Abstract
Purpose
All clinical 89Zr-immuno-PET studies are currently performed with the chelator desferrioxamine (DFO). This chelator provides hexadentate coordination to zirconium, leaving two coordination sites available for coordination with, e.g., water molecules, which are relatively labile ligands. The unsaturated coordination of DFO to zirconium has been suggested to result in impaired stability of the complex in vivo and consequently in unwanted bone uptake of 89Zr. Aiming at clinical improvements, we report here on a bifunctional isothiocyanate variant of the octadentate chelator DFO* and the in vitro and in vivo comparison of its 89Zr-DFO*-mAb complex with 89Zr-DFO-mAb.
Methods
The bifunctional chelator DFO*-pPhe-NCS was prepared from previously reported DFO* and p-phenylenediisothiocyanate. Subsequently, trastuzumab was conjugated with either DFO*-pPhe-NCS or commercial DFO-pPhe-NCS and radiolabeled with Zr-89 according to published procedures. In vitro stability experiments were carried out in saline, a histidine/sucrose buffer, and blood serum. The in vivo performance of the chelators was compared in N87 tumor-bearing mice by biodistribution studies and PET imaging.
Results
In 0.9 % NaCl 89Zr-DFO*-trastuzumab was more stable than 89Zr-DFO-trastuzumab; after 72 h incubation at 2-8 °C 95 % and 58 % intact tracer were left, respectively, while in a histidine-sucrose buffer no difference was observed, both products were ≥ 92 % intact. In vivo uptake at 144 h post injection (p.i.) in tumors, blood, and most normal organs was similar for both conjugates, except for skin, liver, spleen, ileum, and bone. Tumor uptake was 32.59 ± 11.95 and 29.06 ± 8.66 % ID/g for 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab, respectively. The bone uptake was significantly lower for 89Zr-DFO*-trastuzumab compared to 89Zr-DFO-trastuzumab. At 144 h p.i. for 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab, the uptake in sternum was 0.92 ± 0.16 and 3.33 ± 0.32 % ID/g, in femur 0.78 ± 0.11 and 3.85, ± 0.80 and in knee 1.38 ± 0.23 and 8.20 ± 2.94 % ID/g, respectively. The uptake in bone decreased from 24 h to 144 h p.i. about two fold for the DFO* conjugate, while it increased about two fold for the DFO conjugate.
Conclusions
Zr-DFO*-trastuzumab showed superior in vitro stability and in vivo performance when compared to 89Zr-DFO-trastuzumab. This makes the new octadentate DFO* chelator a candidate successor of DFO for future clinical 89Zr-immuno-PET.
Electronic supplementary material
The online version of this article (doi:10.1007/s00259-016-3499-x) contains supplementary material, which is available to authorised users.
Keywords: Immuno-PET, 89Zr, DFO*, DFO, Monoclonal antibodies
Introduction
PET imaging with 89Zr-labeled monoclonal antibodies (mAbs) or other targeted vehicles (e.g., peptides, nanoparticles, and cells), collectively called 89Zr-immuno-PET, can be used for better understanding of disease targets and the in vivo behavior of targeted drugs [1]. 89Zr is ideally suited for this purpose because its physical half-life of 78.4 h matches the residence time of intact mAbs in the body (typically several days). Moreover, 89Zr is a residualising radionuclide; when a mAb internalises, 89Zr-mAbs give higher tumor-to-normal tissue ratios than, e.g., the corresponding 124I-labeled mAbs [2] resulting in better contrast on PET images.
The field of 89Zr-immuno-PET is expanding very rapidly as exemplified by the increasing number of publications in recent years, the types of applications, and the number of clinical trials [1, 3]. Several factors are contributing to this rapid expansion. First, mAbs are entering the mainstream of targeted drug development and therapy [4]. The recent introduction of the next generation of mAbs, characterised by increased potency (e.g., antibody-drug conjugates and immune checkpoint inhibiting mAbs), multiple binding domains, and/or high costs, makes finding answers about their in vivo behavior in individual subjects even more urgent [5]. Second, 89Zr-immuno-PET appears to be ideally suited for quantitative imaging of mAbs, and became technically matured by the commercial supply of 89Zr and chelators, standard protocols for 89Zr coupling to mAbs, and standardisation and harmonisation of 89Zr-quantification by PET imaging [6–8].
The only chelator used thus far in the clinic for the complexation of 89Zr is desferrioxamine (DFO). The fact that DFO was clinically used for many years to counteract iron and aluminum overload, has clearly facilitated the introduction of DFO in clinical 89Zr-immuno-PET. Moreover, the first clinical 89Zr-immuno-PET trial indicated that the chelator is not immunogenic [9], and can, therefore, be used repeatedly. The most often used conjugation procedures employ a 2,3,5,6-tetrafluorophenol (TFP) activated ester of N-succinyl-DFO-Fe (TFP-N-suc-DFO-Fe, in which DFO is protected by Fe3+) or p-isothiocyanatobenzyl-DFO (p-SCN-Bn-DFO = DFO-pPhe-NCS, Macrocyclics), forming stable amide or thiourea bonds, respectively, with lysine residues of the proteins [6, 10–12]. Both bifunctional chelating agents are commercially available and have been applied in clinical trials.
From an inorganic chemistry perspective, however, DFO is not the optimal chelator for stable complexation of zirconium, because DFO consists of three hydroxamate moieties and is a hexadentate chelator, while 89Zr4+ prefers the formation of octadentate complexes [13]. Although the resulting slightly impaired stability of 89Zr-DFO-mAb does not really hamper image quality, it is clear from preclinical studies that in time, some 89Zr4+ becomes released from the conjugate and accumulates in the bones [13–16]. For example, Perk et al. compared the biodistribution of the conjugates 89Zr-DFO-cetuximab, 88Y-DOTA-cetuximab and 177Lu-DOTA-cetuximab in tumor-bearing nude mice [14]. While the overall biodistribution of these conjugates was very similar, a significant higher uptake for 89Zr compared to 88Y and 177Lu was observed in femur and sternum at 48–144 h p.i. Over time, femur levels increased from about 2 %ID/g at 24 h p.i. to 6.9 %ID/g at 144 h p.i., indicating partial instability of the 89Zr-DFO complex. Bone uptake of released 89Zr is unwanted, since it will increase the radiation dose to the bone marrow, impair detection of bone metastases, and disturb 89Zr-mAb quantification in bone, bone marrow, and bone metastases.
An optimal chelating agent should be safe for clinical use, efficiently conjugated to, e.g., mAbs and labeled with 89Zr, not alter the biodistribution of the mAb, and, most importantly, be more stable in vitro and in vivo than DFO to prevent gradual accumulation of 89Zr in the bones. In the past years, several new chelators have been reported aiming at increased 89Zr-complex stability in vivo. Some chelators contain hydroxamate moieties, like the linear and macrocyclic tetrahydroxamate chelators reported by Guerard et al., and the trihydroxamate chelators reported by Boros et al. and Zhai et al. [17–20]. Other examples do not contain hydroxamate moieties like p-SCN-Bn-H6phospha, 3,4,3-(LI-1,2-HOPO), and 3-hydroxypyridin-2-one (2,3-HOPO) [16, 21–23]. However, none of these developments have led to a bifunctional chelator that outperforms 89Zr-DFO-mAb complexes on all abovementioned aspects of an optimal chelating agent.
Recently, we reported the synthesis of a tetrahydroxamate chelator called DFO* (DFO-star), and the high stability of its 89Zr-DFO*-complex as shown in challenging experiments with DFO [24]. In the current paper, we describe the synthesis of the bifunctional DFO*-pPhe-NCS, its coupling to mAbs, and the subsequent labeling with 89Zr. Finally, 89Zr-DFO*-mAb conjugates are compared with 89Zr-DFO-mAb conjugates for stability in vitro, and for biodistribution and PET imaging in vivo in tumor-bearing mice with emphasis on 89Zr bone uptake.
Materials and methods
Details regarding the synthesis and analysis of DFO*-pPhe-NCS, radiolabeling results of cetuximab and rituximab, and examples of SEC-HPLC diagrams of 89Zr-DFO*-trastuzumab, 89Zr-DFO*-rituximab, and 89Zr-DFO*-cetuximab can be found in the supplemental data.
Materials, monoclonal antibodies, cell lines, radioactivity, and nomenclature
All reagents and solvents were purchased from Sigma Aldrich. Trastuzumab (21 mg/mL) directed against human epidermal growth factor receptor 2 (HER2), cetuximab (5 mg/mL) directed against the epidermal growth factor receptor (EGFR), and rituximab (10 mg/mL) directed against CD20, were obtained from the VU University Medical Center pharmacy. In the present study, DFO*-pPhe-NCS is compared with DFO-pPhe-NCS, which is commercially available from Macrocyclics under the name p-SCN-Bn-deferoxamine. Both compounds have the same p-isothiocyanatophenylurea linker attached to DFO or DFO*, respectively, and will be designated 89Zr-DFO(*)-mAb throughout the manuscript. The human gastric cancer cell line NCI-N87 was obtained from ATCC (United Kingdom) after cytogenetic testing and used within 6 months after resuscitation of the frozen cell line. The immunoreactivity was determined using SKOV-3 (HER2), A431 (EGFR), or SU-DHL-4 (CD20) cells essentially as described by Lindmo et al. [25]. 89Zr (≥ 0.15 GBq/nmol in 1 mol/L oxalic acid) was obtained from Perkin Elmer, Boston, USA.
General analyses and procedures
Cetuximab (5 mg/mL) was rebuffered to 0.9 % NaCl with the aid of size exclusion chromatography using PD10 columns (GE Healthcare Life Sciences) and re-concentrated to 5 or 5.77 mg/mL by a spin filter (Microcon-10 centrifugal filter, Merck Millipore). Trastuzumab (21 mg/mL) and rituximab (10 mg/mL) were used as such and diluted to 5 or 5.77 mg/mL with 0.9 % NaCl.
Nanodrop analyses were performed on a NanoVue Plus (GE Healthcare Life Sciences). Spin filter separation was performed to determine the radiochemical purity. To this end 4 μL of sample was diluted with 96 μL eluent (5 % DMSO and 95 % 20 mM histidine/240 mM sucrose buffer pH 5.5–5.8) and applied on a microcon-30 centrifugal filter unit (Ultracel YM-30, regenerated cellulose, 30 kDa cut-off, Merck Millipore). The solution was spun down for 7 min at 14,000 rpm (Eppendorf 5430). The filter was washed twice with 100 μL eluent and spun down at 14,000 rpm for 7 min after each wash step. The filtrate contained free 89Zr/89Zr-DFO(*), while the radiolabeled mAb was left on the filter. Size exclusion (SE)-HPLC was performed to determine protein integrity and radiochemical purity. SE-chromatography was performed on a Jasco HPLC system equipped with a superdexTM 200 10/30 GL size exclusion column (GE Healthcare Life Sciences) or Zenix SEC-300 (3 μm, 300 Å, 7.8x50 mm, Sepax) column including a guard column using a mixture of 0.05 M sodium phosphate, 0.15 M sodium chloride (pH 6.8), and 0.01 M NaN3 as the eluent at a flow rate of 0.5 mL/min or 1 mL/min, respectively. The radioactivity of the eluate was monitored using an inline NaI(Tl) radiodetector (Raytest Sockett). HPLC monitoring of the final products was performed on a Jasco HPLC system using a superdex 200 column. Serum stability samples were analysed on a Zenix SEC-300 using the same eluent as the superdex 200 column. The iTLC analysis was performed to determine the radiolabeling efficiency of DFO*-pPhe-NCS and DFO-pPhe-NCS with 89Zr using iTLC-SG (Agilent Technologies, Santa Clara, CA, USA) and 50 mM DTPA pH = 7 as mobile phase (11.5 cm strip, 8 min run time). 89Zr-DFO*-pPhe-NCS had a Rf = 0–0.25, 89Zr-DFO-pPhe-NCS a Rf = 0, and free 89Zr a Rf = 1.
Preparation of 89Zr-DFO*-mAb and 89Zr-DFO-mAb
The pH of the mAb solution (5 mg/mL) was adjusted to 8.9-9.1 with 0.1 M Na2CO3. This solution was added to 3 eq. of DFO*-pPhe-NCS or DFO-pPhe-NCS in DMSO (2 v/v% compared to aqueous solution) and incubated for 30 min in a Thermomixer (550 rpm) at 37 °C. After 30 min, non-conjugated hydrolysed chelator was removed by size exclusion chromatography using a PD-10 column and 0.9 % NaCl or 20 mM histidine + 240 mM sucrose pH 5.5–5.8 as eluent (same eluent used as after radiolabeling). The concentration of the product was determined by spectrophotometry at 280 nm using NanoDrop or by a calibration curve on a superdex 200 column. Subsequently, DFO*-mAb or DFO-mAb was radiolabeled with 89Zr at room temperature for 60 min. Typically, for a 1 mL reaction the following protocol was used: to 100 μL 89Zr (∼50 MBq) in 1 M oxalic acid solution, 45 μL 2 M Na2CO3 was added and reacted for 3 min. Subsequently, 150 μL 0.5 M HEPES buffer (pH 7.0), 355 μL (0.5 mg) DFO*-mAb, or DFO-mAb and 350 μL 0.5 M HEPES (pH 7.0) were added (the second portion of HEPES may be added with the first portion, as long as the reaction is properly mixed after the addition of mAb). Finally, 89Zr-DFO*-mAb or 89Zr-DFO-mAb was purified by size exclusion chromatography (PD10 column). The concentration of DFO(*)-mAbs was determined by SE-HPLC on a Zenix SEC-300 column. For in vitro stability experiments with trastuzumab, the product was formulated to 0.2 mg/mL and 10 MBq/mL (50 MBq/mg) (see in vitro stability section for buffer composition).
Chelator-to-mAb ratio of 89Zr-DFO*-mAb and 89Zr-DFO-mAb
The chelator-to-mAb ratio was determined by prelabeling the chelator and the subsequent conjugation of 89Zr-chelator as well as by an isotopic dilution assay essentially as described by Meares et al. [26].
Prelabeling the bifunctional chelator: to 20 μL 89Zr (2–5 MBq) in 1 M oxalic acid solution, 100 μL 0.9 % NaCl and 9 μL 2 M Na2CO3 were added and reacted for 3 min. Subsequently, 100 μL 0.5 M HEPES buffer (pH 7.0) and 40 μL DFO*/DFO-pPhe-NCS (5 mM) in DMSO were added. After 5 min, half of the volume (134.5 μL) was transferred to an Eppendorf vial, and to this solution 865.5 μL trastuzumab, rituximab or cetuximab (5.77 mg/mL) were added (final concentration: 5 mg/mL mAb). The pH was set to 8.9-9.1 with 2 M Na2CO3, and the reaction incubated for 30 min in a Thermomixer (550 rpm) at 37 °C. Finally, 89Zr-DFO*-mAb/89Zr-DFO-mAb was purified by size exclusion chromatography (PD10 column) and the conjugation efficiency determined.
Isotopic dilution assay: the DFO(*)-to-trastuzumab and DFO(*)-to-rituximab molar ratios were determined following a general method as described by Meares et al. [26]. In short, conjugates were labelled according to the aforementioned procedure with a known excess of zirconium oxalate spiked with 89Zr.
In vitro stability (aqueous solutions and blood serum)
The in vitro stability was evaluated in two sets of experiments. In a first set of experiments, the products were formulated at 0.2 mg/mL and 10 MBq/mL (50 MBq/mg) and stored at 4 °C and room temperature in two different solutions: 0.9 % NaCl or 20 mM histidine + 240 mM sucrose, pH 5.5-5.8. After 24, 48, 72, and 168 h samples were analysed for radiochemical purity by a spin filter and immunoreactivity by a Lindmo assay. In a second set of experiments, the formulated products in 0.9 % NaCl was mixed with freshly prepared human blood serum at a 1:1 ratio, and 0.02 % sodium azide was added. The samples were incubated at 37 °C in a CO2-enriched atmosphere (5 % CO2). At various time points, aliquots were taken and analysed for radiochemical purity by SE-HPLC on a Zenix SEC-300 column and for immunoreactivity by a Lindmo assay. In the case of serum, SE-HPLC was used for analysis of radiochemical purity, because spin filters gave less reproducible results.
Evaluation of in vivo biodistribution
The biodistribution of both 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab was evaluated in N87 tumor bearing mice. Female mice (HSD:Athymic Nude-Foxn1nu, 21–31 g; Harlan) were 8 to 10 weeks old at the time of the experiments. All animal experiments were done according to the NIH Principles of Laboratory Animal Care and Dutch national law (“Wet op de dierproeven”, Stb 1985, 336). Mice bearing N87 xenografts (two groups of n = 18) were anesthetised by inhalation of 2 % isoflurane and injected intravenously (i.v.) via the retroorbital plexus with either 100 μg 89Zr-DFO-trastuzumab or 89Zr-DFO*-trastuzumab (2 MBq) in 20 mM histidine + 240 mM sucrose, pH 5.5-5.8. Unlabeled trastuzumab was added to the injection mixture to bring the total mAb dose to 100 μg per mouse. At 1, 4, 24, 48, 72, and 144 h p.i., blood samples were drawn to determine the blood kinetics. At 24, 72, and 144 h p.i., six mice of each group were anesthetised, bled, killed, and dissected. After blood, tumor, and normal tissues had been weighed, the amount of radioactivity in each sample was measured in a gamma counter. Radioactivity uptake was calculated as the percentage of the injected dose per gram of tissue (%ID/g).
PET study
PET imaging was performed on a dedicated small animal NanoPET/CT scanner (Mediso Ltd., Hungary, Szanda et al.). N87 xenograft-bearing nude mice (n = 1 for each conjugate) from the 144 h biodistribution timepoint were anesthetised by inhalation of 2 % isoflurane and scanned at 72 h p.i. for 1 h. A CT scan was acquired prior to the PET scan and used for attenuation and scatter correction purposes. Reconstruction was performed with a fully 3-dimensional (3-D) reconstruction (Tera-Tomo; Mediso Ltd.) with four iterations and six subsets, resulting in an isotropic 0.4 mm voxel dimension. The scanner was cross-calibrated with the dose calibrator and well counter, enabling the derivation of accurate SUV measures.
Statistical analyses
Statistical analysis was performed on pharmacokinetics, tissue uptake, and tumor-to-blood ratios between different groups of mice with the Welch’s T-test (SPSS) for paired data. Two-sided significance levels were calculated and p < 0.01 was considered as statistically significant.
Results
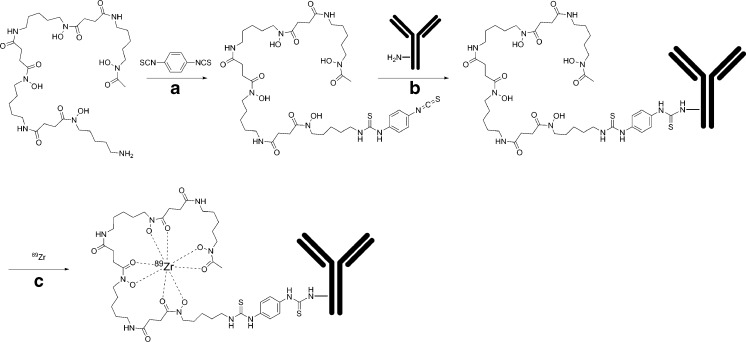
DFO*-pPhe-NCS was prepared from DFO* [24] in a single step by reaction with p-phenylenediisothiocyanate (Fig. 1). The product was purified by preparative HPLC and isolated in a satisfying chemical yield with a purity of >95 % according to HPLC at 275 nm and NMR (Figure S1-4). For conjugation to mAbs, the product was dissolved in DMSO.
Fig. 1.
Synthesis of 89Zr-DFO*-mAbs: a Et3N, iPropOH/H2O/CHCl3, RT, 18 h; b 30 min 37 °C, RT; c 60 min RT
89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab were prepared similarly to postlabeling procedures described by Vosjan et al. [6]. Protein aggregation during modification was observed when using the Vosjan method, which could be minimised by adapting the reagent addition procedure. Instead of adding small portions of the chelator in DMSO to the mAb solution, the mAb was added at once to the chelator in DMSO. This way, the protein integrity was preserved, showing a single IgG peak at 280 nm at SE-HPLC. All conjugates could be radiolabeled efficiently (>80 % radiolabeling yield with 0.5 mg/mL mAb) and had a radiochemical purity of >97.5 % and optimal immunoreactive fraction (>96 %). This is also true for cetuximab and rituximab, which could be radiolabeled with the same efficiency and radiochemical purity and an immunoreactive fraction ≥90 % (see supporting information).
The DFO to mAb molar ratios as determined by the isotope dilution assay were 0.9 ± 0.1 (n = 6) and 1.3 ± 0.1 (n = 3) for trastuzumab and rituximab, respectively, and the DFO* to mAb ratios were 0.6 ± 0.1 (n = 4) and 0.8 ± 0.1 (n = 4) for trastuzumab and rituximab, respectively.
Interestingly, when DFO*-pPhe-NCS and DFO-pPhe-NCS were first radiolabeled with 89Zr and subsequently conjugated to the mAb (prelabeling approach), only for DFO* radiolabeled mAb was obtained. Although DFO*-pPhe-NCS and DFO-pPhe-NCS did radiolabel efficiently (after 5 min at room temperature both compounds were >95 % radiolabeled as determined by iTLC), 89Zr-DFO-pPhe-NCS did not conjugate to mAbs at all. In contrast, in the case of DFO*, the applied 3:1 conjugation ratio of 89Zr-DFO*-pPhe-NCS to mAb resulted in 43 ± 2, 63 ± 1 and 43 ± 2 % radiolabeled product and, thus, a 1.3, 1.9, and 1.3 chelator-to-mAb ratio for trastuzumab (n = 4), rituximab (n = 2), and cetuximab (n = 2), respectively.
89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab obtained by standard post radiolabeling procedures were stored at 4 °C and room temperature in 0.9 % NaCl and 20 mM histidine/240 mM sucrose pH 5.5-5.8 up to 7 days to evaluate the in vitro stability. In 0.9 % NaCl, 89Zr-DFO*-trastuzumab outperformed 89Zr-DFO-trastuzumab in terms of stability (see Table 1 and Table S1); while at 4 °C only 57.6 % intact 89Zr-DFO-trastuzumab was left after 3 days, 89Zr-DFO*-trastuzumab was still 94.5 % intact after the same period of time. At room temperature, the percentage of intact tracer was 41.1 % for 89Zr-DFO-trastuzumab and 90.7 % for 89Zr-DFO*-trastuzumab. In 20 mM histidine/240 mM sucrose, the products exhibited no difference in stability. Both compounds were >92 % intact after 3 days of storage at 4 °C or room temperature. The immunoreactivity of the products, not corrected for free radioactivity, decreased accordingly and was following the same trend as the radiochemical purity. Also in vitro stability studies in human serum/0.9 % NaCl (1:1) showed that 89Zr-DFO*-trastuzumab is more stable than 89Zr-DFO-trastuzumab. After 72 h at 37 °C, 97.5 % and 77.7 % intact tracer was left, respectively. Immunoreactivity determined by the Lindmo binding assay showed the same trend and an immunoreactive fraction of 91 % and 74 % was observed after 72 h at 37 °C for 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab, respectively.
Table 1.
In vitro stability of 89Zr-DFO*-trastuzumab (A) and 89Zr-DFO-trastuzumab (B) at 4 °C in 20 mM histidine + 240 mM sucrose or 0.9 % NaCl or at 37 °C in serum
| A | 20 mM Histidine/240 mM sucrose | 0.9 % NaCl | Serum | |||
| 4 °C | 4 °C | 37 °C | ||||
| Radiochemical purity (%) | Immunoreactive fraction (%) | Radiochemical purity (%) | Immunoreactive fraction (%) | Radiochemical purity (%) | Immunoreactive fraction (%) | |
| 0 h | 99.0 | 98 | 98.0 | 97 | 100.0 | 97 |
| 24 h | 98.4 | 97 | 97.8 | 95 | 100.0 | nd |
| 48 h | 97.7 | 96 | 95.1 | 93 | 99.2 | nd |
| 72 h | 97.0 | 94 | 94.5 | 91 | 97.5 | 91 |
| 168 h | 92.5 | 90 | 89.1 | 82 | 96.3 | 88 |
| B | 20 mM Histidine/240 mM sucrose | 0.9 % NaCl | Serum | |||
| 4 °C | 4 °C | 37 °C | ||||
| Radiochemical purity (%) | Immunoreactive fraction (%) | Radiochemical purity (%) | Immunoreactive fraction (%) | Radiochemical purity (%) | Immunoreactive fraction (%) | |
| 0 h | 97.8 | 96 | 98.6 | 97 | 100.0 | 97 |
| 24 h | 97.2 | 95 | 88.5 | 81 | 88.6 | 85 |
| 48 h | 96.0 | 93 | 65.8 | nd | 82.3 | 79 |
| 72 h | 94.9 | 93 | 57.6 | nd | 77.7 | 74 |
| 168 h | 90.5 | 87 | 50.4 | nd | 72.0 | 64 |
nd not determined
Radiochemical purity of buffer samples determined by a spin filter
Radiochemical purity of serum samples determined by SEC-HPLC
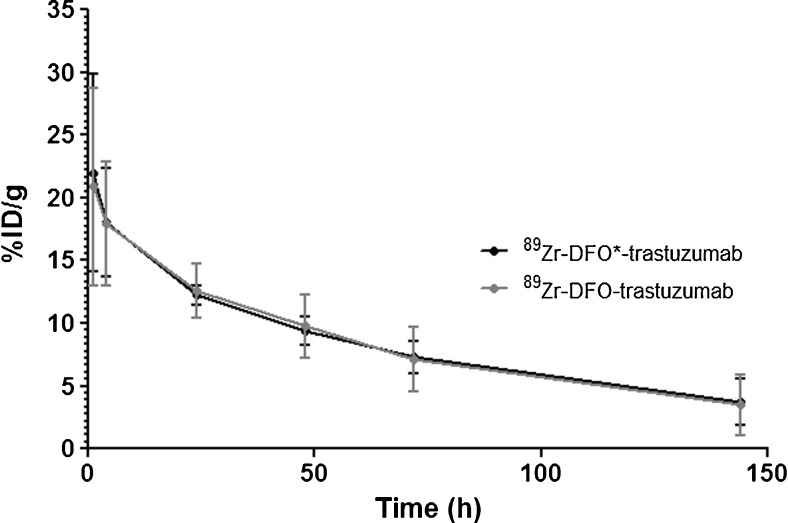
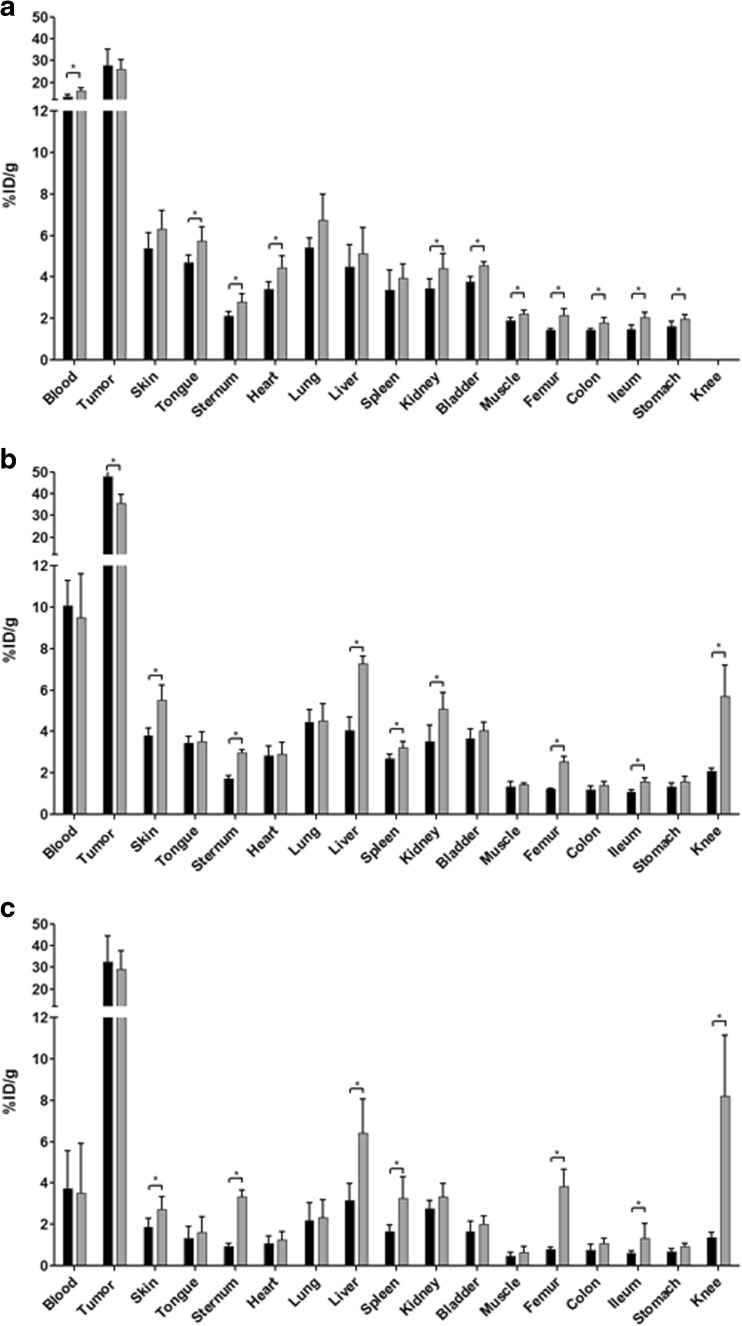
89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab were injected into N87 tumor-bearing nude mice. At 1, 4, 24, 48, 72, and 144 h p.i., blood samples were drawn and the % ID/g determined. At 24, 72, and 144 h p.i., the %ID/g uptake in tumor and normal tissue was determined. Blood kinetics were similar (see Fig. 2). At 144 h p.i., blood kinetics and tumor uptake were not significantly different, while bone-containing organs, skin, liver, spleen, and ileum showed a significantly lower uptake level in case of DFO* (see Fig. 3). Uptake of 89Zr-DFO*-trastuzumab in bone-containing organs decreased over time, while the uptake of 89Zr-DFO-trastuzumab increased (see Figure S5). As a result, ratios of 89Zr-DFO-trastuzumab over 89Zr-DFO*-trastuzumab in bone-containing organs increased over time (see Figure S6). At 24 h p.i., the ratio was 1.3 in sternum and 1.5 in femur; at 72 h p.i. 1.7 in sternum, 2.1 in femur, and 2.8 in knee; and at 144 h p.i., 3.6 in sternum, 5.0 in femur, and 5.9 in knee (see Figure S6).
Fig. 2.
Blood kinetics of 89Zr-DFO*-trastuzumab (black line) and 89Zr-DFO-trastuzumab (grey line) in N87 tumor-bearing nude mice up to 144 h after administration
Fig. 3.
Biodistribution of 89Zr-DFO*-trastuzumab (black bars) and 89Zr-DFO-trastuzumab (grey bars) in N87 tumor-bearing nude mice at 24 h a, 72 h b, and 144 h c after administration. Total administered dose 100 μg. Mean (%ID/g) ± SD at each time point after injection (n = six animals per time point per conjugate). (Significant differences (P < 0.05) in biodistribution between both radioimmunoconjugates are marked with an asterisk)
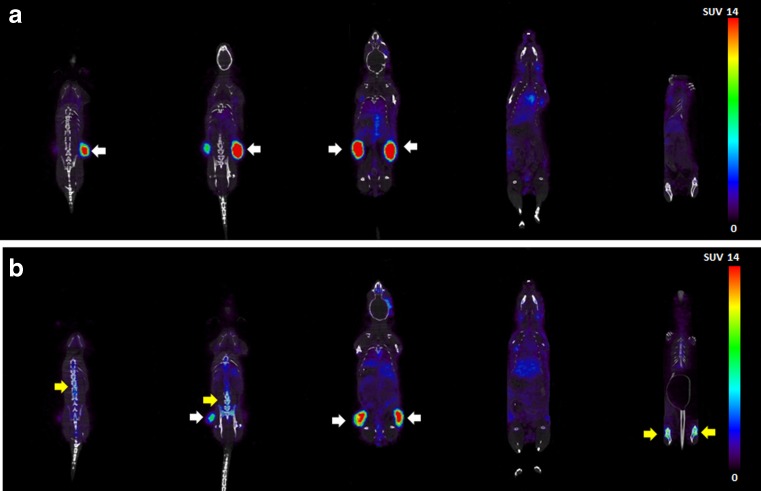
PET imaging studies were performed to evaluate 89Zr-DFO(*)-trastuzumab uptake also in tissues not evaluated in the ex vivo biodistribution experiment. Movies of PET images that were obtained 72 h p.i. are provided in the supporting information. While tumor uptake was clearly visible for both 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab, 89Zr-DFO*-trastuzumab showed less bone uptake than 89Zr-DFO-trastuzumab (see Fig. 4).
Fig. 4.
Coronal PET images of N87 tumor-bearing nude mice acquired 72 h after injection of 100 μg, 2 MBq of either 89Zr-DFO*-trastuzumab a or 89Zr-DFO-trastuzumab b.Tumor are indicated with white arrows, bone uptake is indicated with yellow arrows
Discussion
In the present study, we describe a new bifunctional octadentate chelator for 89Zr, which holds great promise to improve clinical 89Zr-immuno-PET performance.
Previously, we reported the synthesis of DFO* and the superior in vitro stability of its complex with 89Zr in comparison to 89Zr-DFO, also when challenged with an excess of DFO [24]. Here, we describe the synthesis of a bifunctional version of this chelator, DFO*-pPhe-NCS. This bifunctional chelator is prepared starting from the commercially available DFO, which makes DFO*-based chelating agents attractive candidates for commercialization and wide spread use in clinical nuclear medicine. The GMP compliant synthesis of 89Zr-DFO-mAb conjugates has been described before [6] and 89Zr-DFO*-mAbs are prepared in a similar manner. The only difference is a small adjustment of the conjugation protocol, which resulted in improved integrity for the 89Zr-DFO(*)-mAb conjugates. The addition of small portions of the chelator in DMSO to mAbs can result in protein aggregation. To prevent this, the addition order was reversed, and the protein added to the chelator instead. In this way protein aggregation could be minimised. The conjugation of DFO*-pPhe-NCS was slightly less effective, but the subsequent radiolabeling efficiency was similar for both chelators. For DFO-pPhe-NCS, the same chelator-to-mAb ratios and radiolabeling efficiencies were achieved as reported by Vosjan et al. [6]. Using a prelabeling approach, we found that 89Zr-DFO-pPhe-NCS could not be conjugated to mAbs anymore, while 89Zr-DFO*-pPhe-NCS was still capable of conjugation. This indicates that the isothiocyanate linker may be coordinated to 89Zr in case of DFO and, consequently, is not available anymore for bioconjugation. The same accounts for the N-suc-DFO chelator. When N-suc-DFO was first radiolabeled with Zr-89, the COOH group could not be transformed into the TFP-ester anymore [10]. In the case of DFO*; however, 89Zr is coordinatively saturated by the chelator, and; therefore, the isothiocyanate linker is left available for bioconjugation. This interesting difference now allows prelabeling strategies with 89Zr using DFO*, which was not possible before with DFO derivatives. Prelabeling with 89Zr-DFO*-pPhe-NCS could be an advantageous approach in the case of labeling of small amounts of mAb, cells [27], or mAbs with supertoxic payloads, since the number of handlings of the mAb is reduced from two to one.
The coordinative saturation of 89Zr in DFO* manifested itself also in vitro and in vivo. While the stability of 89Zr-DFO-trastuzumab with thiourea linker is strongly dependent on the storage conditions with respect to buffer composition, this is less critical for the thiourea-linked 89Zr-DFO*-trastuzumab. In previous experiments, we found that 89Zr-DFO-mAbs having a thiourea linker between DFO and the mAb should preferably not be stored in sodium chloride-containing buffers. We postulated that the presence of Cl− ions affected the stability of 89Zr-labeled mAb due to radiation-induced formation of hypochlorite ions reacting with the enolised thiourea unit. Mixing of 89Zr-DFO-trastuzumab stored in 0.9 % NaCl with serum resulted in an increased stability, most probably because sulphur atoms of the serum proteins act as scavenging matter for the hypochlorite. The results in Table 1 show that 89Zr-DFO*-mAb with the same thiourea linker is far less sensitive to the presence of Cl−. This indicates that in case of DFO-pPhe-NCS, the enolised sulphur atom of the thiourea unit is coordinated to 89Zr and subsequently oxidised by hypochlorite. These results suggest that the unsaturated coordination of 89Zr is causing the observed impaired stability of the 89Zr-DFO complex, which is not seen with the new chelator DFO*.
Although blood kinetics and tumor uptake in mice were similar for 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab, 89Zr-DFO*-trastuzumab showed a significantly lower uptake in bone containing organs, skin, liver, spleen, and ileum than 89Zr-DFO-trastuzumab (Table 2). Actually, over time the bone uptake of 89Zr-DFO-trastuzumab increased, while the uptake of 89Zr-DFO*-trastuzumab decreased (see Figure S5). Thus, DFO* indeed provides a product that is more stable in vivo, since 89Zr becomes less easily released from the chelator. Recently, much effort has been put in developing improved bifunctional chelators for 89Zr [16, 19, 20, 23]. Focusing on the bifunctional chelators that coordinate 89Zr efficiently, four molecules have been reported. Derivatised fusarinine C (FSC) [20] a cyclic hexadentate chelator, has been tested in combination with a RGD-peptide. Only short-term in vivo experiments are reported, and the performance with longer circulating biologicals is not yet known. Next, there are two 89Zr-HOPO-trastuzumab complexes reported [16, 23]. Deri et al. reported on 89Zr-3,4,3-(LI-1,2-HOPO)-mAb [16]. Although less bone uptake was observed for 89Zr-3,4,3-(LI-1,2-HOPO)-trastuzumab compared to 89Zr-DFO-trastuzumab, the tumor uptake also decreased to half of the uptake observed with 89Zr-DFO-trastuzumab. Tinianow et al. reported on 89Zr-2,3-HOPO-trastuzumab [23]. In vitro serum stability, radiochemical purity of the 89Zr-labeled mAb complex and in vivo bone uptake were less favorable for 89Zr-2,3-HOPO-mAb compared to 89Zr-DFO-mAb. Finally, Boros et al. reported on a macrocycle-based hydroxamate chelator L5 [19]. Although in vitro stability of their 89Zr-chelator was similar to 89Zr-DFO, its bifunctional variant coupled to trastuzumab showed higher bone uptake and faster blood clearance than 89Zr-DFO-trastuzumab. Though a direct comparison of the described hexadentate/octadentate bifunctional chelators for 89Zr is difficult, indications are that DFO* is the best qualified with respect to tumor targeting and in vitro and in vivo stability.
Table 2.
Biodistribution of 89Zr-DFO*-trastuzumab and 89Zr-DFO-trastuzumab in N87 tumor-bearing nude mice at 24, 72, and 144 h after administration
| 24 hr | 72 hr | 144 hr | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFO* | DFO | DFO* | DFO | DFO* | DFO | |||||||||||||
| Blood | 13.45 | ± | 1.20 | 16.16 | ± | 1.47 | 10.06 | ± | 1.22 | 9.49 | ± | 2.13 | 3.73 | ± | 1.84 | 3.50 | ± | 2.41 |
| Tumor | 27.81 | ± | 7.44 | 26.23 | ± | 4.31 | 47.70 | ± | 5.52 | 35.54 | ± | 4.08 | 32.59 | ± | 11.95 | 29.06 | ± | 8.66 |
| Skin | 5.37 | ± | 0.75 | 6.31 | ± | 0.89 | 3.80 | ± | 0.34 | 5.51 | ± | 0.73 | 1.88 | ± | 0.43 | 2.71 | ± | 0.64 |
| Tongue | 4.69 | ± | 0.36 | 5.74 | ± | 0.67 | 3.44 | ± | 0.33 | 3.52 | ± | 0.47 | 1.31 | ± | 0.60 | 1.60 | ± | 0.76 |
| Sternum | 2.10 | ± | 0.24 | 2.79 | ± | 0.39 | 1.72 | ± | 0.16 | 2.97 | ± | 0.15 | 0.92 | ± | 0.16 | 3.33 | ± | 0.32 |
| Heart | 3.41 | ± | 0.34 | 4.45 | ± | 0.58 | 2.82 | ± | 0.49 | 2.90 | ± | 0.56 | 1.07 | ± | 0.35 | 1.25 | ± | 0.41 |
| Lung | 5.41 | ± | 0.48 | 6.73 | ± | 1.28 | 4.43 | ± | 0.62 | 4.52 | ± | 0.83 | 2.18 | ± | 0.88 | 2.32 | ± | 0.87 |
| Liver | 4.47 | ± | 1.07 | 5.11 | ± | 1.26 | 4.05 | ± | 0.63 | 7.27 | ± | 0.35 | 3.17 | ± | 0.82 | 6.43 | ± | 1.64 |
| Spleen | 3.36 | ± | 0.99 | 3.95 | ± | 0.68 | 2.68 | ± | 0.23 | 3.22 | ± | 0.30 | 1.65 | ± | 0.34 | 3.26 | ± | 1.05 |
| Kidney | 3.43 | ± | 0.48 | 4.41 | ± | 0.70 | 3.51 | ± | 0.78 | 5.08 | ± | 0.81 | 2.75 | ± | 0.42 | 3.35 | ± | 0.62 |
| Bladder | 3.75 | ± | 0.28 | 4.56 | ± | 0.19 | 3.65 | ± | 0.46 | 4.04 | ± | 0.41 | 1.65 | ± | 0.50 | 2.01 | ± | 0.40 |
| Muscle | 1.90 | ± | 0.13 | 2.23 | ± | 0.17 | 1.34 | ± | 0.24 | 1.44 | ± | 0.05 | 0.46 | ± | 0.17 | 0.65 | ± | 0.27 |
| Femur | 1.43 | ± | 0.08 | 2.15 | ± | 0.34 | 1.21 | ± | 0.06 | 2.54 | ± | 0.25 | 0.78 | ± | 0,.11 | 3.85 | ± | 0.80 |
| Colon | 1.42 | ± | 0.08 | 1.79 | ± | 0.25 | 1.18 | ± | 0.19 | 1.41 | ± | 0.15 | 0.74 | ± | 0.29 | 1.06 | ± | 0.28 |
| Ileum | 1.48 | ± | 0.21 | 2.03 | ± | 0.28 | 1.09 | ± | 0.09 | 1.57 | ± | 0.20 | 0.60 | ± | 0.10 | 1.34 | ± | 0.70 |
| Stomach | 1.61 | ± | 0.25 | 1.97 | ± | 0.20 | 1.33 | ± | 0.18 | 1.57 | ± | 0.27 | 0.67 | ± | 0.14 | 0.94 | ± | 0.12 |
| Knee | n.d. | n.d. | 2.07 | ± | 0.15 | 5.70 | ± | 1.51 | 1.38 | ± | 0.23 | 8.20 | ± | 2.94 | ||||
Total administered dose 100 μg. Mean (%ID/g) ± SD at each time point after injection (n = six animals per time point and conjugate)
Conclusion
89Zr-DFO*-mAbs can be prepared analogously to 89Zr-DFO-mAbs resulting in radioimmunoconjugates with high radiochemical purity and optimal immunoreactivity. The in vitro serum stability of 89Zr-DFO*-trastuzumab outperformed 89Zr-DFO-trastuzumab. In vivo 89Zr-DFO*-trastuzumab showed comparable tumor targeting and blood kinetics as 89Zr-DFO-trastuzumab, but less uptake in bone-containing organs, skin, liver, spleen, and ileum. This makes DFO* a candidate successor of DFO for clinical 89Zr-immuno-PET.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(GIF 2821 kb)
(GIF 2802 kb)
(PDF 645 kb)
Acknowledgements
The authors thank Carla Gotzmann (University of Zurich, Switzerland) for help towards the preparation of starting material.
Funding
This work was financially supported by the Swiss National Science Foundation (Professorships N° PP00P2_133568 and PP00P2_157545 to G.G and SNSF grant N° 205321–157216 to G.G. and T.L.M), the University of Zurich (G.G), the Stiftung für Wissenschaftliche Forschung of the University of Zurich (G.G), and the Forschungskredit of the University of Zurich (Grant N° K-73532-01-01 to C.M.)
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Contributor Information
Danielle J. Vugts, Phone: +31 20 4445699, Email: d.vugts@vumc.nl
Gilles Gasser, Email: gilles.gasser@chem.uzh.ch.
Thomas L. Mindt, Email: t.mindt@gmx.ch
References
- 1.Van Dongen GAMS, Huisman MC, Boellaard R, et al. 89Zr-immuno-PET for imaging of long circulating drugs and disease targets: why, how and when to be applied? Q J Nucl Med Mol Imaging. 2015;59:18–38. [PubMed] [Google Scholar]
- 2.Verel I, Visser GWM, Boerman OC, et al. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother Biopharm. 2003;18:655–61. doi: 10.1089/108497803322287745. [DOI] [PubMed] [Google Scholar]
- 3.Jauw YWS, Menke-van Der Houwen van Oordt CW, Hoekstra OW, et al. Immuno-Positron Emission Tomography with zirconium-89-labeled monoclonal antibodies in oncology: what can we learn from initial clinical trials? Frontiers in Pharmacol. 2016. doi:10.3389/fphar.2016.00131. [DOI] [PMC free article] [PubMed]
- 4.Evans JB, Syed BA. From the analyst’s couch: next generation antibodies. Nat Rev Drug Discov. 2014;13:413–4. doi: 10.1038/nrd4255. [DOI] [PubMed] [Google Scholar]
- 5.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–8. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 6.Vosjan MJWD, Perk LR, Visser GWM, et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc. 2010;5:739–43. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- 7.Vugts DJ, Visser GWM, Van Dongen GAMS. 89Zr-PET radiochemistry in the development and application of therapeutic monoclonal antibodies and other biologicals. Curr Top Med Chem. 2013;14:446–57. doi: 10.2174/1568026611313040005. [DOI] [PubMed] [Google Scholar]
- 8.Makris NE, Boellaard R, Visser EP, et al. Multicenter harmonization of 89Zr PET/CT performance. J Nucl Med. 2014;55:264–7. doi: 10.2967/jnumed.113.130112. [DOI] [PubMed] [Google Scholar]
- 9.Börjesson PKE, Jauw YWS, Boellaard R, et al. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin Cancer Res. 2006;12:2133–40. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- 10.Verel I, Visser GWM, Boellaard R, Stigter-Van Walsum M, Snow GB, Van Dongen GAMS. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibody. J Nucl Med. 2003;44:1271–81. [PubMed] [Google Scholar]
- 11.Perk LR, Vosjan MJWD, Visser GWM, et al. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur J Nucl Med Mol Imaging. 2010;37:250–9. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen R, Vugts DJ, Stigter-van Walsum M, Visser GWM, Van Dongen GAMS. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-modal fluorescence and PET imaging. Nat Protoc. 2013;8:1010–8. doi: 10.1038/nprot.2013.054. [DOI] [PubMed] [Google Scholar]
- 13.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perk LR, Visser GWM, Vosjan MJWD, et al. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med. 2005;46:1898–906. [PubMed] [Google Scholar]
- 15.Nayak TK, Garmestani K, Milenic DE, Brechbiel MW. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J Nucl Med. 2012;53:113–20. doi: 10.2967/jnumed.111.094169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deri MA, Ponnala S, Kozlowski P, et al. p-SCN-Bn-HOPO: a superior bifunctional chelator for 89Zr immunoPET. Bioconjugate Chem. 2015;26:2579–91. doi: 10.1021/acs.bioconjchem.5b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guérard F, Lee Y-S, Tripier R, Szajek L, Deschamps JR, Brechbiel MW. Investigation of Zr(IV) and 89Zr(IV) complexation with hydroxamates: progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem Commun. 2013;49:1002–4. doi: 10.1039/C2CC37549D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guérard F, Lee Y-S, Brechbiel MW. Rational design, synthesis, and evaluation of tetrahydroxamic acid chelators for stable complexation of zirconium(IV) Chem Eur J. 2014;20:5584–91. doi: 10.1002/chem.201304115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boros E, Holland JP, Kenton N, Rotile N, Caravan P. Macrocycle-based hydroxamate ligands for complexation and immunoconjugation of 89Zirconium for positron emission tomography (PET) imaging. Chem Plus Chem. 2016;81:274–81. doi: 10.1002/cplu.201600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai C, Summer D, Rangger C, et al. Novel bifunctional cyclic chelator for 89Zr labeling-radiolabeling and targeting properties of RGD conjugates. Mol. Pharmaceutics 2015;12-2141-50. [DOI] [PMC free article] [PubMed]
- 21.Price EW, Zeglis BM, Lewis JS, Adam MJ, Orvig C. H6phospa-trastuzumab: bifunctional methylenephosphate-based chelator with 89Zr, 111In and 177Lu. Dalton Trans. 2014;43:119–31. doi: 10.1039/C3DT51940F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deri MA, Ponnala S, Zeglis BM, et al. Alternative chelator for 89Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO) J Med Chem. 2014;57:4849–60. doi: 10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinianow JN, Pandya DN, Pailloux SL, et al. Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) based macrocyclic chelator for 89Zr4+ and its use for immunoPET imaging of HER2 positive model of ovarian carcinoma in mice. Theranostics. 2016;6:511–21. doi: 10.7150/thno.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patra M, Bauman A, Mari C, et al. An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem Commun. 2014;50:11523–5. doi: 10.1039/C4CC05558F. [DOI] [PubMed] [Google Scholar]
- 25.Lindmo T, Boven E, Cuttitta F, Fedoroko J, Bunn PA Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. [DOI] [PubMed]
- 26.Meares CF, McCall MJ, Reardan DT, Goodwin DA, Diamanti CI, McTique M. Conjugation of antibodies with bifunctional chelating agents: isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal Biochem. 1984;142:68–78. doi: 10.1016/0003-2697(84)90517-7. [DOI] [PubMed] [Google Scholar]
- 27.Bansal A, Pandey MK, Demirhan YE, Nesbitt JJ, Crespo-Diaz RJ, Terziv A, et al. Novel 89Zr cell labeling approach for PET-based cell trafficking studies. Eur J Nucl Med Mol Imaging Research. 2015;5:19. doi: 10.1186/s13550-015-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(GIF 2821 kb)
(GIF 2802 kb)
(PDF 645 kb)