, and blood pressure; when they were repeated with inclusion of data from participants who had a previously diagnosed disease other than diabetes or with exclusion of data from the first 5 years of follow-up (data not shown); or when the disease-specific outcome used in the analyses was death from the disease of interest mentioned anywhere in Part I of the death certificate (Fig. S8 in the Supplementary Appendix).
Estimates of Absolute Mortality
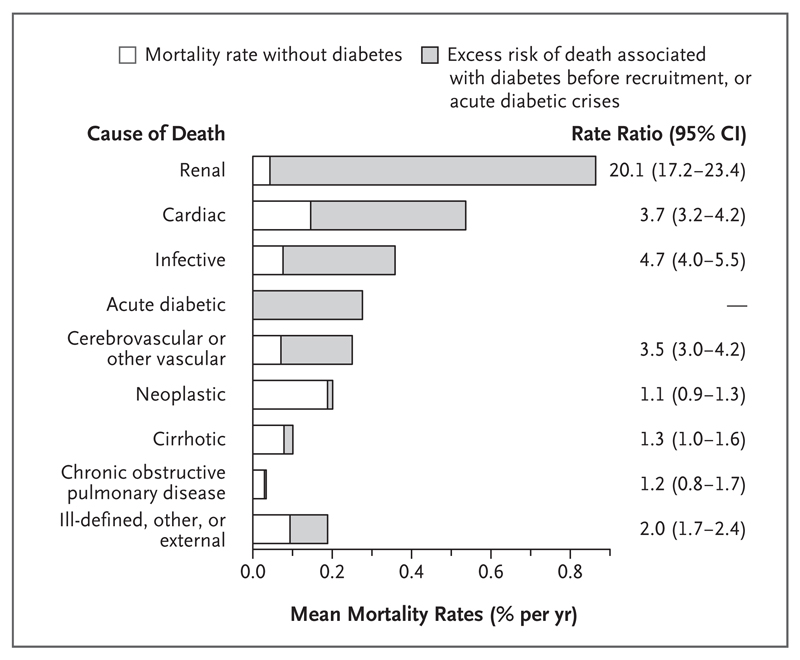
Figure 3 shows absolute estimated disease-specific mortality rates, standardized to national mortality rates in Mexico for the year 2012, among persons with and persons without previously diagnosed diabetes (see the Statistical Analysis section). Between 35 and 74 years of age, the absolute excess risk of death associated with diabetes was greatest for renal disease, followed by cardiac disease, infection, acute diabetic crises, and the aggregate of all other vascular diseases (mainly stroke).
Figure 3. Mortality Rates for Each Cause of Death Among Participants with and Participants without Previously Diagnosed Diabetes.
Shown are the absolute estimated disease-specific rates of deaths according to the cause of death among persons with and persons without previously diagnosed diabetes. The analysis combines the percentages of participants in the current study who died between 35 and 74 years of age from particular diseases, the disease-specific rate ratios for death at 35 to 74 years of age, and 2012 national mortality rates in Mexico. The rates for all bars sum to the rates of death from any cause. The unshaded portions of the bars represent the mortality rate for the specific cause of death among participants without previously diagnosed diabetes. The shaded portions of the bars represent excess risk of death that was either associated with previously diagnosed diabetes or that was due to acute diabetic crises. The weighted average of the death rates shown (for 16% of persons with diabetes plus 84% of persons without diabetes) match uniformly age-standardized 2012 Mexican national rates at 35 to 74 years of age for 50% men plus 50% women. Infective diseases include peptic ulcer disease and exclude any infection in another plotted category. For stroke alone, annual rates of death were 0.05% among persons without previously diagnosed diabetes and 0.19% among persons with previously diagnosed diabetes.
Discussion
Diabetes is more common15 and has a much larger effect on mortality in Mexico than in major high-income countries.3,4,16 By 60 to 74 years of age, approximately one quarter of the participants in the current study had received a medical diagnosis of diabetes, as compared with approximately 7% in the United Kingdom17 and approximately 15% in the United States15 at the time of our baseline survey, and even after adjustment for other risk factors, the rate of death from any cause between 35 and 74 years of age was approximately four times as high among participants with diabetes as among those without diabetes. In contrast, meta-analyses of prospective studies from mostly high-income countries showed that persons with diabetes had less than twice the rate of death from any cause as those without diabetes.3
A probable explanation for our more marked rate ratios for deaths from any cause is inadequate medical care, including poor glycemic control.18,19 More than one third of participants with diabetes diagnosed before recruitment had a baseline glycated hemoglobin level of greater than 10.0%, as compared with only approximately 5% of persons with diagnosed diabetes in cohorts from high-income countries.3,16,20 Furthermore, 8% of all deaths among persons with previously diagnosed diabetes in the current study were due to acute diabetic crises, as compared with less than 1% of deaths among persons with diabetes in the United States.21 In contrast to previous studies,3 our rate ratios for deaths from any cause were similar in men and women, which perhaps reflects similar glycemic control and duration of diabetes (Table 1).
A key feature of our study is that the study clinicians reviewed all death certificates, which contained detailed information about the sequence of events leading up to death.22 World Health Organization conventions for assigning the underlying cause of death attribute to diabetes all deaths from vascular and renal diseases if diabetes was mentioned anywhere in Part I of the death certificate.13 This coding system can result in the undercounting of deaths from vascular and renal disease among persons with diabetes, which in turn can result in artificially low rate ratios for death for the associations of diabetes with these diseases. Hence, in the current study, we accepted diabetes as the underlying cause only for deaths that occurred during acute diabetic crises. Our comparisons of study participants with versus without previously diagnosed diabetes at recruitment therefore yielded appropriately large rate ratios for death from vascular and, particularly, renal disease. Our coding conventions do not, of course, affect the rate ratios for deaths from any cause, which are also large.
Our study has certain limitations. First, we cannot rule out some residual confounding. Second, our study is not representative of Mexico as a whole. The participation rate in the study was high, however, so the study should be reasonably representative of the adults who were contacted at home (two thirds of whom were women) in the two study districts. In addition, our baseline estimates of the prevalence of diabetes are similar to those obtained by a large national survey in Mexico that was conducted at approximately the same time.23 Moreover, in subsequent nationally representative 2006 and 2012 surveys of Mexican adults, glycemic control among persons with diagnosed diabetes was even worse than that reported in our study8; therefore, our estimates of the importance of diabetes to mortality in Mexico as a whole may even be slightly low. As in many prospective studies,3 we did not check self-reports of medically diagnosed diabetes against medical records, but the glycated hemoglobin findings (and high rate ratios for deaths from any cause) suggest that such reports were reasonably reliable, with generally normal glycated hemoglobin levels among persons who reported no diabetes.
Sensitivity analyses in which the definition of diabetes was broadened to include all participants with a glycated hemoglobin level of at least 6.5% at baseline showed a higher percentage of all deaths accounted for by diabetes than the percentage reported in the main analysis (35% vs. 30%). Furthermore, the additional inclusion of deaths related to the effects of diabetes diagnosed after recruitment would result in an even higher percentage of deaths accounted for by diabetes (perhaps approximately 40%; for example, 15% of the deaths from acute diabetic crises occurred in persons who did not have previously diagnosed diabetes and who had a baseline glycated hemoglobin level of less than 6.5%).
The greatest absolute excess risks of death associated with diabetes were from renal disease (primarily chronic kidney disease), vascular disease, infection, and acute diabetic crises. Assuming causality, approximately three quarters of the deaths between 35 and 74 years of age among Mexicans with diabetes were due (directly or indirectly) to their diabetes. Age-specific mortality rates correlated with the duration of diabetes (data not shown), so the lifetime hazard would be even greater for persons in whom diabetes develops in early adult life rather than in later adult life. Overall, we estimate that diabetes was a direct or indirect cause of at least one third of all deaths between 35 and 74 years of age in our study, which is double the indirect estimates for Mexico that relied on rate ratios for death from studies in other countries.1,24 In addition to their relevance to Mexico, our results are relevant to many other populations worldwide, including many millions of U.S. Mexican Americans, among whom the prevalence of diabetes is twice as high as that among U.S. non-Hispanic white persons15 and glycemic control is worse.20
In Mexico, the aggregate of mortality rates from vascular disease, diabetes, and renal disease between 35 and 74 years of age increased slowly from 1998 to 2008, a period during which rates declined steeply in the United States (Fig. S9 and S10 in the Supplementary Appendix). When recruitment into this study ended in 2004, half of all Mexican adults had no health insurance, but during the course of the next 8 years, health care provision improved substantially with the introduction of Seguro Popular,25 which, during the period from 2004 through 2012, extended health insurance nationwide.26 In 2008, mortality rates from vascular, diabetic, and renal disease in Mexico began to decrease, which perhaps reflected better health care provision.8 Nevertheless, mortality rates remain high, as does the prevalence of diabetes.1,8
In this middle-income country, the large excess mortality associated with diabetes reflected both the high prevalence of diabetes, which is partly due to widespread obesity that affects the incidence of diabetes, and the poor prognosis associated with diabetes, which is partly due to inadequate treatment of diabetes, associated risk factors,8 and diabetic complications. Poor glycemic control increases the risk of microvascular disease,27 but during the period of our study, regular testing for albuminuria was rare8 and renal-replacement therapy was limited. Thus, persons with chronic kidney disease often had poor outcomes.28
Mexico recently introduced its National Strategy for Overweight, Obesity and Diabetes, which includes health education, improved opportunities for exercise, taxation of sugary drinks and high-calorie foods, and earlier identification and monitoring of risk factors, including diabetes.29 Within a year after the introduction of this new national strategy, preliminary data suggested that the consumption of sugary drinks had diminished.30 However, despite whatever is achieved in the next few decades by lifestyle interventions (e.g., with respect to adiposity, exercise, and smoking),29–32 diabetes will still affect many people in Mexico and will require treatment to reduce the risk of premature death.
Health care delivery can target both diabetes itself and other determinants of the risk of death or disability from the many different diseases that diabetes can cause.27,33–35 In this middle-income country with a high prevalence of overweight and obesity and with insufficient control of blood glucose, blood pressure, and cholesterol levels, diabetes was a cause of at least one third of all deaths between 35 and 74 years of age (twice the current indirect estimates for Mexico),1,24 with the excess mortality attributed chiefly to renal disease, vascular disease, infection, and acute diabetic crises.
Supplementary Material
Acknowledgments
Supported by grants from the Wellcome Trust (058299/Z/99, 090532, 098381), the Mexican Health Ministry, the National Council of Science and Technology for Mexico, Cancer Research UK, British Heart Foundation, and the UK Medical Research Council Population Health Research Unit.
Dr. Baigent reports receiving grant support from Merck, Novartis, and Pfizer; and Dr. Collins, receiving grant support from BUPA, Abbott/Solvay, Bayer, AstraZeneca, GlaxoSmithKline, Merck, Liposcience, Novartis, and Pfizer.
We thank the study participants; Dr. Linda Youngman (then at the University of Oxford) for planning and supervising sample handling and storage facilities; Dr. Juan Carlos Ramírez Sandoval and Dr. José Gotés Palazuelos (both from Instituto Nacional de Ciencias Medicas y Nutrición Salvador Zubiran, Mexico) for advice on coding of mortality data; and Dr. Hongchao Pan (University of Oxford) for analyzing the national mortality rates for the period from 1973 through 2013.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Jesus Alegre-Díaz, School of Medicine, National Autonomous University of Mexico (UNAM), Mexico City
William Herrington, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford, Oxford, United Kingdom
Malaquías López-Cervantes, School of Medicine, National Autonomous University of Mexico (UNAM), Mexico City
Louisa Gnatiuc, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford, Oxford, United Kingdom
Raul Ramirez, School of Medicine, National Autonomous University of Mexico (UNAM), Mexico City.
Michael Hill, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU); Medical Research Council Population Health Research Unit, University of Oxford, Oxford, United Kingdom
Colin Baigent, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU); Medical Research Council Population Health Research Unit, University of Oxford, Oxford, United Kingdom
Mark I. McCarthy, Nuffield Department of Population Health, and the Oxford Centre for Diabetes, Endocrinology and Metabolism (OCDEM), Nuffield Department of Medicine , University of Oxford, Oxford, United Kingdom
Sarah Lewington, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU); Medical Research Council Population Health Research Unit, University of Oxford, Oxford, United Kingdom
Rory Collins, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford, Oxford, United Kingdom
Gary Whitlock, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford, Oxford, United Kingdom.
Roberto Tapia-Conyer, School of Medicine, National Autonomous University of Mexico (UNAM), Mexico City
Richard Peto, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford, Oxford, United Kingdom
Pablo Kuri-Morales, School of Medicine, National Autonomous University of Mexico (UNAM), Mexico City
Jonathan R. Emberson, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU); Medical Research Council Population Health Research Unit, University of Oxford, Oxford, United Kingdom
References
- 1.International Diabetes Federation diabetes atlas. 7th edition. Brussels: International Diabetes Federation; 2015. http://www.diabetesatlas.org/ [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): a prospective epidemiological survey. Lancet. 2011;378:1231–43. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 6.The World Bank. Mexico data. http://data.worldbank.org/country/mexico.
- 7.World Health Organization. Global database on body mass index. http://apps.who.int/bmi/index.jsp.
- 8.Flores-Hernández S, Saturno-Hernández PJ, Reyes-Morales H, Barrientos-Gutiérrez T, Villalpando S, Hernández-Ávila M. Quality of diabetes care: the challenges of an increasing epidemic in Mexico — results from two national health surveys (2006 and 2012) PLoS One. 2015;10(7):e0133958. doi: 10.1371/journal.pone.0133958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapia-Conyer R, Kuri-Morales P, Alegre-Díaz J, et al. Cohort profile: the Mexico City Prospective Study. Int J Epidemiol. 2006;35:243–9. doi: 10.1093/ije/dyl042. [DOI] [PubMed] [Google Scholar]
- 10.Youngman LD, Clark S, Manley S, Peto R, Collins R. Reliable measurement of glycated hemoglobin in frozen blood samples: implications for epidemiologic studies. Clin Chem. 2002;48:1627–9. [PubMed] [Google Scholar]
- 11.National Glycohemoglobin Standardization Program. International Federation of Clinical Chemistry (IFCC) standardization of HbA1c. http://www.ngsp.org/docs/IFCCstd.pdf.
- 12.Mikkelsen L, Phillips DE, AbouZahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 13.International statistical classification of diseases and related health problems: 10th revision (ICD-10) Vol. 2. Geneva: World Health Organization; 2004. [Google Scholar]
- 14.World Health Organization. Global Health Observatory data repository. life tables by country; Mexico: http://apps.who.int/gho/data/?theme=main&vid=61060. [Google Scholar]
- 15.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 16.Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–32. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 17.Hippisley-Cox J, Ryan R. Diabetes in the United Kingdom: analysis of QRESEARCH data. report to the Department of Health; 2007. http://www.qresearch.org. [Google Scholar]
- 18.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–6. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Diabetes public health resource: mortality due to hyperglycemic crises. http://www.cdc.gov/diabetes/statistics/mortalitydka/index.htm. [Google Scholar]
- 22.Hernández B, Ramírez-Villalobos D, Romero M, Gómez S, Atkinson C, Lozano R. Assessing quality of medical death certification: concordance between gold standard diagnosis and underlying cause of death in selected Mexican hospitals. Popul Health Metr. 2011;9:38. doi: 10.1186/1478-7954-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vázquez-Martínez JL, Gómez-Dantés H, Fernández-Cantón S. Diabetes mellitus in an adult population of the IMSS (Mexican Institute of Social Security): results of the National Health Survey 2000. Rev Med Inst Mex Seguro Soc. 2006;44:13–26. (In Spanish.) [PubMed] [Google Scholar]
- 24.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res Clin Pract. 2010;87:15–9. doi: 10.1016/j.diabres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 25.King G, Gakidou E, Imai K, et al. Public policy for the poor? A randomised assessment of the Mexican universal health insurance programme. Lancet. 2009;373:1447–54. doi: 10.1016/S0140-6736(09)60239-7. [DOI] [PubMed] [Google Scholar]
- 26.Bonilla-Chacín ME, Aguilera N. Universal health coverage studies series 1: the Mexican social protection system in health. Washington, DC: World Bank; 2013. http://documents.worldbank.org/curated/en/2013/01/17286333/mexican-social-protection-system-health. [Google Scholar]
- 27.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Garcia G, Briseño-Rentería G, Luquín-Arellan VH, Gao Z, Gill J, Tonelli M. Survival among patients with kidney failure in Jalisco, Mexico. J Am Soc Nephrol. 2007;18:1922–7. doi: 10.1681/ASN.2006121388. [DOI] [PubMed] [Google Scholar]
- 29.Astudillo O. Country in focus: Mexico’s growing obesity problem. Lancet Diabetes Endocrinol. 2014;2:15–6. doi: 10.1016/S2213-8587(13)70160-8. [DOI] [PubMed] [Google Scholar]
- 30.Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. doi: 10.1136/bmj.h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–41. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 34.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterollowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 35.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.