Abstract
Rationale
GlycA, an emerging inflammatory biomarker, predicted cardiovascular events in population-based studies. Psoriasis, an inflammatory disease associated with increased cardiovascular risk, provides a model to study inflammatory biomarkers in cardiovascular disease (CVD). Whether GlycA associates with psoriasis and how it predicts subclinical CVD beyond hsCRP in psoriasis is unknown.
Objective
To investigate the relationships between GlycA and psoriasis, and between GlycA and subclinical CVD.
Methods and Results
Psoriasis patients and controls (n=412) participated in a two-stage study. We measured GlycA by NMR spectroscopy. NIH participants underwent 18-FDG PET/CT scans to assess vascular inflammation (VI) and coronary CT angiography to quantify coronary artery disease (CAD) burden. Psoriasis cohorts were young (mean age=47.9), with low cardiovascular risk and moderate skin disease. HsCRP and GlycA were increased in psoriasis compared to controls [GlycA: (PENN: 408.8±75.4 vs. 289.4±60.2, p<0.0001, NIH: 415.8±63.2 vs. 346.2±46, p<0.0001)] and demonstrated a dose-response with psoriasis severity. In stage 2, VI (β=0.36, p<0.001) and CAD (β=0.29, p=0.004) associated with GlycA beyond CV risk factors in psoriasis. In ROC analysis, GlycA added value in predicting VI (p=0.01) and CAD (p<0.01). Finally, initiating anti-TNF therapy (n=16) reduced psoriasis severity (p<0.001), GlycA (463.7±92.5 vs. 370.1±78.5; p<0.001) and VI (1.93±0.36 vs. 1.76±0.19; p<0.001), while GlycA remained associated with VI (β=0.56, p<0.001) post-treatment.
Conclusions
GlycA associated with psoriasis severity and subclinical CVD beyond traditional CV risk and hsCRP. Moreover, psoriasis treatment reduced GlycA and VI. These findings support the potential utility of GlycA in subclinical CVD risk assessment in psoriasis and potentially other inflammatory diseases.
Keywords: Inflammation, GlycA, cardiovascular disease, coronary artery disease, FDG PET/CT, computed tomography angiography
INTRODUCTION
Atherogenesis is an inflammatory process1, 2. High-sensitivity C-reactive protein (hsCRP), an extensively studied inflammatory biomarker, predicts long-term cardiovascular risk in individuals with no prior evidence of cardiovascular disease (CVD)3. However, emerging evidence suggests that elevated hsCRP may not be a universal feature of chronic inflammation4 and may inaccurately predict coronary artery disease (CAD) in patients with chronic inflammatory disorders5–8, indicating a need for alternative CV biomarkers in these at-risk populations.
GlycA, a complex heterogeneous nuclear magnetic resonance (NMR) signal originating from mobile glycan residues on plasma glycoproteins, is a novel composite biomarker of systemic inflammation9, 10. Recent studies have demonstrated GlycA to be a strong predictor of future CV events11, 12, incident type 2 diabetes13, 14, long-term risk of severe infection4, and overall mortality12. Moreover, GlycA showed promise in the assessment of disease activity, treatment response, and CAD in patients with inflammatory disorders, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis15–18.
Psoriasis, a chronic inflammatory skin disease affecting 2–3% of US adults19, is associated with chronic systemic inflammation, increased vascular inflammation (VI) by 18-FDG PET/CT20, 21, and a greater risk of incident CV events22–24 and CV mortality25. Traditional risk assessment does not accurately capture the increased CV risk among psoriasis patients26, and many patients with CVD and related events in psoriasis are young23, 25 with low Framingham risk scores26. As such, psoriasis provides a reliable human model to study the utility of novel inflammatory biomarkers for predicting subclinical CVD in chronic inflammatory states.
To understand how GlycA may potentially associate with subclinical CVD in psoriasis, and to compare the association of subclinical CVD to hsCRP, we used a two-stage study design. In the first stage (henceforth PENN cohort), we evaluated psoriasis patients and healthy controls to determine whether GlycA levels were elevated in psoriasis. In the second stage (henceforth NIH cohort), we wished to confirm the association between GlycA and psoriasis and to characterize potential relationships between GlycA and subclinical CVD by assessing VI by 18-FDG PET/CT and CAD by coronary CT angiography (CCTA) to estimate coronary plaque burden. We hypothesized that GlycA would be elevated in psoriasis, associate with skin disease severity, and also directly associate with VI by 18-FDG PET/CT and CAD by CCTA beyond traditional risk factors and hsCRP.
METHODS
A total of 412 participants were included in a two-stage, cross-sectional study design: PENN cohort (n=231; 122 psoriasis patients and 109 controls) and NIH cohort (n=181; 151 psoriasis patients and 30 controls). A detailed description of methods and materials including inclusion/exclusion criteria, clinical assessment, detailed imaging procedures, and statistical analyses for both the cohorts are available in the online-only supplement. STROBE guidelines were followed for reporting the findings from both the stages27.
RESULTS
Characteristics of the study groups
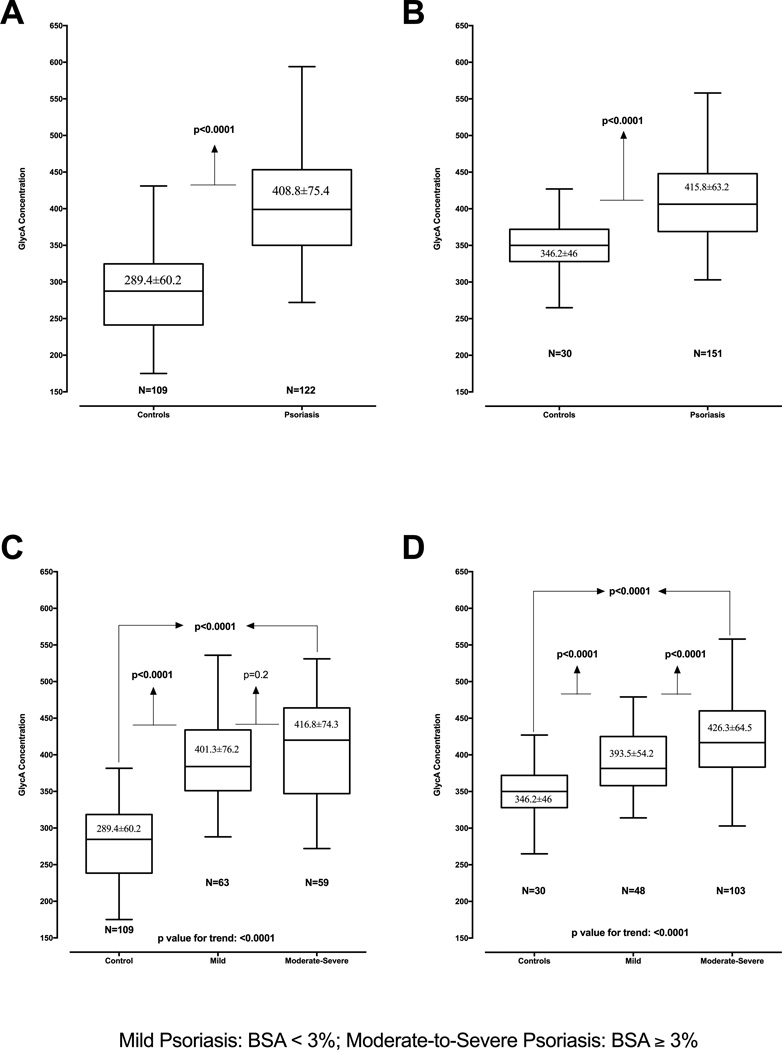
The PENN cohort (Table 1A) contained psoriasis patients (n=122) with mild-to-moderate skin disease (median percent BSA 3, IQR 1–7), and controls (n=109) with similar age and gender. Participants were middle-aged (mean ± S.D.: psoriasis 45.2±13.6 years, controls 48.3±8.3 years), overweight to obese (BMI: psoriasis 30.6±8.1 vs. controls 27.6±4.6), and at low CV risk [Framingham risk score (FRS) median (IQR): psoriasis 5 (3–9); controls 5 (3–8)]. Psoriasis patients had lower levels of total cholesterol (psoriasis 191.1±34.4 vs. controls 211.6±38.0) and LDL cholesterol (psoriasis 111.8±28.8 vs. controls 134.9±35.3), likely corresponding with greater statin use (psoriasis 27%, controls 14%). Finally, psoriasis patients had higher levels of hsCRP [median (IQR) psoriasis 3.3 (0.8–9.9); controls 1.1 (0.5–2.3)] and GlycA (psoriasis 408.8±75.4, controls 289.4±60.2) (Figure 1A) which remained significant after adjustment for age, sex, BMI and traditional CV risk factors.
Table 1.
| A: Demographic and clinical characteristics of the study groups from PENN cohort | |||
|---|---|---|---|
| Parameter | Psoriasis (N=122) | Control (N=109) | P value |
| Demographic and Clinical Characteristics | |||
| Age, years | 45.2±13.6 | 48.3±8.3 | 0.02 |
| Male Gender, N (%) | 68 (60%) | 60 (55%) | 0.49 |
| Hypertension, N (%) | 39 (34.5%) | 31 (29%) | 0.35 |
| Type 2 DM, N (%) | 10 (8.8%) | 0 (0%) | <0.01 |
| Current Smoker, N (%) | 8 (7%) | 0 (0%) | <0.01 |
| Statin use, N (%) | 33 (27%) | 15 (14%) | 0.12 |
| Clinical and Lab Parameters | |||
| Body Mass Index, kg/m2 | 30.6±8.1 | 27.6±4.6 | <0.0001 |
| Systolic blood pressure, mm Hg | 130.2±17.0 | 127.2±16.2 | 0.26 |
| Diastolic blood pressure, mm Hg | 78.9±10.7 | 78.4±10.1 | 0.38 |
| Total Cholesterol, mg/dL | 191.1±34.4 | 211.6±38.0 | <0.0001 |
| Low-Density Lipoprotein cholesterol, mg/dL |
111.8±28.8 | 134.9±35.3 | <0.0001 |
| High-Density Lipoprotein cholesterol, mg/dL |
48.2 ±15.7 | 51.5±14.2 | 0.05 |
| Triglycerides, mg/dL [Median (IQR)] | 127 (78–190) | 122 (88–156) | 0.34 |
| Glucose, mg/dL | 92.5±37.1 | 92.3±11.7 | 0.47 |
| Framingham Risk Score [Median (IQR)] | 5 (3–9) | 5 (3–8) | 0.05 |
| Insulin, µU/mL [Median (IQR)] | 17.7 (11.6–29.7) | 6.7 (4.4–9.8) | <0.0001 |
| HOMA-IR [Median (IQR)] | 3.3 (1.3–6.4) | 1.5 (0.9–2.2) | <0.0001 |
| Hs-CRP, mg/L [Median (IQR)] | 3.3 (0.84–9.85) | 1.1 (0.5–2.3) | 0.02 |
| GlycA, µmol/L | 408.8±75.4 | 289.4±60.2 | <0.0001 |
| Psoriasis Characteristics | |||
| Body Surface Area affected [Median (IQR)] | 3 (1–7) | - | - |
| Systemic or Biologic Treatment, N (%) | 13 (12%) | - | - |
| B: Demographic and clinical characteristics of the study groups from NIH cohort. | |||
|---|---|---|---|
| Parameter | Psoriasis (N=151) | Control (N=30) | P value |
| Demographic and Clinical Characteristics | |||
| Age, years | 50.2±12.9 | 46.8±8.9 | 0.15 |
| Male Gender, N (%) | 85 (56%) | 20 (67%) | 0.73 |
| Hypertension, N (%) | 40 (27%) | 7 (23%) | 0.72 |
| Type 2 DM, N (%) | 14 (9%) | 3 (10%) | 0.9 |
| Hyperlipidemia, N (%) | 71 (47%) | 14 (47%) | 0.98 |
| Metabolic Syndrome, N (%) | 35 (23%) | 5 (17%) | 0.5 |
| Current Smokers, N (%) | 13 (9%) | 1 (3%) | 0.32 |
| Statin Use, N (%) | 48 (32%) | 9 (30%) | 0.9 |
| Clinical and Laboratory Values | |||
| BMI, kg/m2 | 29.1±6.0 | 28.2±5.2 | 0.21 |
| SBP, mm Hg | 123.7±14.5 | 112.4±11.8 | <0.001 |
| DBP, mm Hg | 72.9±10 | 70.3±8.2 | 0.1 |
| Total Cholesterol, mg/dL | 182.4±36.5 | 188.3±38.7 | 0.21 |
| Low-Density Lipoprotein cholesterol, mg/dL | 101.7±29.8 | 105.3±33.3 | 0.29 |
| High-Density Lipoprotein cholesterol, mg/dL | 56.5±18.3 | 53.2±18.2 | 0.18 |
| Triglycerides, mg/dL | 100.5 (76–138) | 108.5 (83–173) | 0.17 |
| Apolipoprotein A1, mg/dL | 158±30.7 | 150±29.1 | 0.1 |
| Apolipoprotein B, mg/dL | 90±19.6 | 87.6±22 | 0.33 |
| Framingham Risk Score, Median (IQR) | 3 (1–6) | 3 (1–5) | 0.87 |
| Glucose, mg/dL | 100.2±16.6 | 97.2±13.4 | 0.17 |
| Insulin, µU/mL [Median (IQR)] | 11.1 (7.2–19.3) | 9.9 (8.2–15.9) | 0.72 |
| HOMA-IR, Median (IQR) | 2.77 (1.58–4.88) | 2.38 (1.74–5.14) | 0.67 |
| Hs-CRP, mg/L [Median (IQR)] | 1.80 (0.71–4.22) | 1.35 (0.79–2.40) | 0.24 |
| GlycA, µmol/L | 415.8±63.2 | 346.2±46 | <0.0001 |
| Psoriasis Characteristics | |||
| Disease duration, years [Median (IQR)] | 20 (8–30) | N/A | |
| Body Surface Area affected, Median (IQR) | 4.1 (2.1–13.5) | N/A | |
| PASI score, Median (IQR) | 5.8 (3–10.1) | N/A | |
| Systemic or Biologic Treatment, N (%) | 55 (37%) | N/A | |
| Vascular Inflammation by FDG PET/CT | |||
| Aortic Vascular Inflammation | 1.70±0.26 | 1.59±0.13 | 0.01 |
| Coronary Artery Disease by CCTA | |||
| Total Burden of Coronary Artery Disease | 1.12±0.46 | 0.99±0.25 | 0.01 |
Continuous variables are expressed as Mean ±S.D unless specified otherwise and categorical variables as %. P values were calculated by Student’s t-test for continuous variable and Pearson’s chi-square test for categorical variables.
DM: Diabetes Mellitus, HOMA-IR: Homeostasis Model Assessment of Insulin Resistance, Hs-CRP: High-sensitivity C-reactive protein, PASI: Psoriasis area and severity index
Figure 1.
GlycA levels are increased in psoriasis vs. controls in a two-stage study design (A: PENN Cohort, B: NIH Cohort), GlycA levels and relationship to psoriasis skin disease severity by body surface area in a two-stage study design (C: PENN cohort, D: NIH Cohort).
The NIH cohort (Table 1B) had psoriasis patients (n=151) with mild-to-moderate skin disease [median (IQR): BSA 4.1 (2.1–13.5) and PASI score 5.8 (3–10.1)], and controls (n=30). Both groups were middle aged (psoriasis 50.2±12.9, controls 46.8±8.9), overweight (BMI: psoriasis 29.1±6.0 vs. controls 28.2±5.2), and at low CV risk by FRS [median (IQR): psoriasis 3 (1–6); controls 3 (1–5)]. Psoriasis patients had higher insulin resistance by homeostatic model assessment of insulin resistance (HOMA-IR) [median (IQR): psoriasis 2.77 (1.58–4.88); controls 2.38 (1.74–5.14)], despite having a low prevalence of type-2 diabetes (psoriasis 9% vs. controls 10%) and near normal fasting blood glucose levels (psoriasis 100.2±16.6, controls 97.2±13.4). They also had an equal prevalence of hyperlipidemia (psoriasis 47% vs. controls 47%) and a similar prevalence of metabolic syndrome (psoriasis 23% vs. controls 17%). HsCRP [median (IQR): psoriasis 1.80 (0.71–4.22); controls 1.35 (0.79–2.40)] and GlycA (psoriasis 415.8±63.2, controls 346.2±46.0) (Figure 1B) were elevated in psoriasis similar to that observed in the PENN cohort.
Psoriasis patients had increased VI by 18-FDG PET/CT (psoriasis 1.70±0.26, controls 1.59±0.13; p=0.01) and CAD by CCTA (psoriasis 1.12±0.46, controls 0.99±0.25; p=0. 01) compared to controls.
GlycA associates with hsCRP, inflammatory cytokines, psoriasis severity and cardiometabolic risk factors
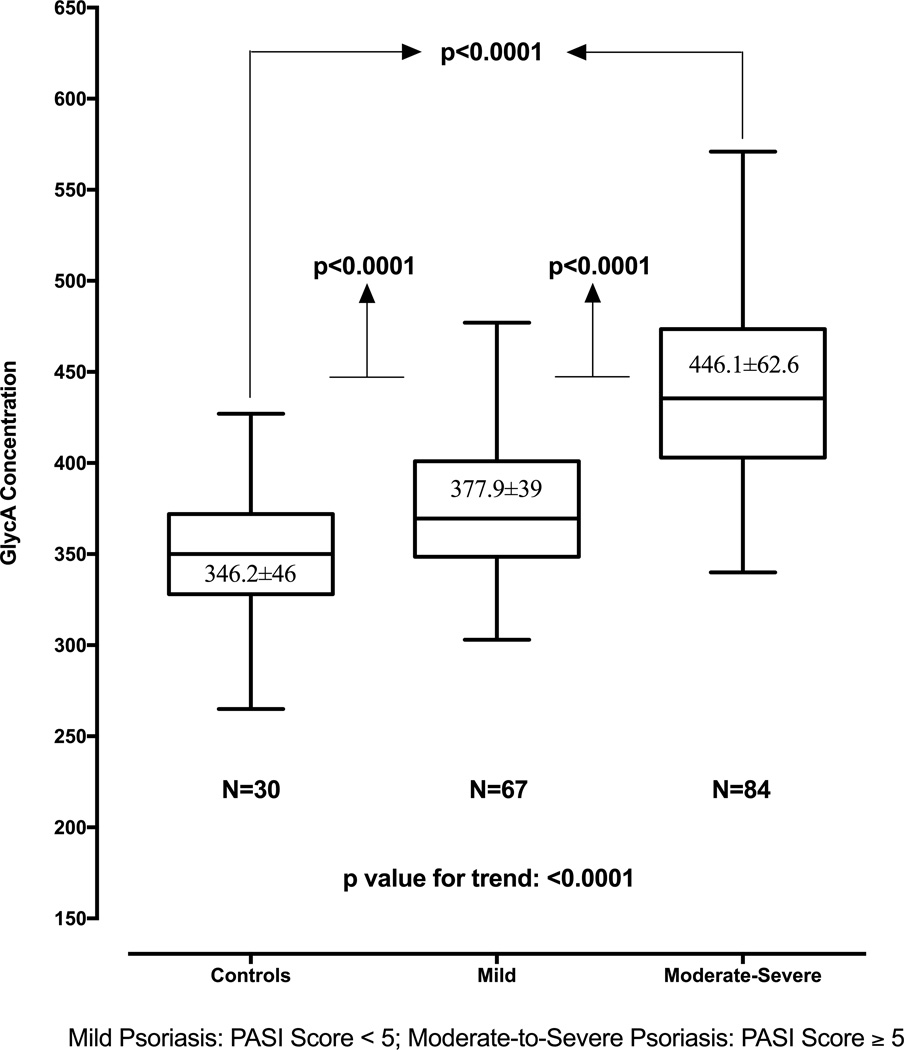
Correlation analyses revealed a relationship between GlycA and hsCRP in psoriasis in both the cohorts (PENN ρ=0.73, p<0.01; NIH ρ=0.50, p<0.001). Furthermore, in the NIH cohort, GlycA correlated with inflammatory cytokines such as IL-6 (psoriasis ρ=0.40, p<0.0001; controls ρ=0.35, p<0.001), IL-16 (psoriasis ρ=0.25, p<0.0001; controls ρ=0.3, p<0.01) and monocyte chemotactic protein-4 (psoriasis ρ=0.12, p<0.05; controls ρ=0.26, p<0.05). GlycA also correlated with psoriasis severity [PENN cohort: BSA (ρ=0.22, p<0.05); NIH cohort: BSA (ρ=0.43, p<0.001) and PASI (ρ=0.57, p<0.001)] (Table 2A&2B, Figure 1 C&D, and Figure 2). The direct association between GlycA and psoriasis severity remained robust beyond traditional CV risk factors [PENN cohort: BSA (β=0.21, p=0.01); NIH cohort: BSA (β=0.40, p<0.001), PASI (β=0.49, p<0.001)] (Table 3 A, B&C). Finally, other cardiometabolic risk factors that significantly correlated with GlycA in psoriasis included metabolic syndrome, waist-to-hip ratio, BMI, LDL-c, HDL-c, FRS, and HOMA-IR (Table 2A&2B).
Table 2.
Correlation Analyses of GlycA with various demographic and cardiometabolic variables in both cohorts
| A: Spearman correlation Analyses of GlycA in the PENN Cohort. | |||
|---|---|---|---|
| Parameter | Total Cohort (N=231) |
Psoriasis (N=122) |
Control (N=109) |
|
Demographic and Clinical Characteristics |
|||
| Age | 0.004 (NS) | 0.08 (NS) | 0.09 (NS) |
| Hypertension | 0.08 (NS) | 0.14 (NS) | 0.04 (NS) |
| Type 2 DM | 0.19 (<0.001) | 0.18 (NS) | No correlation |
| Smoker | 0.23 (<0.001) | 0.10 (NS) | 0.01 (NS) |
| Statin use | 0.07 (NS) | −0.07 (NS) | 0.06 (NS) |
| Clinical and Laboratory Values | |||
| Body Mass Index | 0.33 (<0.001) | 0.43 (<0.001) | 0.29 (<0.001) |
| Systolic blood pressure | 0.21 (<0.001) | 0.14 (NS) | 0.22 (<0.001) |
| Diastolic blood pressure | 0.17 (<0.001) | 0.11 (NS) | 0.21 (<0.001) |
| Total Cholesterol | 0.09 (<0.05) | 0.27 (<0.01) | 0.23 (<0.001) |
| Low-Density Lipoprotein cholesterol | −0.01 (NS) | 0.27 (<0.01) | 0.11 (<0.05) |
| High-Density Lipoprotein cholesterol | −0.08 (NS) | −0.16 (NS) | −0.01 (NS) |
| Triglycerides | 0.30 (<0.001) | 0.35 (<0.001) | 0.36 (<0.001) |
| Framingham risk score | 0.14 (<0.01) | 0.27 (<0.01) | 0.12 (<0.05) |
| Glucose mg/dl | −0.03 (NS) | 0.01 (NS) | 0.05 (NS) |
| Insulin | 0.48 (<0.001) | 0.17 (NS) | 0.25 (<0.001) |
| hsCRP | 0.45 (<0.001) | 0.73 (<0.01) | 0.44 (<0.001) |
| Psoriasis Characteristics | |||
| Body Surface Area | N/A | 0.22 (<0.05) | N/A |
| Systemic or Biologic therapy | N/A | 0.05 (0.58) | N/A |
| B: Spearman correlation Analyses of GlycA in the NIH Cohort. | |||
|---|---|---|---|
| Variable | Total Cohort | Psoriasis | Controls |
| (N=181) | (N=151) | (N=30) | |
| Demographic and Clinical Characteristics | |||
| Age | 0.08 (0.31) | −0.003 (0.95) | 0.24 (0.19) |
| Gender | 0.05 (0.49) | 0.09 (0.06) | −0.23 (0.22) |
| Hypertension | 0.01 (0.86) | −0.03 (0.52) | 0.21 (0.28) |
| Type 2 DM | 0.03 (0.67) | 0.01 (0.81) | 0.19 (0.31) |
| Hyperlipidemia | 0.28 (0.08) | 0.08 (0.08) | 0.24 (0.2) |
| Metabolic Syndrome | 0.14 (0.009) | 0.11 (0.02) | 0.15 (0.46) |
| Waist-to-Hip ratio | 0.16 (0.03) | 0.23 (<0.001) | 0.1 (0.36) |
| Tobacco | 0.12 (0.11) | 0.11 (0.07) | 0.18 (0.3) |
| Current Smokers | 0.08 (0.06) | 0.06 (0.20) | 0.25 (0.19) |
| Statin use | 0.01 (0.9) | 0.01 (0.89) | No correlation |
| Clinical and Laboratory Values | |||
| Body mass index | 0.29 (0.001) | 0.32 (0.001) | 0.20 (0.03) |
| Systolic blood pressure | 0.15 (0.04) | −0.01 (0.91) | 0.24 (0.2) |
| Diastolic blood pressure | 0.07 (0.37) | −0.02 (0.65) | 0.29 (0.12) |
| Total Cholesterol | 0.06 (0.16) | 0.01 (0.75) | 0.29 (0.12) |
| Low-Density Lipoprotein cholesterol | 0.12 (0.09) | 0.16 (<0.001) | 0.17 (0.37) |
| High-Density Lipoprotein cholesterol | −0.14 (<0.007) | −0.17 (<0.001) | −0.11 (<0.01) |
| Triglycerides | −0.02 (0.78) | −0.02 (0.70) | 0.36 (0.04) |
| Apolipoprotein A1 | −0.13 (<0.001) | −0.20 (<0.001) | −0.14 (0.02) |
| Apolipoprotein B | 0.17 (0.02) | 0.17 (0.002) | 0.23 (0.2) |
| Glucose | 0.13 (0.09) | 0.06 (0.19) | 0.20 (0.3) |
| Framingham risk score | 0.12 (0.1) | 0.11 (0.04) | 0.18 (0.42) |
| Insulin | 0.24 (<0.001) | 0.25 (<0.001) | 0.28 (0.02) |
| HOMA-IR | 0.24 (<0.001) | 0.23 (<0.001) | 0.28 (0.03) |
| hsCRP | 0.46 (<0.001) | 0.50 (<0.001) | 0.51 (0.01) |
| Psoriasis Details | |||
| PASI score | 0.57 (<0.001) | 0.57 (<0.001) | N/A |
| Body Surface Area affected | 0.43 (<0.001) | 0.43 (<0.001) | N/A |
| Systemic or Biologic Treatment | −0.12 (0.04) | −0.12 (0.04) | N/A |
| Vascular Inflammation by FDG PET/CT | |||
| Aortic Vascular Inflammation | 0.33 (<0.001) | 0.32 (<0.001) | 0.27 (0.008) |
| Coronary Plaque Burden by CCTA | |||
| Total Burden of Coronary Artery Disease | 0.20 (<0.001) | 0.30 (<0.001) | 0.19 (<0.001) |
Values are expressed as Rho (p value) for all variables.
DM: Diabetes Mellitus, HOMA-IR: Homeostasis Model Assessment of Insulin Resistance, Hs-CRP: high-sensitivity C-reactive protein, PASI: Psoriasis Area Severity Index.
Figure 2.
GlycA levels and relationship with psoriasis skin disease severity measured by Psoriasis Area Severity Index (PASI) score in the NIH cohort.
Table 3.
Relationship between GlycA levels and psoriasis skin disease severity assessed by multivariable linear regression analysis.
| A: GlycA vs. body surface area in the PENN Cohort | |
|---|---|
| Model | β (p value) |
| Unadjusted | 0.29 (0.002) |
| Adjusted for age and gender | 0.34 (0.001) |
| Adjusted for age, gender and FRS | 0.26 (0.007) |
| Adjusted for age, gender, FRS and BMI | 0.25 (0.01) |
| Adjusted for age, gender, FRS, BMI, SBP, LDL-C, HDL-C, HOMA-IR | 0.21 (0.01) |
| B: GlycA vs. body surface area in the NIH Cohort | |
|---|---|
| Model | β (p value) |
| Unadjusted | 0.42 (<0.001) |
| Adjusted for age and gender | 0.42 (<0.001) |
| Adjusted for age, gender and FRS | 0.42 (<0.001) |
| Adjusted for age, gender, FRS and BMI | 0.41 (<0.001) |
| Adjusted for age, gender, FRS, BMI, SBP, LDL-C, HDL-C, HOMA-IR | 0.40 (<0.001) |
| C: GlycA vs. Psoriasis Area and Severity Index score in the NIH Cohort | |
|---|---|
| Model | β (p value) |
| Unadjusted | 0.52 (<0.001) |
| Adjusted for age and gender | 0.53 (<0.001) |
| Adjusted for age, gender and FRS | 0.52 (<0.001) |
| Adjusted for age, gender, FRS and BMI | 0.50 (<0.001) |
| Adjusted for age, gender, FRS, BMI, SBP, LDL-C, HDL-C, HOMA-IR | 0.49 (<0.001) |
All values reported as ‘Standardized β (p value)’.
FRS: Framingham Risk Score, BMI: Body Mass Index, SBP: Systolic Blood Pressure, HOMA-IR: Homeostasis Model Assessment of Insulin Resistance.
GlycA associates with vascular disease independent of traditional CV risk factors
In unadjusted linear regression models, stage 2 demonstrated that GlycA associated with VI (psoriasis β=0.30 p<0.001, controls β=0.26, p<0.001) (Table 4A). This association remained significant beyond traditional CV risk factors in psoriasis (β=0.26, p=0.004) and in controls (β=0.18, p=0.03) (Table 4A). However, similar associations were not found for hsCRP with VI in neither psoriasis (β= − 0.02, p=0.76)) nor controls (β= − 0.02, p=0.93).
Table 4.
Relationship between GlycA and Vascular disease
| A: Multivariable regression analyses show a direct relationship between vascular inflammation and GlycA. | |||
|---|---|---|---|
| Model | Total Cohort | Psoriasis | Control |
| Unadjusted | 0.30 (<0.001) | 0.30 (<0.001) | 0.26 (<0.001) |
| Adjusted for age and gender | 0.29 (<0.001) | 0.28 (<0.001) | 0.22 (<0.001) |
| Adjusted for age, gender and FRS | 0.25 (<0.001) | 0.25 (<0.001) | 0.22 (<0.001) |
| Adjusted for age, gender, FRS and BMI | 0.26 (<0.001) | 0.22 (<0.001) | 0.17 (0.045) |
| Adjusted for age, gender, FRS, BMI, SBP, LDL-C, HDL-C, HOMA-IR, Smoking and Statins |
0.21 (0.002) | 0.26 (0.004) | 0.18 (0.03) |
| B: Multivariable regression analyses show a direct relationship between total burden of coronary artery disease quantified by CCTA and GlycA. | |||
|---|---|---|---|
| Model | Total Cohort | Psoriasis | Control |
| Unadjusted | 0.17 (<0.001) | 0.31 (<0.001) | 0.13 (0.002) |
| Adjusted for age and gender | 0.15 (<0.001) | 0.25 (0.005) | 0.12 (0.003) |
| Adjusted for age, gender and FRS | 0.16 (0.003) | 0.26 (0.004) | 0.15 (0.007) |
| Adjusted for age, gender, FRS and BMI | 0.12 (0.007) | 0.24 (0.002) | 0.10 (0.02) |
| Adjusted for age, gender, FRS, BMI, SBP, LDL-C, HDL-C, HOMA-IR, Smoking and Statins |
0.14 (0.001) | 0.34 (0.01) | 0.13 (0.04) |
All values reported as ‘Standardized β (p value)’.
CCTA: Coronary Computed Tomography Angiography, FRS: Framingham Risk Score, BMI: Body Mass Index, SBP: Systolic Blood Pressure, HOMA-IR: Homeostasis Model Assessment of Insulin Resistance.
GlycA also associated with CAD (psoriasis β=0.31, p<0.001, controls β=0.13, p=0.002) (Table 4B) in unadjusted models. After adjustment for traditional CV risk factors these relationships remained significant in psoriasis (β=0.34, p=0.01), as well as in controls (β=0.13, p=0.04) (Table 4B). Finally, CAD (β=0.05, p=0.30) did not associate with hsCRP in psoriasis, however, was strongly associated with hsCRP in controls (β=0.23, p=0.03).
GlycA provides value in assessing VI and CAD burden beyond traditional CV risk factors and hsCRP in psoriasis
The contribution of GlycA in assessing VI and CAD beyond traditional CV risk factors and hsCRP was first determined using likelihood ratio testing in nested models and second by analyzing receiver operating characteristic (ROC) curves. GlycA provided maximum value in estimation of VI beyond hsCRP, when added to fully adjusted models, in both psoriasis (χ2=19.59, p<0.0001) and controls (χ2=8.95, p=0.003) (Table 5A). GlycA also provided incremental value in measuring CAD burden in psoriasis (χ2=7.88, p=0.008) (Table 5B) beyond hsCRP and traditional CV risk factors. As expected in controls, hsCRP strongly predicted CAD burden (χ2=7.22, p=0.02) (Table 5B).
Table 5.
Incremental Value of GlycA beyond traditional cardiovascular risk factors and Hs-CRP in assessing both Vascular Inflammation and Coronary Artery Disease
| A: Incremental value provided by GlycA in assessing aortic vascular inflammation by 18-FDG PET/CT. | |||
|---|---|---|---|
| Model | Total Cohort | Psoriasis | Control |
| Chi-square (p value) |
Chi-square (p value) |
Chi-square (p value) |
|
| GlycA added to Model 1 | 23.47 (0.0007) | 24.83 (<0.0001) | 5.8 (0.02) |
| Hs-CRP added to Model 1 | 0.96 (0.33) | 0.1 (0.8) | 1.01 (0.32) |
| GlycA added to Hs-CRP in Model 1 | 20.06 (<0.0001) | 19.59 (<0.0001) | 8.95 (0.003) |
| Hs-CRP added to GlycA in Model 1 | 2.54 (0.11) | 0.7 (0.4) | 0.22 (0.64) |
| B: Incremental value provided by GlycA in assessing total burden of coronary artery disease by CCTA. | |||
|---|---|---|---|
| Model | Total Cohort | Psoriasis | Control |
| Chi-square (p value) |
Chi-square (p value) |
Chi-square (p value) |
|
| GlycA added to Model 1 | 15.80 (0.0003) | 8.8 (0.003) | 7.78 (0.003) |
| Hs-CRP added to Model 1 | 1.09 (0.3) | 0.64 (0.42) | 8.12 (0.04) |
| GlycA added to Hs-CRP in Model 1 | 14.34 (0.0003) | 7.88 (0.008) | 6.49 (0.01) |
| Hs-CRP added to GlycA in Model 1 | 0.63 (0.43) | 0.4 (0.55) | 7.22 (0.02) |
Model 1 is adjusted for age, gender, FRS, BMI, HOMA-IR, SBP, LDL-C, HDL-C, smoking and Statin use.
Hs-CRP: High-sensitivity C-reactive protein, FRS: Framingham Risk Score, BMI: Body Mass Index, SBP: Systolic Blood Pressure, HOMA-IR: Homeostasis Model Assessment of Insulin Resistance.
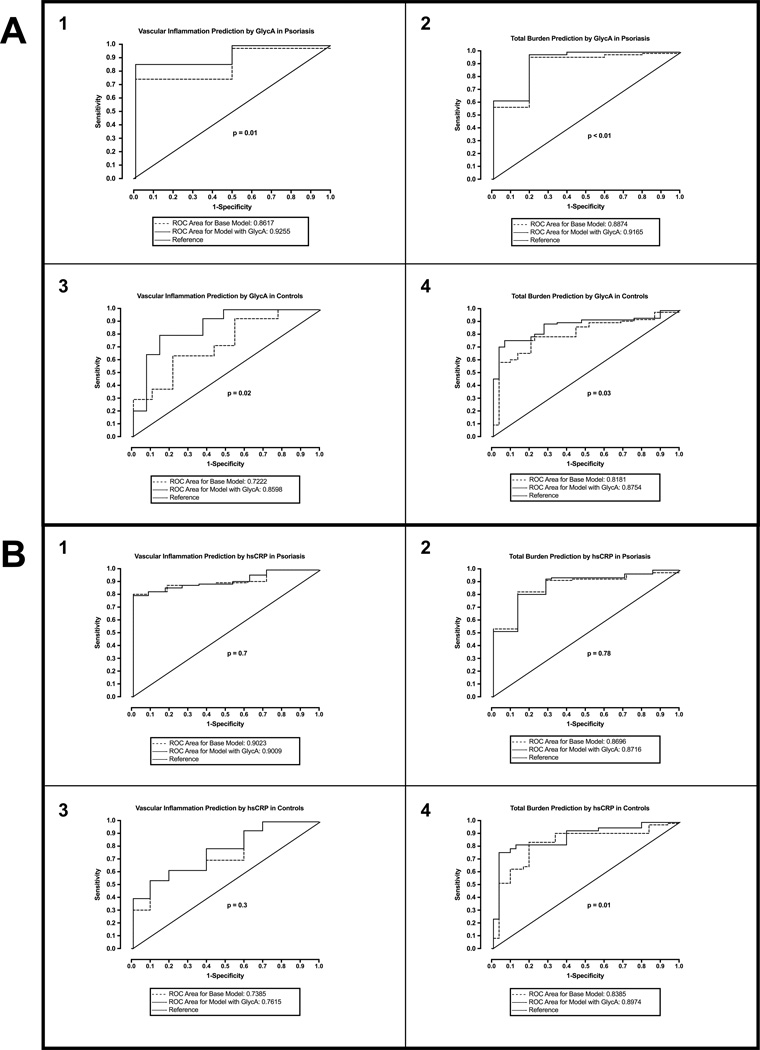
ROC analyses demonstrated that GlycA added value to the base model adjusted for traditional CV risk factors in predicting VI above the cohort mean in psoriasis (AUC for base model: 0.86, 95% CI 0.85–0.87 vs. AUC for model with GlycA: 0.93, 95% CI 0.91–0.94; p=0.01) (Figure 3A1). Similar results were observed in predicting CAD (AUC, 95% CI: 0.89, 0.88–0.89 vs. 0.92, 0.91–0.92; p<0.01) (Figure 3A2). Furthermore, GlycA also added incremental value in predicting these measures of subclinical CVD in controls (Figure 3A 3&4). HsCRP did not add value in predicting CAD or VI above the cohort mean in psoriasis (Figure 3B 1&2). Finally, in controls, though hsCRP did not add value to predicting VI (Figure 3B3), it provided value in predicting CAD above the cohort mean (Figure 3B4).
Figure 3.
Receiver operating characteristic (ROC) curves demonstrating incremental values added by GlycA and hsCRP: (A) ROC analyses demonstrate that GlycA adds value in predicting higher vascular inflammation and greater total burden of coronary artery disease in both psoriasis (1 and 2) and controls (3 and 4); (B) ROC analyses demonstrate that hsCRP adds value in predicting greater total burden of coronary artery disease (4) but not vascular inflammation (3) in controls, however, it fails to add any value in predicting vascular inflammation and coronary artery disease in psoriasis (1 and 2). Mean values of vascular inflammation and coronary artery disease burden were used to convert these continuous variables into dichotomous variables, such that values ≤ mean were designated as 1 and values < mean were designated as 0.
Successful treatment of psoriasis with anti-TNF therapy decreases GlycA and aortic vascular inflammation by FDG PET/CT
Given the association between GlycA and systemic inflammation, we hypothesized that the treatment of psoriasis would lead to a reduction in GlycA. We initiated 16 treatment-naïve patients on anti-TNF therapy and followed them longitudinally for improvement in psoriasis and potentially VI. In these 16 patients, GlycA decreased significantly at follow-up compared to baseline (baseline 463.7±92.5 vs. post-treatment 370.1±78.5, p<0.001), whereas hsCRP reduction did not achieve statistical significance [median (IQR): baseline 2.3 (0.8–9.3) vs. post-treatment 1.3 (0.6–3.2); p=0.054]. Furthermore, we observed an improvement in both PASI score and VI (baseline vs. post treatment VI: 1.93±0.36 vs. 1.76±0.19; p<0.001). Strikingly, the strong association between VI and GlycA persisted post-treatment beyond traditional CV risk (β=0.56, p<0.001), whereas hsCRP was not associated with VI post-treatment (β=0.01, p=0.95).
DISCUSSION
Using a two-stage study design in psoriasis and healthy controls, we demonstrated the following major findings: 1) GlycA levels were elevated in psoriasis compared to healthy controls with a dose-response relationship between GlycA and psoriasis skin disease severity; 2) GlycA significantly correlated with hsCRP, inflammatory cytokines, and markers of cardiometabolic disease; 3) GlycA associated with VI by 18-FDG PET/CT and CAD by CCTA beyond traditional CV risk factors in psoriasis; 4) GlycA provided maximum value in the assessment of VI and CAD beyond hsCRP in models adjusted for traditional CV risk in psoriasis; 5) successful treatment of psoriatic skin inflammation with anti-TNF therapy decreased GlycA levels and VI with persistence of the association between VI and GlycA post-treatment. Collectively, these findings support the value of GlycA as a promising biomarker of inflammation and subclinical CVD risk in psoriasis.
In recent years, the inflammatory hypothesis of atherosclerosis has generated interest in several potential inflammatory biomarkers for CV disease. These include cytokines such as IL-6, TNF-α, IFN-γ and monocyte chemoattractant protein-1 (MCP-1); mediators of endothelial activation such as VCAM-1, ICAM-1 and E-selectin; and acute phase reactants such as serum amyloid A and hsCRP. HsCRP, a marker of systemic inflammation, has become a promising candidate as it is a validated prognostic biomarker of CV disease. However, recent evidence demonstrates that hsCRP may not accurately predict CV disease in patients with inflammatory conditions such as SLE6, psoriasis7, rheumatoid arthritis5 and HIV8. Furthermore, a large population based longitudinal study of >10,000 patients showed evidence to suggest that elevated hsCRP may not be a universal feature of chronic inflammation4. Collectively, these findings indicate that hsCRP may perform suboptimally in CV risk prediction in patients with chronic inflammatory diseases and suggest a need for alternative CV biomarkers in these vulnerable populations.
Contemporary studies to identify new inflammatory biomarkers for CV disease, demonstrate the potential for measuring GlycA. GlycA, an NMR signal originating from a subset of glycan N-acetylglucosamine residues on enzymatically glycosylated acute-phase proteins, is a biomarker of systemic inflammation9, 10. Recent studies involving >25,000 subjects found GlycA to be predictive of 15-year CV events11, incident diabetes mellitus13, and all cause mortality12, 28. Fischer et al. also found a 67% and 55% increase in mortality for every standard deviation increase in GlycA, in two independent populations totaling > 17,000 healthy adults29. Furthermore, these large population studies in subjects without pre-existing inflammatory diseases have revealed that GlycA either conferred additional value beyond traditional biomarkers of inflammation, such as hsCRP, IL-6, ICAM-1 and fibrinogen, or that it was equivalent to these traditional biomarkers in predicting long-term CV and all-cause mortality11, 12, 28. Strikingly, GlycA also showed associations with BMI and fitness among adolescents30, suggesting its role even in the early stages of cardiometabolic dysfunction. Additionally, in inflammatory states, GlycA associated with disease activity as well as CHD among rheumatoid arthritis patients15, 18, and was elevated in patients with SLE compared to controls16. Finally, GlycA also associated with disease activity in SLE, and revealed improved levels subsequent to treatment17.
With increased systemic inflammation and VI20, 21, higher prevalence of diabetes and other traditional CV risk factors31, 32, and a greater risk of CV disease33 as well as CV23, 25 and cerebrovascular events22 psoriasis provides a reliable human model to understand how inflammatory biomarkers perform in CV risk assessment in an inflammatory disease state. In the present study, we demonstrated that GlycA associated with VI and CAD beyond hsCRP and traditional risk in psoriasis, and GlycA provided value in the assessment of subclinical CVD independent of traditional risk factors. Since GlycA provides a composite measurement of human inflammatory glycoproteins, it may capture a broader, summative profile of systemic inflammation10. Together, these findings suggest that GlycA may assess both systemic inflammation and CVD risk more accurately when compared to hsCRP in chronic inflammatory conditions.
GlycA levels recently were shown to be stable in healthy individuals for > 10 years, barring a clinical change4. However, whether GlycA levels respond to anti-inflammatory treatment is unknown. We found that treatment of skin disease with anti-TNF therapy led to reductions in GlycA and VI, suggesting that GlycA may be a reliable biomarker for disease severity, subclinical CVD risk and treatment response in psoriasis. Furthermore, the association between VI and GlycA persisted post-treatment suggesting that it may be a robust biomarker of CVD. Given the known relationship between VI by 18-FDG PET/CT and prospective CV events34, 35, our results suggest that this achieved reduction in GlycA may correspond with a concomitant reduction in CV risk, however, randomized interventional trials are required to answer this question.
There are limitations to the current study which warrant mention. First, the sample size of controls in the second stage of the study was limited, as was the sample size of the treatment cohort. Furthermore, given the cross-sectional design, our study cannot assess causality. Finally, we did not examine the association of GlycA and hard CV endpoints. Despite these limitations, this is the first study, to our knowledge, of GlycA in psoriasis, to show an association between GlycA and subclinical CVD using multi-modality imaging in an inflammatory state, and also the first study to perform a systematic comparison between GlycA and hsCRP in subclinical CVD assessment. Additionally, both VI by 18-FDG PET/CT34, 36 and CAD by CCTA37, 38 are validated surrogates for prospective CV outcomes. Utilizing these markers in deeply-phenotyped cohorts, we provide novel insight into the value of GlycA for assessing subclinical CVD, building upon the previously demonstrated association of GlycA with prospective CV events11, 12.
As GlycA NMR signal is composed of several acute phase reactant glycoproteins, deeper physiologic studies to enhance our understanding of GlycA pathophysiology are warranted. Furthermore, from the emerging evidence and findings of our study, GlycA may be utilized as a risk factor in epidemiologic studies as well as a potential surrogate endpoint in clinical trials of anti-inflammatory treatment. However, larger studies will be needed to confirm these findings before broad scale use of GlycA in trials. Moreover, GlycA measurement may become more widespread with the recent FDA-approval of NMR-based lipoprotein measurement such as LDL-particle number in clinical laboratories. Finally, though large population studies have shown GlycA as a marker of prospective cardiovascular events, prospective cardiovascular event studies with large sample size will be needed to determine its utility as a clinical biomarker of cardiovascular outcomes.
In conclusion, our study provides strong evidence for an association between GlycA and psoriasis as well as GlycA and subclinical CVD in psoriasis. Furthermore, GlycA predicted VI and CAD beyond hsCRP. Finally, following anti-TNF treatment, we found a decrease in GlycA levels and VI with persistence of the relationship between GlycA and VI. Taken together, these findings support a potential role of GlycA in CV risk assessment in an inflammatory state beyond hsCRP. Eventually, GlycA may be considered in psoriasis patients in addition to hsCRP for CVD assessment, however, larger studies are needed to further confirm these findings.
Supplementary Material
Novelty and Significance.
What Is Known?
GlycA is a nuclear magnetic resonance (NMR)-derived signal, originating from mobile glycan residues on plasma glycoproteins, is an emerging biomarker of systemic inflammation associated with all-cause mortality and future cardiovascular events.
Psoriasis is a chronic inflammatory skin disease associated with greater risk of myocardial infarction and provides a reliable human inflammatory model to understand how GlycA may associate with subclinical vascular diseases.
Vascular inflammation (VI) quantitatively assessed by 18-F Fluorodeoxyglucose Positron Emission Tomography Computed Tomography (18-FDG PET/CT) and coronary artery disease (CAD) burden by coronary CT angiography (CCTA) provide surrogate markers of future cardiovascular events.
What New Information Does This Article Contribute?
GlycA was associated with severity of psoriasis skin disease in a dose-dependent fashion measured by body surface area and psoriasis area severity index (PASI).
GlycA was associated with both vascular inflammation by 18-FDG PET/CT and coronary artery disease burden by CCTA beyond traditional cardiovascular risk factors, and added incremental value beyond high-sensitivity C-reactive protein (hsCRP) in psoriasis.
Treatment of psoriasis with anti-TNF therapy led to a decrease in GlycA levels and VI, and GlycA maintained a strong relationship with VI following treatment.
Recent studies have suggested that hsCRP, a biomarker of systemic inflammation which provides value in CV risk prediction, may not accurately capture risk in patients with chronic inflammatory disorders. GlycA is an emerging biomarker of systemic inflammation associated with CV events in population-based studies, but has not been systematically characterized in an inflammatory disease state. Therefore we examined whether in psoriasis, a chronic inflammatory skin disease, GlycA is associated with skin inflammation and vascular diseases. We found that GlycA was associated with psoriasis severity and also with vascular inflammation by 18-FDG PET/CT and burden of coronary artery disease by CCTA. Moreover, treatment of skin disease with anti-TNF therapy was associated with reduction in GlycA levels and VI, with GlycA maintaining its association with vascular inflammation following therapy. This study provides novel insights into the potential utility of GlycA in assessing subclinical CVD, as well as to follow the effects of anti-inflammatory therapy. These findings pave the way for future studies to examine the use of GlycA in studying other inflammatory disease states and in large studies of anti-inflammatory therapy to investigate whether the use of GlycA translates into clinical outcomes.
Acknowledgments
We would like to thank Dr. James Otvos and Dr. Irina Shalaurova for their contribution in obtaining GlycA values for the participants in this study.
SOURCES OF FUNDING:
The study was supported by the NIH intramural research program.
Dr. Gelfand served as a consultant for AbbVie, AstraZeneca, Celgene Corp, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis Corp, Valeant, and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Amgen, Eli Lilly, Janssen, Novartis Corp, Regeneron, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr. Gelfand has also received a grant from the National Institute of Arthritis and Musculoskeletal Diseases (5K24AR064310–03). Dr. Nehal Mehta is a full-time US Government employee.
Nonstandard Abbreviations and Acronyms
- VI
Vascular Inflammation
Footnotes
AUTHOR CONTRIBUTIONS
AAJ and NNM had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: AAJ, JBL, TMA, MA, MPP, JMG and NNM.
Acquisition, analysis and interpretation of the data: AAJ, JBL, TMA, MA, JAR, HLT, PK, QN, TA, TS, BN, MPP, JMG and NNM.
Drafting of the manuscript: AAJ, JBL, TMA, MA, HLT, MPP, JMG, and NNM.
Critical revision of the manuscript for important intellectual content: AAJ, JBL, TMA, MAA, MPR, DAB, JMG and NNM.
Statistical analysis: AAJ, JBL, TMA, PK, JMG and NNM.
All authors contributed in finalizing the manuscript for submission.
CONFLICT OF INTEREST AND FINANCIAL DISCLOSURES:
All other authors declare no conflicts of interest to disclose.
REFERENCES
- 1.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. The New England journal of medicine. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. American heart journal. 2004;148:S19–S26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie Scott C, Würtz P, Nath Artika P, Abraham G, Havulinna Aki S, Fearnley Liam G, Sarin A-P, Kangas Antti J, Soininen P, Aalto K, Seppälä I, Raitoharju E, Salmi M, Maksimow M, Männistö S, Kähönen M, Juonala M, Ripatti S, Lehtimäki T, Jalkanen S, Perola M, Raitakari O, Salomaa V, Ala-Korpela M, Kettunen J, Inouye M. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Systems. 1:293–301. doi: 10.1016/j.cels.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Emami H, Vijayakumar J, Subramanian S, Vucic E, Singh P, MacNabb MH, Corsini E, Hoffmann U, Bathon JM, Solomon DH, Tawakol A. Arterial 18F-FDG uptake in rheumatoid arthritis correlates with synovial activity. JACC Cardiovascular imaging. 2014;7:959–960. doi: 10.1016/j.jcmg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kay SD, Poulsen MK, Diederichsen AC, Voss A. Coronary, Carotid, and Lower-extremity Atherosclerosis and Their Interrelationship in Danish Patients with Systemic Lupus Erythematosus. The Journal of rheumatology. 2016;43:315–322. doi: 10.3899/jrheum.150488. [DOI] [PubMed] [Google Scholar]
- 7.Staniak HL, Bittencourt MS, de Souza Santos I, Sharovsky R, Sabbag C, Goulart AC, Lotufo PA, Bensenor IM. Association between psoriasis and coronary calcium score. Atherosclerosis. 2014;237:847–852. doi: 10.1016/j.atherosclerosis.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. Jama. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell JD, Brown JC, Nicholson JK, Sadler PJ. Assignment of resonances for 'acute-phase' glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Lett. 1987;215:311–315. doi: 10.1016/0014-5793(87)80168-0. [DOI] [PubMed] [Google Scholar]
- 10.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clinical chemistry. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 11.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association. 2014;3:e001221. doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR., Jr Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clinical chemistry. 2016 doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 13.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1544–1550. doi: 10.1161/ATVBAHA.115.305635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connelly MA, Gruppen EG, Wolak-Dinsmore J, Matyus SP, Riphagen IJ, Shalaurova I, Bakker SJ, Otvos JD, Dullaart RP. GlycA, a marker of acute phase glycoproteins, and the risk of incident type 2 diabetes mellitus: PREVEND study. Clin Chim Acta. 2016;452:10–17. doi: 10.1016/j.cca.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett DB, Connelly MA, AbouAssi H, Bateman LA, Tune KN, Huebner JL, Kraus VB, Winegar DA, Otvos JD, Kraus WE, Huffman KM. A novel inflammatory biomarker, GlycA, associates with disease activity in rheumatoid arthritis and cardio-metabolic risk in BMI-matched controls. Arthritis research & therapy. 2015;18:86. doi: 10.1186/s13075-016-0982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung CP, Ormseth MJ, Connelly MA, Oeser A, Solus JF, Otvos JD, Raggi P, Stein CM. GlycA, a novel marker of inflammation, is elevated in systemic lupus erythematosus. Lupus. 2016;25:296–300. doi: 10.1177/0961203315617842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durcan L, Winegar DA, Connelly MA, Otvos JD, Magder LS, Petri M. Longitudinal Evaluation of Lipoprotein Variables in Systemic Lupus Erythematosus Reveals Adverse Changes with Disease Activity and Prednisone and More Favorable Profiles with Hydroxychloroquine Therapy. The Journal of rheumatology. 2016;43:745–750. doi: 10.3899/jrheum.150437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ormseth MJ, Chung CP, Oeser AM, Connelly MA, Sokka T, Raggi P, Solus JF, Otvos JD, Stein CM. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis research & therapy. 2015;17:117. doi: 10.1186/s13075-015-0646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. Journal of the American Academy of Dermatology. 2014;70:512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, Baer A, Antigua J, Van Voorhees AS, Torigian DA, Alavi A, Gelfand JM. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Archives of dermatology. 2011;147:1031–1039. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, Ng Q, Joshi AA, Krishnamoorthy P, Dave J, Rose SM, Doveikis J, Playford MP, Prussick RB, Ehrlich A, Kaplan MJ, Lockshin BN, Gelfand JM, Mehta NN. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arteriosclerosis, thrombosis, and vascular biology. 2015 doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, Troxel AB. The risk of stroke in patients with psoriasis. The Journal of investigative dermatology. 2009;129:2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 24.Menter A, Griffiths CE, Tebbey PW, Horn EJ, Sterry W International Psoriasis C. Exploring the association between cardiovascular and other disease-related risk factors in the psoriasis population: the need for increased understanding across the medical community. Journal of the European Academy of Dermatology and Venereology : JEADV. 2010;24:1371–1377. doi: 10.1111/j.1468-3083.2010.03656.x. [DOI] [PubMed] [Google Scholar]
- 25.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. European heart journal. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta NN, Krishnamoorthy P, Yu Y, Khan O, Raper A, Van Voorhees A, Troxel AB, Gelfand JM. The impact of psoriasis on 10-year Framingham risk. Journal of the American Academy of Dermatology. 2012;67:796–798. doi: 10.1016/j.jaad.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 28.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, Vandenburgh MJ, Schaumberg DA, Lee IM, Glynn RJ, Ridker PM, Buring JE, Mora S. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circulation research. 2016;118:1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer K, Kettunen J, Wurtz P, Haller T, Havulinna AS, Kangas AJ, Soininen P, Esko T, Tammesoo ML, Magi R, Smit S, Palotie A, Ripatti S, Salomaa V, Ala-Korpela M, Perola M, Metspalu A. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. 2014;11:e1001606. doi: 10.1371/journal.pmed.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jago R, Drews KL, Otvos JD, Willi SM, Buse JB. Novel measures of inflammation and insulin resistance are related to obesity and fitness in a diverse sample of 11–14 year-olds: The HEALTHY study. Int J Obes (Lond) 2016 doi: 10.1038/ijo.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, Margolis DJ, Gelfand JM. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. The Journal of investigative dermatology. 2012;132:556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. Journal of the American Academy of Dermatology. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Ahlehoff O, Gislason GH, Charlot M, Jorgensen CH, Lindhardsen J, Olesen JB, Abildstrom SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. Journal of internal medicine. 2011;270:147–157. doi: 10.1111/j.1365-2796.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- 34.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, Tawakol A. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovascular imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 36.Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, Tahara N, Fuster V, Warburton EA, Fayad ZA, Tawakol AA. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? Journal of the American College of Cardiology. 2010;55:2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 37.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. Journal of the American College of Cardiology. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 38.Versteylen MO, Kietselaer BL, Dagnelie PC, Joosen IA, Dedic A, Raaijmakers RH, Wildberger JE, Nieman K, Crijns HJ, Niessen WJ, Daemen MJ, Hofstra L. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. Journal of the American College of Cardiology. 2013;61:2296–2305. doi: 10.1016/j.jacc.2013.02.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.