Abstract
Introduction
Mediators in pain transmission are the targets of a multitude of different analgesic pharmaceuticals. This review explores the most significant mediators of pain transmission as well as the pharmaceuticals that act on them.
Areas Covered
The review explores many of the key mediators of pain transmission. In doing so, this review uncovers important areas for further research. It also highlights agents with potential for producing novel analgesics, probes important interactions between pain transmission pathways that could contribute to synergistic analgesia, and emphasizes transmission factors that participate in transforming acute injury into chronic pain.
Expert Commentary
This review examines current pain research, particularly in the context of identifying novel analgesics, highlighting interactions between analgesic transmission pathways, and discussing factors that may contribute to the development of chronic pain after an acute injury.
Keywords: Analgesia, Anesthesia, Pain, Pain transmission, Cannabinoids, Opioids, Pain chronicity, Analgesic synergy, Novel analgesics
1. Introduction
Pain is a defining feature of medical pathology and one of the most common reasons for seeking health care services. Subjective pain levels give important insights into the course and severity of medical illnesses. Interestingly, self-reported pain has also been found to be a potent predictor of long-term usage of health care resources [1]. Many pharmacological agents have been devised to reduce the severity of pain. Mediators of pain transmission are a common target of these agents and represent several areas of active anti-nociceptive research. After a patient encounters an aversive stimulus, a sensory signal must be transmitted to the brain before the stimulus can be perceived as painful. Pain transmission refers to the system of mediators, pathways, tracts, and nervous structures that achieve this communication.
In this review, we have critically evaluated high-impact research articles from PubMed that examined the pharmacology of pain transmission to answer the following questions: What are the clinically significant mediators of pain transmission? And, how do pharmacological interventions take advantage of these mediators of pain transmission? Finally, we provided an expert commentary and five-year review of pain transmission research.
2. What are the clinically significant mediators of pain transmission?
Pain transmission includes the nervous tracts and synapses that relay a nociceptive signal from the periphery to the brain. The transmission system includes the mediators that propagate signals between neurons. Pharmacologically, these mediators and receptors underlie many of the analgesics in the pharmacopeia. The key mediators involved in the pain transmission process are summarized in Table 1 and are discussed below. The role of the mediators mentioned in Table 1 is critically discussed in the following sections.
Table 1.
Summary of Key Pain Mediators and their receptors in Pain Transmission
| Transmission Mediator | Abbreviation (if applicable) | Examples (if applicable) | Receptor(s) |
|---|---|---|---|
| Adenosine | ADO | A1, A2A, A2B, A3 (or ADORA 1, 2A, 2B, and 3); P2X3 | |
| Bradykinin | BK | B1 and B2 Receptors, TRPV1 (also called vanilloid) | |
| Cytokines | Various | TNF-α | Receptor names are specific to the cytokines. For example: IL-1β receptor is IL-1R1 |
| IL-1β | |||
| IL-6 | |||
| Calcitonin Gene-Related Peptide | CGRP | Calcitonin-like receptor and CGRP receptor (G-protein-coupled 7 transmembrane) | |
| Cannabinoids | CB1, CB2, WIN, abn-CBD | ||
| Eicosanoids | Various | Leukotrienes (LT) | EP (for PGE2), IP (PGI2), DP (PGD2), TP (TXA2) and others |
| Prostaglandins (PG) | |||
| Prostacyclins (PGI) | |||
| Thromboxanes (TX) | |||
| Endogenous Opioids | Endorphins | Opioid Receptors: Mu, Kappa, and Delta; FQ (NOP/ORL Receptors) | |
| Enkephalins | |||
| Dynorphin | |||
| Excitatory Amino Acids | GLU or E (glutamate) | Glutamate (most important) | NMDA, AMPA, Kainate receptors and metabotropic glutamate receptors |
| ASP or D (aspartate) | Aspartate (role as transmitter is debated) | ||
| γ-aminobutyric acid | GABA | GABA-A, GABA-B, and GABA-C receptors | |
| Glycine | GLY or G | NMDA, α3-glycine receptors, other glycine receptors (GlyRα and GlyRβ) | |
| Histamine | HIST | H1, H2, H3, H4 Receptors | |
| Low pH | H+ | ASIC (acid sensing ion channels), TRPV1 (vanilloid). | |
| Nerve Growth Factor | NGF | TrkA [high affinity]; p75 [low affinity] | |
| Neuropeptide Y | NPY | NPY receptors Y1, Y2, Y4, and Y5 | |
| Nitric Oxide Synthase | iNOS | Nitric Oxide (NO) | Guanylate Cyclase |
| Norepinephrine (α2 agonists) | Norepior NE | α-adrenergic receptors, particularly α2. | |
| Reactive Oxygen Species | ROS | Various | Intracellular Proteins |
| Serotonin | 5-HT | 5-HT Receptors examples: 5-HT2A and 5-HT2C. | |
| Tachykinins/Neurokinins | Neurokinin A | Neurokinin 1, 2, and 3 Receptors (with differing affinities). | |
| Substance P (SP) | |||
| Vasoactive Intestinal Polypeptide | VIP | PAC1, VPAC1 and VPAC2. |
3. How do pharmacological interventions take advantage of pain transmission mediators?
This review critically evaluated the analgesic agents that use these transmission pathways involving key mediators, as shown in Table 1. Specifically, the effects of the following transmission mechanisms are evaluated: adenosine and adenosine agonists, bradykinin and bradykinin agonists, calcitonin gene-related peptide, cannabinoids, eicosanoids, endogenous opioids and opioid agonists, γ-aminobutyric acid, glutamate antagonists, glycine, histamine, nerve growth factor, neuropeptide Y, nitric oxide, norepinephrine, serotonin, tachykinins and neurokinins, and vasoactive intestinal polypeptide.
3.1 Adenosine and adenosine agonists
Adenosine is the endogenous molecule that activates a variety of receptors including A1, A2A, A2B, A3, and P2Y receptors, with A1 and A2A, A3, and P2Y being particularly applicable to pain transmission research [2]. Through these G protein-coupled receptors, cyclic adenosine monophosphate (cAMP) is either increased (A2A, A2B) or decreased (A1, A3), resulting in changes in the release of excitatory and inhibitory neurotransmitters. Due to ubiquity of adenosine in the body, pain-specific induction of these pathways has been judged clinically impractical by few investigators [3]. However, others have identified compounds that could provide clinically efficacious pain relief via activity at specific adenosine receptors. Imlach et al [4], for example, recently identified a specific, positive allosteric modulator for the A1 receptor. They observed that many adenosine agonists have systemic activity; however, an A1-specific receptor agonist was found to show increased activity at primary afferent synapses [4]. In a rat model of neuropathic pain, the A1-specific adenosine agonist increased adenosine, gamma amino butyric acid (GABA), and glycine in the dorsal horn [4]. Ford et al [2] observed that activation of A3 receptors has analgesic effects that are unrelated to the A1 or A2A receptors and cause fewer side effects. These results suggest that specific adenosine agonists could have activity in relieving neuropathic-related pain.
Similarly, Otsuguro et al [5] suggested that inhibitors of adenosine kinase could find clinical use in relieving pain. Adenosine kinase is involved in reactions that transform adenosine into other products, particularly AMP. While many adenosine kinase inhibitors have adverse effects, temporary use of adenosine kinase inhibitors in a rat model could provide significant analgesia by increasing local adenosine concentrations [5]. Otsuguro et al [5] did observe a “time-dependent” relationship between adenosine kinase inhibitors: longer use corresponded to more significant side effects.
Theoretically, caffeine would complicate any effort to use adenosine agonists clinically. Caffeine, an adenosine antagonist, is broadly used in the society and could theoretically attenuate the activity of adenosine agonists. Thus, though caffeine, like other methylxanthines, has the potential to affect pain pathways, regular consumption may negate any measurable clinical effect it might have [6]. Interestingly, despite antagonism of adenosine receptors, caffeine has been found to potentiate pain-relieving drugs. Derry et al [7] conducted a review of 20 studies that have evaluated the efficacy of caffeine as an adjuvant to analgesics, and found a significant, albeit small, benefit to adding caffeine to analgesic preparations. Interestingly, this benefit was not dependent on the primary analgesic or root cause of the pain [7].
Currently, agonists of adenosine A3 receptors represent a relatively new and promising avenue in pain research. One study that evaluated the activity of a specific adenosine A3 receptor agonist noted that, in a rodent model of neuropathic pain, A3 agonism produced powerful analgesia [8]. This effect disappeared in A3 receptor knockout mice [8]. Equally interesting, mice without neuropathic pain were not observed to have changes in nociception in response to A3 agonists, demonstrating activity at A3 receptors to have potential for neuropathic pain-specific therapy [8]. In another study that probed paclitaxel-induced chronic neuropathic pain, A3 agonists were noted to block the process of chemotherapy-induced neuropathic pain without affecting the anti-neoplastic properties of paclitaxel [9]. A3 agonists were noted to work by inhibiting NADPH oxidase and by modulating redox-dependent pathways, including glutamine transport via GLT-1 and glutamine synthetase [9, 10]. Importantly, part of the efficacy of A3 agonists may be related to its effect on GABA signaling (via GAT-1) or enhancement of chloride anion gradients via activity at KCC2 [10]. Based on these studies, it is likely that adenosine agonists have both an intrinsic capacity for controlling pain and efficacy via interactions with other pain mediators.
While managing side effects and reconciling societal consumption of adenosine antagonists could make the clinical use of adenosine agonist pain-relievers difficult, new research suggests a potential role for adenosine agonists, particularly A3 agonists, and adenosine kinase inhibitors in the pharmacopeia.
3.2 Bradykinin and bradykinin agonists
Bradykinin plays a role in cardiovascular function, inflammation, and homeostasis [11]. Within the kinin family, bradykinin has been shown to be particularly important in mediating pain and inflammation [12]. Its effects are mediated though cell surface receptors, including the Bradykinin B1 and B2 G-protein coupled receptors [12]. Aspirin has been shown in recent studies to reduce the affinity of the B2 receptor for bradykinin by accelerating its dissociation rate, which could potentially account for some of its analgesic effect [12].
Although there is evidence to suggest that B1 and B2 receptors are involved in the inflammatory pain response, there have been relatively few human studies evaluating compounds that could mediate analgesia by acting on bradykinin receptors [11]. Similarly, while this area represents a potential source of novel analgesics, few clinical trials examining the role of bradykinin in pain have been conducted in the last ten years.
Interestingly, injection of bradykinin is a common practice for inducing pain in research models. Surprisingly, relatively little research has been done to evaluate the mechanism by which bradykinin induces pain in test subjects [13]. While many new research studies use agonism of bradykinin receptors to induce pain, there is a paucity of new research evaluating ways to antagonize bradykinin receptors to alleviate pain. Given this observation, bradykinin receptor antagonism represents an interesting corridor in pain research.
3.3 Calcitonin gene-related peptide (CGRP)
Calcitonin gene-related peptide (CGRP) has long been recognized as a potent vasodilator. Recent studies, however, have highlighted its role in wound healing [14], involvement in migraine headaches [15], and its involvement in pain and inflammation [16]. Its roles in pain and inflammation are particularly pertinent to this review, especially as it pertains to migraine.
Hansen et al [17] demonstrated that CGRP injection could induce migraines with aura in study participants. Conversely, Marcus et al [18] showed that selective antagonism of CGRP receptors was superior to placebo in providing relief to patients with severe migraine. Interestingly, the CGRP antagonist chosen by Marcus et al [18] was known to lack vasoconstrictive properties, making its therapeutic action more likely to be the result of effects on the pain pathway than vasoconstriction. Finally, Greco et al [19] found CGRP to mediate analgesia in a rodent model of hyperalgesia. Specifically, Greco et al [19] administered nitroglycerin, a vasodilator known to induce migraine-like pain, to rats. Nitroglycerin injection was followed by administration of CGRP antagonists [19]. The CGRP antagonist was found to decrease the pain response of the rats by reversing the effects of nitroglycerin [19].
It was previously thought that CGRP had pro-inflammatory properties; however, recent research suggests that CGRP does not induce inflammation [16]. In fact, Romero-Reyes et al [16] showed that, in mice, CGRP receptor antagonists completely failed to induce inflammation. It is worth noting that Romero-Reyes et al [16] identified a reduction in the pain-related behaviors of mice that were treated with CGRP receptor antagonists [16].
Though clinical trials of some CGRP antagonists (like Tolcegipant) have not demonstrated measurable clinical benefit as migraine treatments, CGRP receptor antagonists continue to show potential as anti-nociceptive agents that function without directly affecting inflammatory pathways [20].
3.4 Cannabinoids
While the use of smoked marijuana remains controversial, cannabinoid-based pharmaceutical interventions are becoming more common in certain clinical settings. Moreover, cannabinoids represent an important frontier in analgesic research. The mechanism of action of cannabinoids in the process of pain transmission is summarized in Figure 1.
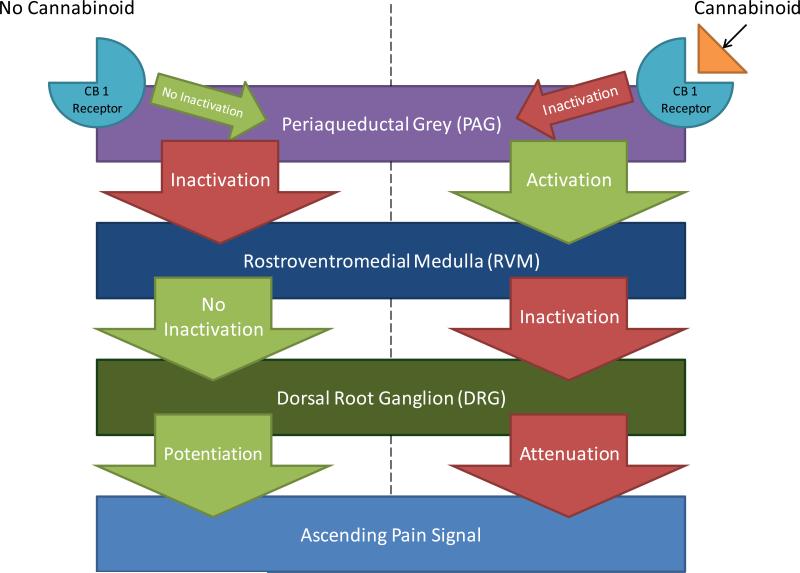
Figure 1. Action of Cannabinoid in Descending Modulatory Tracts.
Activation of CB1 receptors that are present on the periaqueductal grey (PAG) leads to PAG inhibition. The PAG tonically inhibits the action of the rostroventromedial medulla (RVM), which in turn weakens pain transmission through the dorsal root ganglion (DRG) via descending modulatory fibers. Attenuation of the pain circuitry of the DRG decreases nociception, and ultimately, the perception of pain. Cannabinoids, then, work by inhibiting the inhibitor of a nociceptive inhibitor. In the absence of cannabinoids, the PAG inactivates the RVM, preventing its inhibitory action on the DRG. The net result is a relatively higher level of nociceptive signaling.
Cannabinoids may be useful because of their analgesic properties as well as the effects they exert on other analgesics. For example, Altun et al [21] conducted studies that evaluated the effect of cannabinoid receptor agonists on opioids. Using a model that gauged pain in morphine-tolerant rats, Altun et al [21] found that activating CB1 and CB2 (cannabinoid receptors) increased the anti-nociceptive effect of morphine in rats that were exposed to a hot plate. Activating cannabinoid receptors was also found to mediate analgesia [21]. Thus, it was proposed that cannabinoid receptors play a role in mediating tolerance to opioids. Similarly, Pecina et al [22] found that genetic variants with different activity of fatty acid amine hydrolase (FAAH), an enzyme that breaks-down cannabinoids, were found to have a potentiated response to placebos. In other words, the effect of endogenous opioids (mediators of the placebo effect) was increased by cannabinoids [22]. FAAH has been identified as an interesting way to treat pain via endogenous cannabinoids. For example, Pawsey et al [23] noted that phase 1 trials of an FAAH inhibitor demonstrated pain syndrome treatment with a very tolerable side effect profile. Manipulation of endogenous cannabinoids, rather than supplementation with exogenous cannabinoids alone, may become an important avenue for providing cannabinoid-mediated analgesia.
In another study, Cooper et al [24] compared the analgesic effects of Dronabinol (oral cannabinoid) to smoked marijuana, and found that, relative to placebo, both smoked marijuana and Dronabinol produced analgesia and increased pain tolerance (in response to the cold pressor test). The effect of Dronabinol to mediate longer-term analgesia was much more significant than smoked marijuana and carried lower risk of abuse-related effects [24]. Cooper et al [24] also noted that the analgesic effects and potential side effects of cannabinoids differ depending on the patient's history of marijuana exposure. These findings support the use of cannabinoids as analgesics. This statement is bolstered by the concept of cannabimimetic compounds such as N-palmitoyl-ethanolamine that has shown analgesic properties [25]. Their greatest usefulness is likely to be in a chronic, rather than acute, pain setting. This suggestion is further evidenced by the work of Ostenfeld et al [26] who showed that cannabinoids failed to demonstrate a significant analgesic benefit in patients with acute dental pain.
Wilsey and co-investigators [27] studied the effect of different doses of vaporized cannabinoids in patients with neuropathic pain. When compared to placebo, both low and medium doses of vaporized cannabinoids provided pain relief to the test subjects with either absent or tolerable side effects [27]. These findings support the use of cannabis for treating neuropathic pain and advocate for better quality control and standardization in cannabis production. Johnson et al [28] made similar findings about the efficacy of cannabinoids in treating chronic pain. Specifically, these investigators found significant effect of cannabinoids in treating patients with opioid refractory, cancer-induced pain. Johnson et al [28] further observed that the efficacy of the cannabinoid treatment had tolerable side effects and did not lose efficacy over long-term use; patients using the study medication long-term were unlikely to seek dosage increases.
Cannabinoids may also have clinical use in preventing an acute injury from developing into chronic pain [29 - 31]. Alkaitis et al [29] used a rat model to demonstrate that post-operative administration of cannabinoid antagonists resulted in significantly increased hypersensitivity and allodynia when compared to the control group [29]. Importantly, this effect persisted even after discontinuation of the cannabinoid antagonists [29]. Moreover, the group of rats treated with the cannabinoid antagonists had associated increases of glial fibrillary acidic protein (GFAP) in the dorsal horn. Increased GFAP expression is associated with the development of chronic pain states, suggesting that one possible mechanism by which cannabinoids decrease pain chronification is by limiting protein expression in spinal astrocytes. Landry et al [30] demonstrated that a possible mechanism involves the MAP kinase (MAPK) pathway. Persistently, elevated levels of spinal MAPK have been associated with chronic pain states and, in this study, it was established that activation of CB2 receptors leads to increased activity of MAPK phosphatases and corresponding decreases in spinal levels of MAPK. These two mechanisms were also supported in a similar study completed by Paszcuk et al [31]. The proposed mechanism by which cannabinoids may diffuse the process of pain chronicity is summarized in Figure 2. However, more work is warranted to elucidate the process by which cannabinoids influence pain chronicity.
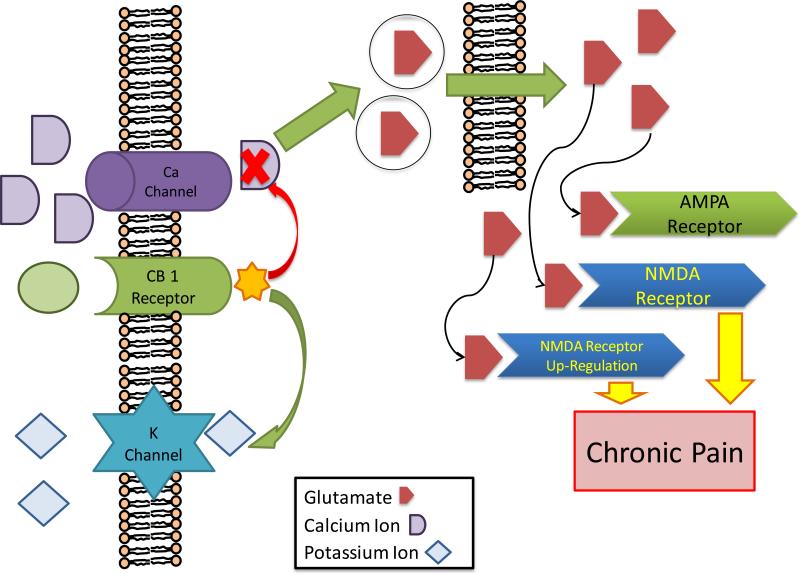
Figure 2. Effect of Cannabinoid on Pain Chronicity.
Cannabinoids are derived from arachidonic acid, and cannabinoid receptors are expressed in many nociceptive pathways in the central nervous system and peripheral nervous system. When cannabinoid receptors are bound by cannabinoids they cause an inhibition of of presynaptic calcium uptake, and an increase in inward-rectifying potassium channel uptake of potassium. Calcium is needed for vesicular release of glutamate, thus this change in ion concentration leads to a decrease in glutamate release from presynaptic neurons. This is one proposed mechanism that gives cannabinoids their anti-nociceptive effects. This inhibition of glutamate release may be important for preventing the formation of a chronic pain pathway by preventing overstimulation of NMDA receptors. This model also supports the observation that cannabinoids have analgesic synergy with the opioid pathway.
A body of literature (described above) seems to mechanistically support the use of cannabinoids in pain treatment; however, a recent systematic review and meta-analysis of randomized double-blind trials questions their clinical utility [32]. In fact, based on this meta-analysis, the Neuropathic Pain Special Interest Group (NeuPSIG) recommends against the use of cannabinoids in neuropathic pain. This recommendation is related to risk of mental illness, misuse, and negative trials. Although relatively few cannabinoid-related trials were included in the analysis, the majority failed to positively treat neuropathic pain with some even demonstrating negative outcomes [32].
Another avenue that needs to be addressed is related to dose-efficacy of cannabinoids. A controlled trial by Portenoy et al [33] was designed to partially address this area. Here, opioid-treated cancer patients with chronic pain were randomized into three dosage groups and treated with oro-mucosally delivered cannabinoid preparations (nabiximols). Nabiximols are fixed ratio formulations of tetrahydrocannabinol (THC) and cannabidiol (CBD). Primary outcomes included overall subjective measurements of pain as well as alterations in fixed-dose or breakthrough opioid use. Analysis of the primary outcomes, combined dose groups versus placebo, revealed nonsignificant relief in baseline pain [33]. Interestingly, however, analysis of the 2 lower dose groups did reveal significant reductions from baseline pain. This study suggests that there may be a dosage ceiling on the potential analgesic properties of cannabinoid (nabiximol) agents.
While controversial in some settings, a significant body of literature describes the interactions of cannabinoids in pain transmission pathways (see Figure 1). Despite the controversy, cannabinoids may represent an important source of novel treatments for the management of clinical pain and may potentially be useful for avoiding the development of chronic pain states (see Figure 2). Whether for increasing the effects of opioids, managing opioid tolerance, treating neuropathic pain, or treating chronic pain, cannabinoids possess desirable analgesic qualities. Because marijuana includes more than 60 identified cannabinoids (e.g., THC, CBD), it is likely that marijuana has an “entourage effect.” Therefore, as was the case in the work by Portenoy et al [33], carefully controlled studies that either acknowledge the “entourage effect” or rigorously control individual cannabinoids or cannabinoid ratios are needed. Additional research will further refine and elucidate the current understanding of cannabinoid pain transmission pharmacology.
3.5 Eicosanoids
Eicosanoids are a class of signaling molecules that include hydroperoxy fatty acids, leukotrienes, prostaglandins, and thromboxanes [34, 35]. During an inflammatory event, arachidonic acids are released and subsequently converted into eicosanoids by enzymes such as cyclooxygenase (COX) -1 and COX-2, the cytochrome P450 family, and lipoxygenase [35]. Non-steroidal anti-inflammatory drugs (NSAIDs), which are the most widely used analgesics, decrease inflammatory pain via inhibition of COX and a subsequent reduction in prostaglandin production [35]. These drugs can be quite effective, particularly in an inflammatory setting; COX-2 production of prostaglandins has been shown to be a major driver of inflammatory pain [35].
Prostaglandins are involved in the regulation of many physiologic functions including vascular tone, platelet aggregation, gastrointestinal motility, and the inflammatory response [36]. Prostaglandin E2 (PGE2) is a particularly important mediator of both acute inflammatory pain and chronic neuropathic pain [37]. PGE2 acts on the G-protein coupled receptors that excite nociceptive neurons, sensitizing them to the effects of pain mediators (including ATP, bradykinin, and capsaicin) [37]. Additionally, as discussed below, PGE2 inhibits the anti-nociceptive properties of glycine in the spinal cord.
While typically linked to acute inflammatory pain, there is evidence that PGE2 is an important mediator in the chronification of pain. St-Jacquesa et al [37] compared the effects of short- and long-acting PGE2 analogs on pain chronicity in rats. Injection of a long acting form of PGE2 (dmPGE2) produced allodynia that lasted significantly longer than the short-acting form [37]. Interestingly, the length of the allodynia increased at an accelerating, non-linear pattern with subsequent injections of dmPGE2 [37]. This result suggests that repeated or prolonged exposure to PGE2 facilitates long-term allodynia, an effect that is analogous to chronic pain [37]. The proposed time-dependent effect in PGE2-induced pain chronicity is summarized in Figure 3. More work is needed to determine the specific role of PGE2 in the chronification of pain. Similarly, more research is necessary to evaluate the potential for NSAID-use to avert pain chronicity in those that have suffered acute injury.
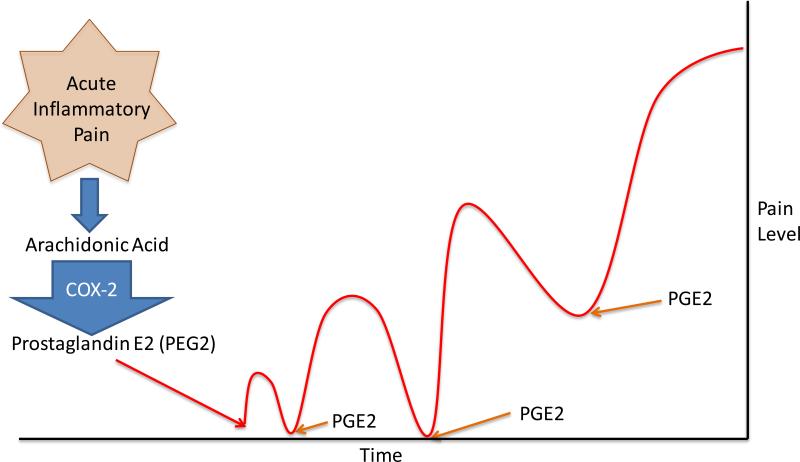
Figure 3. The role of PGE2 in Pain Chronicity.
Recent research has suggested that prolonged and/or repeated exposure to Prostaglandin E2 (PEG2) can lead to the development of chronic pain. Researchers have compared the effects of short- and long-acting PGE2 analogs on pain chronicity in rats. An injection of a long acting form of PGE2 (dmPGE2) produced allodynia that lasted significantly longer than the short-acting form. Repeated injections of dmPEG2 lead to an increased and prolonged pain response. This result suggests that repeated or prolonged exposure to PGE2 facilitates long-term allodynia, an effect that is analogous to chronic pain. Researchers have suggested the adequate pain control with PEG2 inhibitors (such as non-steroidal anti-inflammatory) can help prevent the development of chronic pain.
COX inhibitors, including ibuprofen, aspirin, and naproxen, non-selectively inhibit COX-1 and COX-2, causing a reduction in inflammatory eicosanoids. Because eicosanoids work in pathways other than inflammation, the action of NSAIDs can lead to significant side effects [33, 38, 39]. In an effort to reduce unwanted off-target effects, selective COX-2 inhibitors were developed [38]. Despite being excellent inhibitors of inflammatory eicosanoid and prostaglandin production, studies showed that rofecoxib (a COX-2 inhibitor) led to increased risk of adverse cardiovascular events (including stroke and myocardial infarction) [33, 38, 39]. Increased risk of cardiovascular events is believed to be due to the selective inhibition of endothelial prostacyclin (PGI2; a potent vasodilator) and the unopposed production of thromboxane A2 by COX-1 (an activator of platelet aggregation and vasoconstriction) [33, 38, 39]. Thus, despite their efficacy in controlling inflammatory pain, rofecoxib was removed from the market in 2004 [38] and other –coxibs have the distinction of carrying a black box warning for cardiovascular risk.
Recently, the Coxib and traditional NSAID Trialists’ Collaboration (CNTC) conducted a meta-analysis that compared traditional NSAIDs to COX-2 inhibitors. The goal of the analysis was to evaluate the cardiovascular and gastrointestinal risk of both traditional NSAID (tNSAIDs) medications and selective COX-2 inhibitors (coxibs) [40]. This analysis showed that high doses of some tNSAIDs had cardiovascular risks that were indistinguishable from the coxib drugs [40]. Their analysis also showed that tNSAID medications increased the risk of heart failure and upper gastrointestinal complications by 2-4 fold. Coxibs, like tNSAIDs, increased cardiovascular risk; however coxibs had fewer gastrointestinal complications than tNSAIDs [40]. Interestingly, CNTC identified no increased risk of stroke in any of the tNSAIDs or coxibs studied [40]. This meta-analysis suggests that COX-2 inhibitors may have a more advantageous risk-benefit ratio than most other NSAIDs.
Nonetheless, NSAIDs remain the most frequently used analgesics. While there is ongoing discussion surrounding potential NSAID side effects and the potential clinical use of COX-2 specific inhibitors, inhibition of eicosanoids remains a key target site to intervene and alleviating acute and chronic pain.
3.6 Endogenous opioids and opioid agonists
While volumes could be written about the analgesic action of opioids, the main objective of this article is to summarize some of the recent studies into their pain-relieving properties, limitations, and interactions with other pain pathways. Agonists of opioid receptors are well-known for their ability to modulate pain. Recently, Mizoguchi et al [41] noted cessation of pain-associated behaviors in rats after the infusion of endomorphins. Specifically, after acid-sensing ion channel agonists were administered to the rats (inducing pain) it was found that agonism of μ-opioid receptors inhibited spinal pain transmission in the subjects [41].
While widely used and highly valued for their pain-relieving properties, opioids carry negative side effects including respiratory depression and adverse gastrointestinal effects. However research is being conducted to find solutions to these adverse events. Dual opioid therapy, for example, has been shown to decrease rates of gastrointestinal effects compared to mono-opioid therapy. In one study, patients treated with a combination of morphine-oxycodone were compared to patients treated with oxycodone-acetaminophen following total knee arthroplasty [42]. Patients in the dual opioid group reported a 15% rate of adverse gastrointestinal effects compared to a 50% rate in the oxycodoneacetaminophen group [42]. A similar study in patients following bunionectomy has shown comparable results [42]. Together, these studies suggest that dual opioid therapy may have advantages postoperatively in treating pain while reducing side effects [43]. Similarly, Tapentadol is a μ-opioid agonist and norepinephrine reuptake inhibitor that has been found to have fewer gastrointestinal effects, such as constipation and vomiting [44]. Imanaka et al [44] found that Tapentadol was able to induce pain control in 84% of patients with terminal cancer-related pain according to self-reported scores [44]. The majority of the un-controlled patients did report some degree of improvement in their condition on Tapentadol [44]. Due to an improved side effect profile and good analgesic efficacy, it may be clinically appropriate to consider using Tapentadol before traditional opioid agonists whenever possible.
Traditional opioid medications exert their action through μ-opioid receptors with seven transmembrane domains. Recent research has confirmed the existence of splice variants of the seven transmembrane domain receptors, specifically six transmembrane μ-opioid receptors [45]. Importantly, a new opioid medication, 3-iodobenzoyl-6β-naltrexamide (IBNtxA), works exclusively through the six transmembrane domain receptor [45]. One study found that IBNtxA achieves analgesia without the characteristic opioid medication side effects of respiratory depression and physical dependence [45]. Accordingly, developing new drugs that act on μ-opioid receptors with six (rather than seven) transmembrane domains may lead to opioid analgesics that achieve pain relief with neither respiratory depression nor addiction potential.
Tramadol has activity as an opioid agonist, norepinephrine re-uptake inhibitor, and serotonin re-uptake inhibitor [46]. As it has been noted to cause nausea and vomiting, particularly in a post-operative setting, it has been used less than morphine post-operatively [46]. Pang et al [46] combined Tramadol with Metoclopramide (anti-emetic and analgesic) and found the efficacy of the combination to be equal to morphine with comparable side effects. Pang et al [46] proposed the combination of Tramadol and Metoclopramide to be a suitable substitute for patients that cannot receive morphine.
Another well-known complication of chronic opioid use is the development of tolerance and hyperalgesia. It has been shown that when ultra-low dose naloxone is administered with morphine it may restore the analgesic effects of morphine [47]. Recently studies have begun to elucidate the mechanism by which this occurs. One study implicated the involvement of microglial activation in the development of morphine-induced hyperalgesia [48]. Microglial cells are thought to cause abnormal pain signaling following injury as they activate and secrete inflammatory cytokines. In this study, the authors demonstrated that ultra-low dose naloxone resulted in decreased microglial activation and inflammation via an opioid-receptor independent mechanism [48]. Lin et al [49] found that ultra-low dose naloxone combined with morphine injected intrathecally into rats was associated with an increase in IL-10 [49]. Injecting recombinant rat IL-10 with morphine alone restored sensitivity to morphine analgesia [49]. Thus it is likely that one mechanism by which ultra-low dose naloxone resensitizes rodent test subjects to morphine is via upregulation of IL-10 expression and decreased neuroinflammation. The development of drugs that inhibit microglial cell activation may yield exciting combination medications when added to opioids for the treatment of chronic pain.
Interestingly, research has shown the existence of dimerization between different opioid receptors and between opioid receptors and other pain receptors. Jordan et al [50], for example, demonstrated dimerization of δ and κ opioid receptors. Other combination receptors include μ-nociception receptor (NOP, previously named opioid-receptor like-1 receptor) and μ-NK1 receptors [50, 51, 52]. One study showed that, when compared to μ-opioid receptor 1, μ-NK1 receptors showed a distinct pathway of receptor internalization and delayed re-sensitization following exposure to NK1-selective ligands [52]. This suggests a possible role for NK1 receptors in developing tolerance to opioid medications when coupled with μ-opioid receptors as a heterodimer. Recently, hybrid molecules have been developed to take advantage of these dimers with the aim of improving opioid pain control. In one study, a hybrid was made from an opioid pharmacophore and an NOP ligand [53]. When compared directly to morphine, this hybrid showed later onset of action, but longer acting pain relief in a rodent pain model [53]. Opioid receptor dimers and associated hybrids provide a new area within opioid pain research. These agents have the potential to generate efficacious analgesics, minimize opioid side effects, and may shed light on the mechanisms of opioid tolerance and addiction.
Research suggests that long-term opioid use can lead to epigenetic changes. Doehring et al [54] found that, compared to non-addicted controls, opioid addicts have changes in DNA methylation in OPRM1, the gene that encodes the mu opioid receptor. In addition, patients treated for pain with opioid agonists for at least one year were found to have increased DNA methylation in the OPRM1 gene compared to non-opioid treated patients [54]. It is speculated that the correlation between DNA methylation and opioid use is associated with decreased μ-opioid receptor transcription. Similarly, Chao et al [55] found that epigenetic modification of the brain-derived neurotrophic factor (BDNF) gene in dorsal root ganglion (DRG) neurons following repetitive morphine exposure was associated with the development of hyperalgesia in rats. Specifically, methylation of an exon promoter in the BDNF gene was decreased following repeat morphine administration leading to an increased expression of BDNF in DRG neurons which correlated with increased nociceptive behavior in rats [55]. The increased response to pain was mitigated with administration of anti-BDNF IgG [55]. In addition, administration of 5-aza-2′-deoxycytidine, an inhibitor of DNA methylation, also increased BDNF expression in DRG neurons and was associated with the development of pain hypersensitivity in the rats [55]. The findings of these studies support epigenetic causes of opioid resistance in response to chronic opioid administration. New therapies that manipulate epigenetic processes may have the potential to increase opioid effectiveness via changes in opioid receptor density.
In addition to decreased effectiveness with chronic use, many clinicians have noticed that the effect of opioids is attenuated by the presence of nerve injury. This observation is validated and supported by the research. For example, one study tested the expression of μ-opioid receptors in rats. In this study, the rats were subjected to nerve injury via spinal nerve ligation [56]. Importantly, in response to nerve injury (spinal nerve ligation), the expression of both μ-receptor mRNA and mu receptor-related proteins decreased [56]. More recently, a group has managed to characterize and describe these changes [57]. This study observed that down-regulation of mu opioid receptors occurs in the dorsal root ganglion, but not the spinal cord [57]. The authors of this study also used mice with a knockout Ehmt2 (a histone methyltransferase) and observed that the knockout mice did not experience nerve-injury related resistance to opioid therapy [57]. This finding suggests a possible epigenetic cause of nerve injury related opioid resistance. Other research suggests that early management of nerve injury-related pain with opioid agonists prolongs chronic pain and may in fact interfere with endogenous pain mediators. In one study rats were treated with sciatic chronic constriction injury (CCI) or sham injury followed by treatment with morphine [58]. Rats treated with morphine showed a significantly longer duration of lower pain threshold in both the CCI and sham group, with much larger effects noted in the CCI group [58]. Thus, it is theoretically possible to prolong conditions of chronic pain with early introduction of opioid medications.
While opioids represent the gold standard for pain relief, the authors note that the majority of recent articles on opioid analgesia focus on management of side effects and addiction, as well as finding suitable non-opioid substitutes. A summary of the studies investigating the use of other drugs to modulate opioid effectiveness, addiction, or side effects is shown in Figure 4. Opioid drugs provide powerful analgesia; however, the potential for addiction, tolerance, overdose, and other negative effects hinder their utility, demonstrating the need for development of other classes of analgesics.
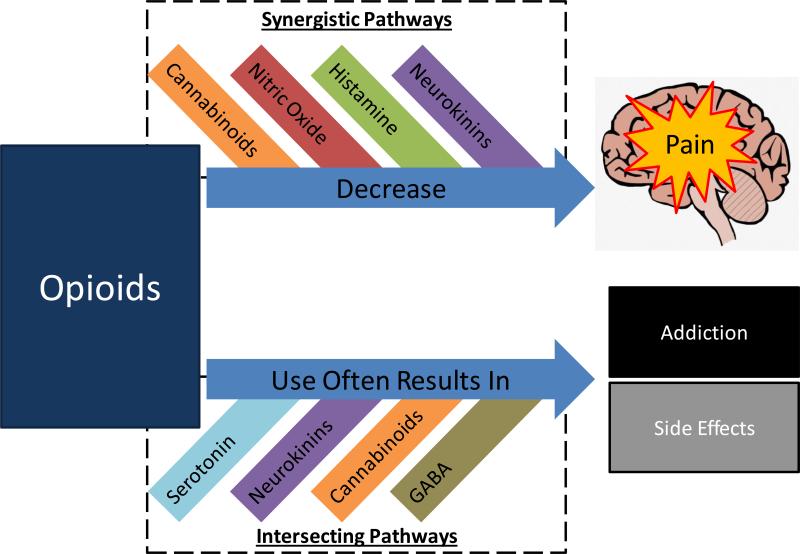
Figure 4. Interactions in the Opioid Pathway.
A variety of pain pathways intersect with the opioid pain pathway. While this figure is not necessarily comprehensive, these “intersections” represent important research targets. For example, histamine agonists have been shown to increase the efficacy of opioid analgesics. Could histamine agonists and opioids be combined into an analgesic with increased efficacy? One downside of opioids includes the potential for significant side effects, including addiction. For pathways that intersect in the processes of side effects, can these pathways be used to alleviate side effects or decrease the chances of addiction? Interactions between pain pathways, particularly the opioid pathway, represent an important area for providing clinical pain relief to patients that are experiencing significant pain with lower risk of adverse outcomes.
3.7 Gamma aminobutyric acid (GABA)
GABA is the most widely distributed inhibitory neurotransmitter in the CNS and plays an important role in CNS nociception. The inhibitory action of GABA in maintaining subthreshold membrane potentials includes inhibition of pain transmitting fibers: activation of both GABAa and GABAb receptors is linked to decreases in nociceptive signaling.
The current GABA agonists in the pharmacopeia (e.g., benzodiazepines) are not typically clinically used for analgesia primarily due to potent sedative-hypnotic profiles. Because sedation is primarily produced by activation of GABAa-α1 receptors, it follows that work related to subunit selective GABA agonists with different pharmacologic profiles would attract significant attention [59]. The potential for GABAergic analgesic development is bolstered by the identification of distinct GABAa subunit populations in the dorsal horn of the spinal cord and on primary afferent neurons [60]. Significant work has been performed in this area. As an example, researchers have described the GABAa-α5 selective actions of NS11394 relative to a number of therapeutic areas including inflammatory and neuropathic anti-nociception in rat models [61, 62, 41]. In response to nociceptive stimuli, they demonstrated decreased activity of pain transmitting C-fibers, nociceptive spinal nerves, and possibly also central circuits [61, 62]. Unlike typical benzodiazepines, these agents are able to produce significant analgesia without producing sedation. Ultimately, such an agent would have tremendous clinical potential. GABA remains effective until sodium chloride dependent GABA transporters (GAT) remove GABA from the synaptic cleft. Four subtypes of GATs have been described (GAT-1, GAT-2, GAT-3, and BGT-1). Some researchers have evaluated the effects of increasing GABA by decreasing the activity of GATs. Two such studies demonstrated a decreased sensitivity to pain in GAT-1 knockout rats [63, 64]. Additionally, both of these studies showed that rats with overexpression of GAT-1 experienced hyperalgesia [63, 64]. Furthermore, the role of GAT antagonism in the treatment of human neuropathic pain is enhanced by the off-label use of the anticonvulsant drug, tiagabine [65]. These studies are conclusive: increased GABA in pain transmission systems leads to decreased pain sensation. Conversely, decreased GABA in pain transmission systems contributes to hyperalgesia.
A study by Kataoka et al [66] investigated the role of GAT-3 by administering differing doses of a GAT-3 inhibitor to different, rodent-based pain models. Specifically, Kataoka et al [66] applied thermal, mechanical, and chemical stimuli to induce pain in the subjects, followed by administration of a GAT-3 inhibitor. The result was an increase in the withdrawal threshold (indicative of pain) in the thermal pain model without an observed change in the rat's response to mechanical pain [66]. Interestingly, while GAT-3 inhibition did not affect chemically-induced pain in the early model, it was observed to inhibit the nociceptive response to persistent chemically-induced pain [66]. Katoaka et al [66] concluded that GAT-3 inhibitors could have utility in treating pain in clinical practice, especially in the setting of acute thermal injury and chronic neuropathic pain.
Because the physiologic action of GABA is the result of receptor-binding and ion flux, factors that affect ion (e.g., chloride) gradients influence the strength of GABA action. Relevant to the discussion of neuropathic pain transmission are the actions of two chloride cotransporters within the spinal cord: Na+-K+-2Cl− cotransporter (NKCC1) and K+-Cl− cotransporter (KCC2). NKCC1 serves to transport chloride into the cell, thus decreasing the chloride gradient and decreasing GABA action [67]. Conversely, KCC2 transports chloride out of cells effectively increasing the chloride gradient allowing for a more robust GABA response [67]. In the context of neuropathic pain, increased NKCC1 or decreased KCC2 activity results in enhanced pain transmission due to less profound GABAergic neurotransmission [67]. These physiologic pathways reveal an interesting avenue for anti-nociception research. The human diuretic bumetanide, a known NKCC1 antagonist, when intrathecally administered to rats with capsaicin-induced neuropathic pain, caused the subjects to exhibit less neurogenic inflammation and hyperalgesia [68]. While mechanistically interesting, the human use of bumetanide for neuropathic pain is not yet optimized as NKCC1 is widely expressed and has varying functions. However, a promising approach to the treatment of neuropathic pain was revealed by Gagnon et al [69] who identified CLP257 as a selective KCC2 activator. In a rat model, the effects of CLP257 were limited to the spinal cord and caused increased KCC2 expression, and alleviated pain hypersensitivity [69]. Similar physiological interactions with ion cotransporters are seen in glycine neurotransmission.
Zhang et al [70] investigated epigenetic changes that impair GABAergic synapses, leading to pain hypersensitivity. Interestingly, isolated suppression of central GABAergic synapses l eads to a chronic pain state [70]. Tao et al [71] added to this work, noting that alteration of the histones suppressing the expression of the Gad65 gene, which is essential to the pain-relieving function of GABA, reduces the pain-related behavior of Gad65 deficient test subjects. By showing the presence of prolonged pain states in GABA deficient subjects, these studies underscore the importance of GABA in pain suppression and chronicity.
In another study, Zhang et al [72] tested the effect of the brain's reward system on opioid addiction. Significantly, Zhang et al [72] showed that inflammatory pain predisposes sufferers to increased reward-center responses to opioids. Moreover, down-regulation of GABA receptors in the amygdala is involved in this process [72]. Simultaneously, pharmaceutical activation of GABA receptors in the amygdala was observed to decrease pain and reduce the reward-center response to opioids [72]. This significant study suggests that GABA receptors could play an important role in both management and prevention of opioid addiction.
As is the case with other factors in pain transmission, GABA interacts and intersects with other pain transmission pathways. For example, Yuan et al recorded inhibitory and excitatory nociceptive conduction currents while exposing rodent spinal cords to phenylephrine (an alpha-1 adrenergic activator). They noted that alpha-1 signaling significantly decreased excitatory transmission [73]. Adrenergic effects on pain transmission are discussed separately; however, the anti-nociceptive effect observed by Yuan et al [73] was observed to be blocked by GABAa receptor antagonists and, to a lesser degree, GABAb antagonists. This study supports a role for alpha-1 acting pharmaceuticals for use in generating pain relief via GABA pathways.
A vast literature addresses potential agents that provide analgesia by increasing inhibitory signals in pain transmitting neurons. In the future, GABA activity will likely underlie new pharmacological pain medications.
3.8 Glutamate
While there are many excitatory amino acids that could contribute to nociception, this review will focus on glutamate and its receptors as a prototype of excitatory amino acids. Glutamate is the most common excitatory neurotransmitter in the central nervous system. Four glutamate receptors are important in the role of glutamate in pain transmission: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), kainate, and metabotropic glutamate receptors [74 - 76]. In addition to its role in pain transmission, a significant body of research investigates the role of glutamate and its receptors in pain modulation. As pain modulation lies outside the scope of this review, this information is not treated here.
As the most prevalent excitatory amino acid, agents that affect glutamate pathways can directly affect pain transmission. For example, alpha-2 adrenergic receptors are known to produce analgesia by inhibiting glutamate-mediated pain signals [77]. To elucidate this process, Wang et al studied alpha-2 antagonists in a mouse model. They noted calcium/calmodulin-dependent protein kinase II (CaMKII) to be an abundant protein of the nociceptive synapse [77]. They showed that alpha-2 antagonists inhibit the auto-phosphorylation of CaMKII, proposing that reduced CaMKII activity may lead to dephosphorylation of NMDA and AMPA glutamate receptors and a corresponding decrease in nociceptive transmission [77].
Glutamate injection into the masseter muscle of human test subjects has been noted to increase perceived pain quantity, distribution, and intensity [78]. A study of monosodium glutamate (MSG) reached similar conclusions: orally administered MSG invoked muscle tenderness and headache in test subjects [79]. Studies like these make it clear that glutamate and its receptors play an important role in the process of pain transmission.
In a human model, Truini and colleagues [80] showed that N-actetylcysteine (NAC) inhibits nociceptive transmission via activity at glutamate receptors. Truini et al [80] propose testing and ultimately using NAC for the treatment of pain. Similarly, Nam et al [81] found specific antagonists of metabotropic glutamate receptors to mediate analgesia in rodent test subjects.
Ketamine is an established anti-nociceptive agent that works at NMDA receptors. One study evaluated the effect of ketamine on a post-stroke model of pain in rats. In this study, central sensitization of NMDA receptors was proposed to be related to neuropathic pain [82]. To investigate this hypothesis, the authors administered ketamine to post-stroke rats and observed improvement in the rats’ allodynia [82]. This study would support further investigation of a role for NMDA receptors the treatment of neuropathic pain.
A concern surrounding ketamine use is related to its safety profile. For example, decreased motor coordination was noted in rats that received ketamine treatment [82]. Ketamine safety has been probed in a variety of clinical studies. One study that evaluated intranasal ketamine for pain control in an emergency department setting cited concerns about ketamine safety [83]. The study concluded that, based on a visual analog pain score, most of the study subjects received pain relief from the ketamine [83]. At the same time, they noted only mild or transient side effects in this population [83].
A systematic review [84] of several randomized, controlled trials of perioperative ketamine use as an adjunct to existing pain methods (opioids) demonstrated significant efficacy. In fact, the analysis showed decreased postoperative total opioid use and increased time to first analgesic consumption [84]. Interestingly, the analgesic effect of ketamine was greatest following major surgery (e.g., cardiothoracic). Side effects such as hallucinations and nightmares were increased but sedation and postoperative nausea and vomiting were decreased secondary to corresponding decreases in opioid use [84]. While ketamine's clinical use continues to be hampered by concerns over potential side effects, an increasing group of studies support the use of ketamine for providing analgesia.
Other agents have been noted to target NMDA receptors. These agents, including Src and Casein Kinase II (CK2 kinase), may also be potential targets for achieving therapeutic analgesia, particularly in the setting of chronic pain. CK2 kinase, for example, has been noted to be involved in the process of pain hypersensitivity that can occur in the setting of calcineurin inhibitor use [85]. Similarly Src phosphorylation of NMDA receptors has been linked to intractable pain [86]. Additionally, the actions of the magnesium ion is known to be inhibitory to NMDA activity. This likely explains its sedative, relaxant, and analgesic effects. A meta-analysis [87] evaluating the outcomes of perioperative magnesium infusion showed decreased total morphine use, decreased pain by visual analog score 4-6 hours after surgery, but no decreased pain by visual analog score 20-24 hours postoperatively. NMDA receptor modulators, including both ketamine and non-ketamine agents, may hold clinical potential for treating pain.
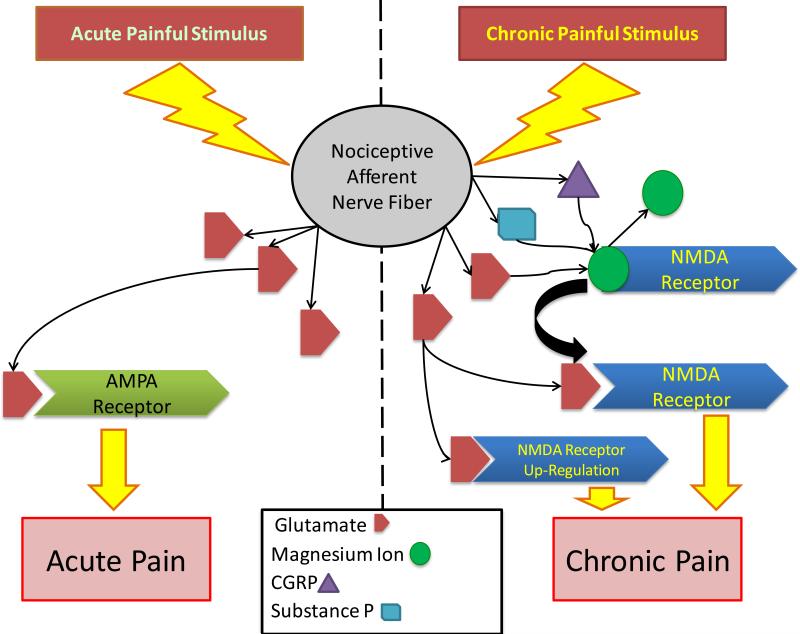
Ko et al [88] observed an increase in metabotropic glutamate receptor expression in response to neuron damage and demyelination in ligated rat nerves. Ko et al [88] identify glutamate receptors as a primary potential target in the pharmaceutical treatment of pain hypersensitivity due to nervous system damage. Similarly, Qiu et al [89] showed that ligation of rat nerves led to up-regulation of AMPA receptors and increased excitatory activity at pain synapses. The role of glutamate receptors in the development of chronic pain is summarized in Figure 5. Given that up-regulation of glutamate receptors contributes to hyperalgesia, increased glutamate receptor concentrations on pain-sensing neurons could be a factor in the initiation of chronic pain states.
Figure 5. Glutamate Receptors in Pain Chronification.
Prolonged firing of C-fiber nociceptors causes release of glutamate, which binds to post-synaptic NMDA and AMPA receptors. Glutamate release by sensory afferents acts on AMPA receptors if the impulse is more acute. If a repetitive and high-frequency glutamate impulse is repetitive, NMDA receptor are stimulated and up-regulated. Normally, the NMDA receptor is blocked by Mg2+ ions. Under repetitive and high frequency stimulation from glutamate Mg2+ blockade removed. The removal of Mg2+ blockade is likely facilitated by substance P and CGRP release from C-fibers. With the removal of Mg2+ from NMDA receptors, there is enhanced NMDA receptor activation, which leads to enhanced inflammatory pain, neuropathic pain, and hyperalgesia. This process over time leads to the development of chronic pain states. Researchers have suggested that the use of low dose NMDA receptor antagonists may play a role in the prevention of chronic pain.
Like NMDA receptors, AMPA receptors are known to play an important role in glutamateric pain transmission. Chen et al, for example, studied nerve injury-mediated changes in the AMPA receptors of rats. They found that the GluA2 subunit of the AMPA receptor becomes internalized in response to nerve injury [90] Moreover, they link this internalization process to the development of chronic pain in the test subject population [90]. This study suggests that the composition of subunits of the AMPA receptor may be a factor in pain chronicity.
Microglia are well known to contribute to the development of neuropathic pain (Figure 6). Among other mediators, microglia releases TNF-α in response to nerve damage. The release of TNF-α increases the excitatory, glutamatergic effect on pain transmission in the substantia gelatinosa while simultaneously decreasing the GABAergic effect [91]. An important intermediate in this process is CC chemokine ligand 2 (CCL2). Remarkably, Huang et al [91] found that both inhibition of microglial activation and the use of selective TNF-α antagonists could prevent CCL2-mediated hyperalgesia in a rat model of thermal hyperalgesia.
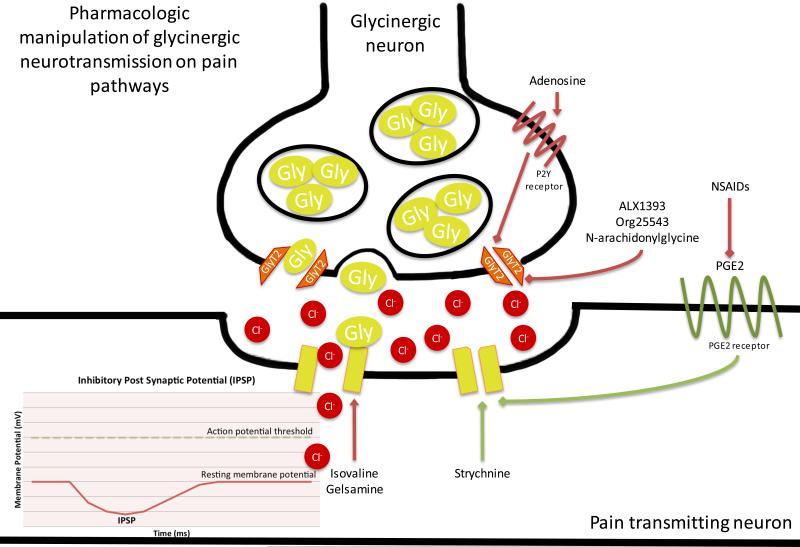
Figure 6. Pharmacologic Manipulation of Glycinergic Nociceptive Neurotransmission.
The action of glycine on glycine receptors is inhibitory to the postsynaptic neuron. Glycine receptor activation results in the influx of hyperpolarizing chloride and the generation of an inhibitory postsynaptic potential (IPSP). GlyT2 functions to reuptake glycine, thus reducing the synaptic concentration of glycine. In the case of pain transmission, glycine results in the generation of an IPSP on a pain transmitting and thus decreases pain transmission. Conversely, activation of GlyT2 enhances glycine reuptake that results in disinhibition of pain transmission. Pain transmission is increased when strychnine or PGE2 receptor signaling closes glycine receptors. Pain transmission is decreased when NSAIDs decrease production of PGE2. Pain transmission is also decreased when adenosine-activated P2Y receptor signaling closes GlyT2, and when ALX1393, Org25543, or N-arachidonyl glycine close GlyT2. Green arrows indicate processes that result in increased pain transmission. Red arrows indicate processes that result in decreased pain transmission.
As an important excitatory pain neurotransmitter, glutamate receptor antagonists present a particularly interesting area for the development of novel pain relieving pharmaceuticals.
3.9 Glycine
Glycine is an important neurotransmitter that induces widely variable physiologic responses depending on the receptor to which it binds. Glycine binds to glycine receptors resulting in influx of hyperpolarizing, inhibitory chloride. Glycine also acts as a co-ligand with glutamate to the excitatory N-methyl-D-aspartate (NMDA) receptors. Accordingly, glycine has been shown to play a role in both activating and inhibiting pain transmission throughout the central nervous system.
In the spinal cord, glycine (along with GABA) is crucial to the inhibition of pain transmission (Figure 7). The dorsal horn of the spinal cord, which represents an early synaptic center of nociception, is rich with inhibitory glycine receptors [92]. The peripheral transmission of pain is met by inhibitory glycinergic influences to limit central pain transmission [92]. This relationship is pathophysiologically important: the genesis of chronic pain is thought to be related to imbalances of inhibitory and excitatory neurotransmission [93]. The concept of glycine receptor agonists is a theoretically attractive avenue to be pursued because the effects of these drugs could likely be restricted to the spinal cord [94]. The local effects of glycine agonists stand in contrast to the systemic effects of benzodiazepines (GABAa receptor agonists) that are commonly used for their sedative-hypnotic profile.
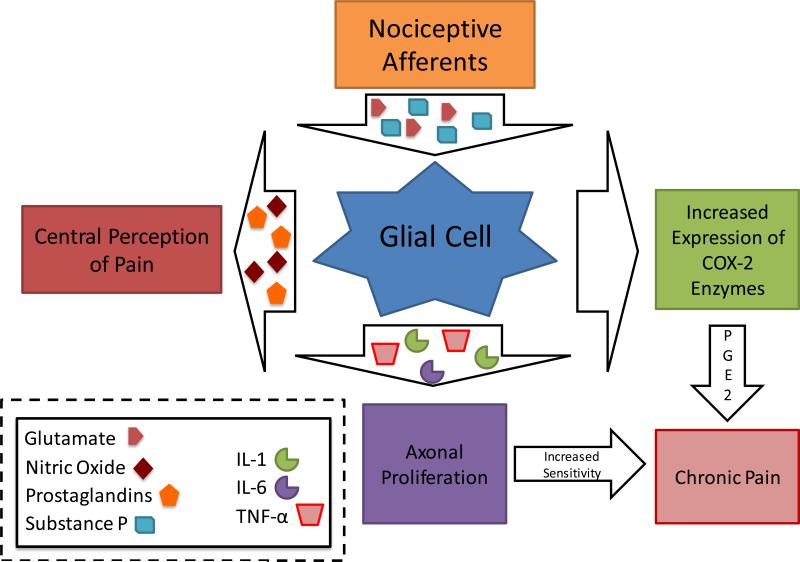
Figure 7. Glial Cells in the Pain Chronification Process.
Microglial cell activation in the CNS plays a central role in the development of chronic pain states. Signaling molecules including substance P and excitatory amino acids (e.g. glutamate) are released from primary afferent neuron terminals, causing the activation of glial cells. Activated glial cells then from and release second order signaling molecules such as nitric oxide and prostaglandins. These second order signals lead to central pain perception. Additionally, activated glial cells then cause the up-regulation the expression of central cyclooxygenase-2 enzymes, which in turn increases the production of prostaglandin E2 (a pro-inflammatory signal also implicated in the development of chronic pain). Activated glial cells may also directly release interleukin-1 (IL-1), interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α). Collectively, IL-6, IL-1, and TNF-α cause proliferation of axons and primary afferent neuron terminals. In summary, continuous glial cell activation produces pro-inflammatory mediators that contribute to the development of a chronic neuropathic pain state. This model is also supported by the fact that glial cell activation is found in many conditions that lead to chronic pain such as post-traumatic recovery, pro-inflammatory states, central demyelinating disorders, and diabetes mellitus.
Agonists of spinal glycine receptors are few in number but still represent an active area of research. Isovaline, an amino acid with analgesic properties, acts at a number of receptor types, including glycine receptors [95, 96]. MacLeod et al [95] experimented with isovaline to inhibit strychnine-induced pain in mice. Strychnine, a glycine receptor antagonist, causes pain via disinhibition of pain transmitting neurons [95]. Intravenous and intrathecal administration of isovaline showed promising analgesic effects; notably, without considerable systemic toxicity [95]. Asseri et al [96] demonstrated similar results, again without considerable CNS toxicity. Researchers have also used rats to study the anti-nociceptive effects of gelsemine, a plant-derived alkaloid with a mechanism similar to isovaline [97, 98]. Their work noted both analgesic efficacy and identified the specific glycine receptor subunit to which gelsemine binds [97, 98]. These results suggest that glycine agonists could find clinical use in a human model, with isovaline being the prototypical agent.
Synaptic concentrations of glycine are greatly influenced by the glycine transporter GlyT2. GlyT2 functions in the reuptake of glycine from the synaptic cleft, effectively reducing the concentration and action of glycine. GlyT2 has been the recipient of academic attention because inhibition of glycine reuptake increases glycinergic neurotransmission and reduces pain. As an example, Haranishi et al [99] administered intrathecal GlyT2 antagonists in a rat model of pain and observed decreased pain-related behavior. Numerous other studies, ranging from chemical antagonists to knockout studies, showed similar analgesic results [100, 101, 102, 103]. Unfortunately, due to concerns of toxicity, lethality, and narrow therapeutic windows, pharmaceutical companies discontinued their work in this area. Despite this significant setback, Meur et al [103] maintained interest in this field and discovered that the irreversible mechanism of action of GlyT2 antagonists was largely responsible for their toxic effects. Their work suggested that reversible antagonists of GlyT2 may demonstrate a more palatable side-effect profile [103]. With time and considerable effort, GlyT2 may become a safe target for the treatment of pain in humans.
Briefly, it should be mentioned that the activity of GlyT2 is regulated by the activity of adenosine P2Y receptors [104]. The activation of P2Y receptors, via a cascade of cell signaling molecules, results in decreased GlyT2 activity [104]. It follows, then, that increased P2Y activity may result in increased concentrations of glycine in the synaptic cleft thereby inhibiting pain transmission. Indeed, the work of Ando et al [105] demonstrated that selective agonism of P2Y1 receptors significantly alleviated neuropathic pain in rats. This alternative approach to GlyT2 receptors may represent yet another method for achieving glycine-related analgesia.
Inflammatory pain is partially caused by prostaglandin E2 (PGE2) mediated inhibition of glycine receptors in the spinal cord [35, 37]. The resultant disinhibition of pain transmission results in the central perception of pain. It is therefore logical to assume that the anti-nociceptive action of NSAIDs, to some extent, is permissive to the action of glycine receptors via the down regulation of PGE2 production. In other words, the pain relief associated with NSAIDs may depend on the anti-nociceptive action of glycine.
Despite its intricacy, the physiology of glycine in pain transmission is well understood (Figure 7). In this article, we emphasize the potential for glycine-related therapies to provide relief for the debilitating complications of pain.
3.10 Histamine
Due to a variety of histamine receptors and anatomical distribution, histamine and histamine receptors play a complex role in pain-related pathways. To study the nociceptive role of peripheral H1 receptors, Mobarakeh et al [106] evaluated reactions to painful stimuli in H1-knockout mice. Mice that lacked the H1 receptor consistently displayed decreased pain-related behavior when subjected to painful stimuli [106]. Additionally, after administration of H1 receptor antagonists to wild-type mice, Mobarakeh et al [106] noted delayed responses to painful stimuli. Anoush et al [107] induced pain by injecting formalin into the paws of rats and evaluated the effects of two H1 receptor antagonists: ketotifen and fexofenadine. They found ketotifen to have an effect equal to diclofenac (an NSAID) in both the acute and chronic phases of pain [107]. Similarly, fexofenadine provided significant, albeit less, pain relief [107].
The use of antihistamine medications for pain management is not a new practice. Roughly fifty years ago intravenous hydroxyzine was evaluated for efficacy during minor, but painful, dermatologic procedures [108]. The majority of subjects reported decreased pain [108]. Intramuscular administration of hydroxyzine, in combination with morphine, was found to have superior analgesic effects to morphine alone [109]. A drawback, however, to administration of antihistamine medications in pain management is added sedation [109]. Another study evaluated the analgesic efficacy of hydroxyzine versus meperidine, an opioid, in patients with metastatic cancer [110]. Hydroxyzine displayed superior efficacy [110]. In an emergency department-based study, patients with migraine headaches were randomized to receive ketorolac, an NSAID, or a meperidine-hydroxyzine combination [111]. The efficacy of pain relief was found to be equal between the two groups [111].
Interestingly, the peripheral and central effect of histamine receptors appears to exert opposite effects on pain transmission. Erfanparast et al [112] found local agonism of H1 and H2 receptors in thalamic nuclei of rats to be anti-nociceptive to formalin induced pain. They also noted an interaction between thalamic H1 agonists and opioids. Anti-nociception was greater when H1 agonists were administered simultaneously with morphine [112]. The anti-nociceptive effects of the H1 and H2 agonists were attenuated in the presence of naloxone, an opioid receptor antagonist [112]. The opposite was also true: the anti-nociceptive effect of morphine was attenuated in the presence of H1 and H2 receptor antagonists [112]. These findings are consistent with those of other studies that demonstrated that microinjection of histamine into specific areas of the brain produce analgesia [113 - 117]. Tamaddonfard et al [118] explained that morphine, primarily via mu-receptors, induces the release of histamine in various regions of the brain, leading to synergistic pain relief.
Sanna et al [119] studied the effects of agonism of neuronal H4 receptors in pain transmission. They found that activation of H4 receptors, primarily in the dorsal root ganglia, resulted in altered MAP kinase signaling and ultimately decreased pain transmission [119]. H4 receptor agonists are currently an active area of research, with some compounds being studied in clinical trials [120]. It is believed that, with time, H4 receptor agonists may find use in the treatment of a number of pathologies, including inflammatory and neuropathic pain [120].
Histamine also upregulates expression of the voltage-gated sodium channel 1.8 (Nav 1.8) and is therefore further implicated in painful processes. Specifically, Yue et al [121] cultured dorsal root ganglia neurons in the presence of histamine and noted a significant increase of Nav 1.8 channels. Interestingly, this increased expression was inhibited only by H2 receptor blockers (e.g., famotidine) and not by antagonists of H1 or H3 receptors. These findings are consistent with those of Yu et al [122].
Further still, Wei et al [123] noted increased noradrenergic activity in the locus coeruleus of rats when treated with histamine. This increased noradrenergic activity ultimately resulted in increased descending pain inhibition. This relationship appears to be related to the activity of H2 receptors as zolantidine, an antagonist of H2 receptors, inhibited any noradrenergic-related decrease in pain hypersensitivity [123].
In summary, histamine agonism and antagonism both represent viable pharmacologic targets for inducing analgesia. Additionally, histaminergic interaction with other pain pathways, particularly opioids, could become clinically significant in achieving better control of painful symptoms.
3.11 Nerve growth factor
Neurotrophins are a family of proteins that are involved in many neural and physiological processes including apoptosis, axonal growth, neuropathic pain, integrin activation, muscle soreness, endometriosis-related pain, and the development of chronic pain states (such as osteoarthritis and lower back pain) [124 - 131]. Nerve growth factor (NGF) is the prototypical member of the neurotrophin protein family. NGF has been implicated in inflammatory and non-inflammatory, mechanical and thermal hyperalgesia [125, 132]. However, NGF has also been demonstrated to have an anti-nociceptive effect when administered in smaller quantities [133]. Interestingly, in the right location and at the right concentration, NGF may even be cardioprotective [126].
The dichotomous nature of NGF has not hindered development of NGF blocking drugs which are effective in the treatment of several types of pain, including chronic low back pain and osteoarthritic hip and knee pain [134, 135]. Importantly, anti-NGF antibodies are currently in trials for clinical use [136]. Gow et al [136] showed in phase 1 trials that anti-NGF antibodies are well tolerated and could be efficacious in the treatment of osteoarthritic pain via antagonism of NGF and hampering NGF action with its receptors. The promising results of anti-NGF antibodies, however, may only be the beginning of research into NGF as an important factor in pain. Isa and colleagues [132], for example, used high molecular weight hyaluronic acid hydrogels (hydrogels) to alleviate inflammation and decrease NGF, and found that inflammation causes degeneration of the nucleus pulposus and increased generation of NGF. These hydrogels were shown to decrease inflammation, decrease NGF expression (which subsequently decreases NGF activity at its receptors), and provide a good environment for regeneration of the nucleus pulposus [132].
NGF has been implicated in the process of pain chronicity. For example, Lopez-Alvarez et al [130] showed that NGF release in hyper-excitable neurons may contribute to chronic pain states. Interestingly, increasing-intensity treadmill exercise helps to avoid neuronal “sprouting” and increased NGF secretion that contribute to this type of neuropathic pain [130]. Eskander et al [131] went one step further by determining the mechanism of NGF-mediated pain chronification. In a rat model of NGF-induced persistent hyperalgesia, NGF was observed to contribute to a hyperalgesic state by incre asing the activity of pain-transducing TRPV1 channels and through oxidative processes [131]. Investigating methods to attenuate the NGF response to decrease the incidence of neuropathic pain is another interesting avenue in neurotrophin research.
Significant work has been done in evaluating the role of NGF in endometriosis-related pain. Chen et al [128] used a mouse model of endometriosis to test the effect of a treatment on NGF. This study found that the group's treatment led to decreased pain behaviors and decreased NGF in the mice [128]. Luvone et al [129] found NGF to play a role in both endometriosis- and ureteral calculosis-related pain. Specifically, ultramicronized palmitoylethanolamide (PEA-um) was noted to decrease pain-related behaviors in rat models of endometriosis and ureteral calculosis [129]. The effect of PEA-um was linked to decreases in NGF (as well as other factors) [129].
Unfortunately, NGF has been associated with rapidly progressive osteoarthritis (OA) in a number of studies. One study mentioned a “causal link” between increased NGF and the pain associated with osteoarthritis [137]. This study went on to evaluate the structural changes that occur in OA and noted increased NGF to be a feature of OA [137]. Significantly, NGF accumulation in OA and acceleration of bone damage can occur in the absence of joint pain [137]. In a review that combined the findings of several studies the authors noted that, despite its promise as a potential analgesic, NGF-targeting agents increase the risk of both OA and potential joint replacement [138]. Interestingly, this paper observed the bone destructive effects of NGF to be more significant when paired with NSAIDs [138]. Thus, despite evidence showing NGF to be an attractive pharmaceutical target for pain, side effects may limit its potential usefulness.
Research in NGF and other neurotrophins has identified novel targets for providing significant pharmacologic pain relief. Continued research into the role of neurotrophins in pain chronicity may yet yield additional methods for treating or avoiding pain, particularly if agents with a favorable side effect profile can be identified.
3.12 Neuropeptide Y
Many research groups have used knockout mice to study the role of Neuropeptide Y (NPY) in pain transmission. Naveilhan et al [139], for example, observed mechanical hypersensitivity and increased development of hyperalgesia in response to acute thermal and chemical stimuli in mice lacking the NPY receptor Y1. Naveilhan et al [139] alluded to NPY-induced inhibition of substance P release (and perhaps other pain-related neurotransmitters) as a potential cause of NPY-related hyperalgesia. Similarly, Shi et al [140] observed increased mechanical hypersensitivity in their NPY knockout mouse model. Shi et al [140] considered the role of NPY in cell survival and influence on dorsal horn neurotransmitters as potential causes of the hyperalgesia. Interestingly, Shi et al [140] also noted a significant increase in the body weight of NPY knockout mice, an observation that has been verified in other NPY knockout studies. Other studies have emphasized the role of NPY in a human model. For example, Wang et al [141] found the concentration of NPY to be significantly higher in the knees of patients suffering from knee osteoarthritis. Perhaps more interestingly, as the pain level (as judged by two different pain score systems) increased, the concentration of NPY showed concomitant increase [141]. A study by Bokarewa et al [142] evaluated the role of tobacco (cigarette smoking) in women with fibromyalgia. Interestingly, the women who smoked reported significantly higher levels of pain [142]. At the same time, these women who smoked were found to have altered levels of leptin and NPY [142].
Boateng et al [143] identified ATF-3, GAP-43, NPY, and galanin as indicators of nerve damage in a mouse model of neuropathic pain. Interestingly, these authors suggested that increases in NPY, which is generally anti-nociceptive, may serve the physiologic purpose of decreasing the increased pain sensitivity that is tied to new-onset neuropathic pain.
Sathyanesan et al [144] showed that indomethacin (an NSAID) changes the transcription of a variety of genes, including those linked to NPY. NSAIDs decrease NPY concentrations in the central nervous system, a result that would be seemingly be pro-nociceptive [144]. Sathyanesan et al [144] propose that, centrally, NPY may play a role in the body's response to stress and that indomethacin may lead to sub-ideal behavioral responses to stress.
While often linked to pain, NPY has been shown to play a role in a variety of pathologies and processes. One study even suggested that polymorphisms in genes related to NPY may have predictive power for identifying women at risk for osteoporosis [145]. Similarly, Carvajal et al [146] noted that, in NPY Y2 receptor knockout mice, NPY seemed to play a role in anxiety and depression. Moreover, they suggested that NPY Y2 receptors could be a potential target for human anxiolytics and antidepressants [146].
While active in a variety of settings, evidence exists for action at NPY receptors to have possible clinical utility. Further research in human models are warranted to evaluate the role of NPY in pain chronicity and the possibility of using drugs that act at NPY receptors to mediate analgesia.
3.13 Nitric oxide synthase (NOS)
Nitric oxide (NO), synthesized in response to the activation of NOS, is an important player in both inflammatory and neuropathic pain. Gautam et al [147] evaluated the changes in NO tied to incisions into the paws of rats. After establishing increases in NO in response to incision, there was decreased post-incision nociceptive behavior in rats that were treated with nitric oxide synthase (NOS) inhibitors. In a study with a similar emphasis on the importance of inflammation in the pain response, Khan et al [148] studied the effect of two distinct coumarin drugs on mediators of inflammation. Significantly, these drugs decreased the expression of NOS mRNA as well as other mediators of inflammation, including COX enzymes [148]. The findings from these studies strongly support the role of NOS as a player in the process of inflammatory pain.
Hervera et al [149] hypothesized that NO and NOS play a role in the efficacy of cannabinoids and opioids. Using a mouse model of neuropathic pain, Hervera et al [149] found that administration of nitric oxide synthases increased the efficacy of both cannabinoids and opioids in reducing neuropathic pain. This response, they determined, is linked to the activation of spinal nitric oxide-cGMP-PKG pathways [149]. Significantly, it appears that NO increases the effectiveness of cannabinoids and opioids by increasing the transcription of their receptors [149].
The potential clinical utility of pharmaceuticals that act on NOS is complicated by a variety of factors. In some settings, like the increased transcription of cannabinoid and opioid receptors in response to NO, NOS can be anti-nociceptive [149]. Conversely, inhibition of NOS has been linked to decreased inflammatory pain [147]. Jin et al [150] explained that NO has different effects on the nociceptive symptoms at different concentrations, in different locations, and under different circumstances. These investigators found that NO increases glycinergic (inhibitory) pain transmission while simultaneously reducing glutamatergic (activating) transmission, a fact that may explain its anti-nociceptive action at the level of the spine.
While a variety of studies have identified and characterized the action of NOS in pain transmission, problems remain in using NOS as a pharmaceutical target. Further research is needed to identify therapies for achieving analgesia via action on NO and NOS.
3.14 Norepinephrine
Norepinephrine and other agonists of α-receptors, particularly α2 receptors, have been shown to mediate analgesia. Maire et al [151], using a rat model, showed that descending pain modulatory neurons from the amygdala affect pain transmission via the activation of central alpha-2 adrenergic receptors. This effect mediates decreased pain sensation [151]. Maire et al [151] observed that, while descending opioid modulation uses a similar pathway, analgesia mediated by clonidine (an α2 agonist) is not inhibited by naloxone (an opioid receptor antagonist) indicating an analgesic mechanism distinct from that of opioids.
Clonidine has been evaluated for use in spinal anesthesia. Singh et al [152] studied Clonidine as an adjuvant to Bupivicaine in patients undergoing abdominal surgery. They showed that spinal anesthesia that contained Clonidine and Bupivicaine provided longer-lasting blockade of motor movement and sensation, as well as longer-term analgesia [152] compared to giving Bupivicaine alone. Similarly, Sen and Sen [153] found Clonidine to be an effective adjuvant to Bupivicaine and noted that patients who received Clonidine with Bupivicaine waited longer after surgery before requesting additional analgesia. In fact, a meta-analysis by Blaudszun et al [154] that evaluated systemic α2-adrenergic agonists found clonidine and dexmedetomidine (discussed below) to have significant analgesic efficacy.
Dexmedetomidine, another α2-adrenergic agonist, is widely used for the induction of anesthesia. It has also been found to have analgesic properties. For example, in non-cooperative pediatric patients, Surendar et al [155] found Dexmedetomidine, Midazolam (benzodiazepine), and Ketamine (NMDA antagonist) to be similar in inducing anesthesia. However, Surendar et al [155] noted that Dexmedetomidine and Ketamine produced analgesia both intra- and post-operatively. In a dog model, Soto et al [156] provided intra-articular injections of morphine, dexmedetomidine, saline, or a combination of morphine and dexmedetomidine. While Soto et al [156] noted improved analgesia when a combination of morphine and dexmedetomidine were given, Soto et al [156] noted no difference between morphine and dexmedetomidine when given alone. If, in a human model, its analgesic effects rival morphine, dexmedetomidine would represent a significantly under-utilized analgesic.
The clinical effectiveness of α2 agonists is not limited to drugs that exclusively act on α2 receptors. As mentioned in the section exploring opioids, both Tramadol and Tapentadol have norepinephrine reuptake inhibition properties which results in the stimulation of alpha receptors. Additionally, pharmaceuticals like Duloxetine (a serotonin-norepinephrine reuptake inhibitor; discussed in the Serotonin section) have been used to provide pain relief, particularly in those with diabetic neuropathy [157]. The demonstrated effectiveness of existing α2-adrenergic agonists [154], combined with their role in anesthesia makes α2 agonists a potential source for generating new, powerful analgesic drugs.
3.15 Serotonin
Serotonin plays an important role both in providing analgesia and in modulating the effects of other analgesic drugs. Bhosale et al [158], for example, studied the effect of serotonin antagonists (Ondansetron) on Paracetamol (Acetaminophen) analgesia in patients that had undergone ear, nose, and throat procedures. While they mentioned other sources that conclude the opposite, Bhosale et al [158] concluded that Ondansetron increases the analgesic action of Paracetamol in their study population: using two different pain scales, they noted a significantly higher pain score in the placebo group. This study supports the use of serotonin antagonists for increasing patient comfort and decreasing patient pain in a post-operative setting. Importantly, there are also studies that have failed to support their use in this setting [159, 160].
In a rat model, Ren et al [161] investigated the role of serotonin channels in reversing the respiratory depression that can be caused by opioid agonists. Interestingly, Ren et al [161] found 5-HT1A agonists (Befiradol) to reverse Fentanyl-induced respiratory depression relative to a saline control. Befiradol was also found to reduce the duration of Fentanyl analgesia [161]. Kim et al [162] evaluated the role of 5-HT1A receptors in the process of inflammation-induced allodynia noting that, relative to other serotonin receptors, 5-HT1A receptors affect nociceptive processing. Drugs that act at the 5-HT1A receptors could have clinical potential to change the action of opiates or alter pain transmission.
Using mice, Yesilyurt et al [163] investigated the role of serotonin receptors in stress-induced analgesia. After forcing mice to endure a stressor (swimming in cold water), tail flick response (indicative of pain) upon exposure to a hot plate was decreased [163]. Yesilyurt et al [163] further showed that both endogenous opioid and non-opioid effects played a role stress-induced analgesia. Specifically, when serotonin receptors were blocked in test subjects, the effect of stress-induced analgesia was observed to decrease. This research supports the role of serotonin receptors in the pain transmission system. It also highlights the potential for pharmaceutical action on specific serotonin receptors to mediate clinical analgesia.
Important classes of medications that affect serotonin levels are anti-depressants. In a study of patients with neuropathic pain, Gao et al [157] found that patients receiving Duloxetine (inhibits the re-uptake of serotonin and norepinephrine) reported significantly improved pain relative to a placebo control group. In other settings, the use of serotonin re-uptake inhibitors is more controversial. Leombruni et al [164] found that both L-carnitine and Duloxetine have efficacy in treating the pain and improving the quality of life for patients with fibromyalgia. Conversely, in a review of studies that evaluated the role of serotonin reuptake inhibitors in fibromyalgia patients, Wallitt et al [165] concluded that there is no evidence that serotonin reuptake drugs are more efficacious than placebo for treating fibromyalgia. Given the fact that serotonin reuptake inhibiting drugs have been found to be efficacious in certain pain syndromes and settings, further research should be done to characterize their role in providing clinical analgesia.
3.16 Tachykinins and neurokinins
Tachykinins are a family of neuropeptides that include Substance P, Neurokinin A, and Neurokinin B [166]. Substance P binds to the Neurokinin-1 (NK-1) receptor, Neurokinin A binds to the Neurokinin-2 (NK-2) receptor, and Neurokinin B binds to the Neurokinin-3 (NK-3) receptor [166]. Significantly, however, none of these tachykinins are highly selective for their respective NK receptors [166]. For this reason, each of the tachykinins is known to have activity at any of the neurokinin receptors [166].
Due to the concentration of NK receptors in the dorsal horn of the spinal cord, and other anatomical locations associated with pain sensation, it has historically been thought that tachykinins play a key role in pain transmission. This is demonstrated by a multitude of animal studies from the past that focus on the role of NK receptors in pain transmission [167 - 169]. However, when the results of animal studies were applied to a human model, action at neurokinin receptors was not found to be more effective than established pain treatments (ibuprofen and pregabalin) [170, 171]. Further, NK-1 receptor antagonists were shown to be ineffective for treating neuropathic pain [172].
Since one of the primary effects of opioids is significant inhibition of Substance P release, research suggesting a limited role of neurokinins in pain transmission seemed somewhat perplexing [173]. More recent studies by Chen et al [173] showed that only inflammatory (and not neuropathic) pain responded to Substance P inhibition in rat test subjects. This further supports the established role of Substance P as an inducer of inflammation [176]. Moreover, Foran et al [174], using a rat model, found that increased expression of Substance P in the spinal cord augments the response to opioid anti-nociception by attenuating the development of opioid tolerance. In another study, Tumati et al [175] showed that intrathecal administration of an NK1 receptor antagonist reduced opioid withdrawal-mediated hyperalgesia in rats, suggesting that NK1 blockade may prove useful in treating the symptoms of opioid withdrawal.
The most significant analgesic properties of neurokinin receptor antagonists are limited to the realm of inflammatory pain. Studies have shown that administration of NK1 receptor antagonists decrease pain sensation in rats with induced monoarthritis [176, 177]. Uematsu et al [176] induced inflammation in rats, then conducted histological analysis of rodent cartilage. Significantly, Uematsu et al [176] observed less cartilage destruction in rats that had been treated with NK1 receptor antagonists. Thus tachykinin antagonism may have a significant clinical role in protecting joint architecture in the setting of chronic inflammatory disease. Further evidence of the role of tachykinins as inflammatory mediators was identified by Chauhan et al [178]. In a mouse model of pneumococcal meningitis, they found that antagonism of NK1 receptors decreased meningeal inflammation and reversed infection-related neuronal demyelination.
Tachykinins have also been associated with normal hypothalamic functioning. Simavli et al [179] found that agonism of NK1 receptors led to early onset of puberty. Similarly, absence of the Tac1 gene (which encodes Substance P) led to delayed puberty onset and subfertility in female mice [179]. Using Tac1 knockout mice, Niedermair et al [180] observed that Substance P deficiency is associated with decreased bone formation. These studies raise the possibility of significant side-effects related to the clinical use of tachykinin and neurokinin receptor modulators.
Drugs with action at tachykinin and neurokinin receptors remain an interesting avenue for providing inflammatory pain relief, protection of connective tissue, and for treatment of opioid withdrawal. More research is needed to elucidate potential clinical efficacy and side effects of these agents.
3.17 Vasoactive intestinal polypeptide
Vasoactive intestinal polypeptide (VIP), while traditionally described as a neurotransmitter involved in gastrointestinal tract motility, plays a role in a number of physiologic processes. While the exact mechanism and function of VIP in pain transmission is unclear, it is clear that VIP is involved in pain pathways and that its receptors could prove to be beneficial therapeutic targets. VIP is expressed in Lissauer's tract of the spinal cord, particularly in the lumbosacral region [174]. The anatomical distribution of VIP expression is believed to be related to a possible physiologic role for VIP in afferent pain neurotransmission from pelvic viscera. This observation is supported by increased VIP expression, in association with decreased pain thresholds, prior to parturition in female rats [174].
VIP has been implicated in the genesis of osteoarthritic pain. McDougall et al [181] induced osteoarthritis in rats and, after delivery of a VIP-receptor antagonist (VPAC), noted cessation of pain-related behaviors in the test subjects. A related follow-up study by Schuelert and McDougall [182] showed that local VIP administration into joints caused sensitization of nociceptive afferent neurons. Furthermore, they provided electrophysiological evidence of pain reduction following injection of VPACs [182]. These findings demonstrate a potential use for VPACs to treat osteoarthritic pain. Other VIP antagonists, including somatostatin, may eventually find clinical use as analgesic agents.
VIP and the structurally related pituitary adenylate cyclase-activating polypeptide-38 (PACAP) play a role in the pathophysiology of migraines. Cernuda-Morollón et al [183], for example, measured serum VIP levels in patients with chronic and episodic migraine. Their research showed consistent, significant elevations in serum VIP among test subjects that suffered with migraines. Furthermore, Amin et al [184] demonstrated that intravenous infusion of VIP and PACAP can induce migraine headaches. Although VIP and PACAP have vasodilatory properties, it is clear that these substances play a role in migraine genesis and migraine-related pain [184]. Additionally, Amin et al [184] identify the PAC1 receptor as an important potential pharmacological target for treating migraines.
Alterations of VIP expression have also been demonstrated to contribute to the genesis of ne uropathic pain. Shehab et al [185], for example, induced peripheral nerve injury by ligating L5 spinal nerves in rats. After ligation, they observed the resultant neurochemical changes in the spinal cord. VIP expression, and other markers, increased in both the L5 region and adjacent spinal cord segments [185]. The author suggested that changes in neurochemicals (like VIP) play a role in the hyperalgesia incident to neuropathic pain [185]. Similarly, Sun-Joo Son et al [186] induced peripheral nerve injury in an animal model and demonstrated upregulated expression of VIP and neuropeptide Y in the dorsal root ganglia. Significantly, however, the work of Kim et al [187] demonstrated that isolated increases in VIP expression in rats with nervous injury did not fully explain the generation of neuropathic pain. Rather they suggested that the generation of neuropathic pain is probably multifactorial [187]. While it is likely that the induction of neuropathic pain is multifactorial, VIP seems to play a role in this process.
Pharmacologic research on the agonism and antagonism of VPAC receptors is currently in its early stages. Li et al [188] performed chemical research of several VIP derivatives to classify them as agonists or antagonists at VPAC receptors. This work may provide crucial information for future research aimed at identifying the effects that result from the use of these agents. The full extent of the physiology of VIP in pain transmission remains unsettled; however, it is clear that VIP represents a new field for generating agents to treat clinical pain.
4. Expert Commentary
Despite a truly impressive understanding of pain pathways and processes, pain remains a crippling complication of disease for thousands of patients. Targeted emphasis on therapies and strategies for providing clinically significant analgesia should remain a priority in pain research, as well as performing studies that observe long term safety data for use of these analgesics. By identifying novel agents to treat pain, discovering synergistic combinations between existing analgesics, confronting pain management from a multimodal perspective and by improving ability of medicines to prevent acute pain from becoming chronic, the scourge of pain can be more effectively alleviated. Pain management cannot be empirically treated based on disease classification, but must be approached in a patient- specific manner focusing on the patient's constellation of presenting symptoms. Choosing effective agents must take into accounts all aspects of the pain patient's particular pain phenotype.
5. Five-year view
While vast, the current pharmacopeia has failed to meet the pain-related needs of many patients. Developing new agents, methods, and strategies for treating pain remains essential in importance. This review highlights many pathways and agents that show potential for treating pain in novel ways. For example, drugs that selectively act at specific adenosine pathway receptors may be able to provide clinically significant analgesia, with some specific acting examples already being used for research [3]. Similarly, bradykinin antagonists could be explored as novel pain-relieving agents. Medications that act on the nerve growth factor (NGF) pathways are already in the process of being approved for clinical use [136]; however, more work could certainly be done to identify unique ways of alleviating through action on the NGF pathway.
Cannabinoids represent one of the best-recognized sources of potential new agents for treating pain. Wide potential variability in the concentration, quality, and contents of “street” marijuana makes the development of pharmaceutical grade alternatives highly desirable for treating clinical nausea, decreased appetite, and pain. Research has shown limited efficacy for cannabinoids in an acute setting [25]; however, they seem to be better-suited for chronic pain states [23, 26, 28]. Cannabinoids have also been shown to be effective in a neuropathic pain picture [26]. Regardless of these findings, other studies have not found significant benefits to cannabinoid-based analgesics [32]. Further work should be invested in describing the usefulness of cannabinoids in other clinically significant settings, like inflammation.
Work in area of chloride extrusion activators may show eventual utility. The physiologic activity of GABA in decreasing neuropathic pain transmission is enhanced by the activity of the KCC2 chloride cotransporter [67]. The recent identification of the novel CLP257 as a KCC2 activator and the selective activity within the spinal cord makes this an avenue to pursue [67]. We encourage further work in this area as it may prove fruitful with eventual clinical utility.
In vivo studies have uncovered evidence that Angiotensin II, the receptors of which are known to be present in the DRG neurons of rats, is involved in neuronal hyperexcitabilty [189]. Angiotensin II receptor antagonists have shown to have significantly improved analgesia when compared to placebo in rat studies, making it an encouraging prosepct for providing neuropathic pain control in a novel way [189].
Another target that has been recently explored is the sigma-1 receptor (σ1R). The σ1R has been demonstrated to play a role in the sensitivity of both acute and chronic pain, with σ1R knockout mice showing decreased pain responses [190]. Currently, a σ1R antagonist is in phase II clinical trials for neuropathic pain. Early studies support a potential role for the use of σ1R antagonists in the treatment of chronic neuropathic pain [190].
Another limitation of the current pharmacopeia deals with differing actions of drugs in different body compartments. For example, NO has different effects depending on its site of action in the pain transmission circuitry [150]. Certain drugs, like glycine transporter inhibitors, currently have limited utility because of poor blood-brain-barrier penetration. Advances in drug delivery methods could potentially remedy this obstacle. The use of exosomes, which are cell secreted lipid-coated vesicles, to deliver drugs across the blood-brain-barrier has recently shown promising results [179 - 181]. By encapsulating the drug within exosomes it is possible to deliver drugs to the CNS that would otherwise have had poor penetration. While these methods remain expensive and impractical, further advances in drug delivery systems could expand the pharmacopeia by enabling analgesics to be targeted to specific sites. Novel analgesics could theoretically be generated by simply re-targeting existing pharmaceuticals.
5.1 Pain Chronification
One interesting frontier in pain medicine deals with finding ways to prevent acute injury and pain from becoming chronic. Research suggests that pain transmission pathways may play a significant role in this process. For example, neurokinin has been shown to both decrease inflammatory pain and joint cartilage destruction in rats [176, 177]. Preventing joint destruction in this case may also prevent osteoarthritis-related pain. More elegantly, it has been shown that glutamate receptors are upregulated in response to nervous system damage [88, 89]. Drugs that could prevent this up-regulation may be able to decrease the rate at which injury becomes chronic. Prostaglandins, particularly PGE2, have been implicated in the process of pain chronification. Specifically, up-regulation of PGE2 is linked with the development of chronic pain in rats [37]. Research could be done to see if concentrated doses of selective PGE2 antagonists would decrease the incidence of chronic pain after an acute injury.
Work focused on the connection between NSAIDs and other transmission pathways has shown that NSAIDs may be able to avoid the development of chronic pain states by changing the transcription of specific genes, including the gene that codes for NPY [144]. NPY may play a role in the body's response to stress, potentially allowing it to contribute to the production of a chronic pain state [144].
Nerve growth factor (NGF) has been implicated in the process of pain chronicity. In a rat model of NGF-induced persistent hyperalgesia, NGF was observed to contribute to a hyperalgesic state by increasing the activity of pain-transducing TRPV1 channels and through oxidative processes [131]. Potentially, pharmaceuticals that act in the NGF pathway could reduce the density of TRPV1 channels post-injury, decreasing long-term hyperalgesia in response to acute injury.
Another contributor to the process of pain chronification appears to be linked to the balance of inhibitory and activating neurotransmitters in pain synapses. For example, just as up-regulation of pain-activating glutamate receptors has been linked to chronic pain [88, 89], suppression of GABAergic synapses (inhibitory) leads to a chronic pain state [72]. TNF-α release in the substantia gelatinosa (in response to inflammation) increases glutamate while simultaneously decreasing GABA concentrations [69]. Agents that selectively inhibit TNF-α in the substantia gelatinosa could theoretically prevent the development of GABA- or glutamate-rooted causes of chronic pain. Similarly, GABA-acting drugs, which are not currently designed for providing pain relief, could theoretically be used to alleviate chronic pain or balance imbalanced pain neurotransmitters [59].
A systematic review [191] analyzed randomized controlled trials that studied the effect of perioperatively administered medications on long term pain outcomes. Among 40 included randomized trials, the majority pertained to ketamine, gabapentin, and pregabalin. Analysis revealed statistically significant reduced development of chronic pain in subjects treated with ketamine, but not gabapentin or pregabalin. This finding, however, is not conclusive due to potential confounding factors. The authors cautioned against the low number of subjects included in the ketamine trials. Furthermore, dose, duration of treatment, and surgical procedures varied widely from trial to trial. This meta-analysis indicates the need for high-powered and well-controlled trials that evaluate these medications in limiting post-surgical chronic pain. This statement is further evidenced by the conflicting findings of a meta-analysis by Clarke et al [192] who found significant reductions in chronic postsurgical pain following perioperative administration of both gabapentin and pregabalin.
5.2 Interactions among Analgesics
The most powerful analgesics in current clinical use are opioids. Concerns with the use of opioids in treating pain have focused on diminishing effects, avoiding addiction, and decreasing negative side effects. As demonstrated by Soto et al [156], it may be possible to combine the administration of opioids with other analgesic drugs to decrease the dose of opioids required to provide effective pain relief. As effective alternatives to opioids are unavailable in many cases, identifying synergistic effects between pain transmission pathways could improve the clinical use of opioids.
Multimodal pain therapy is also of utmost importance. As discussed in a review by Kerfeld et al [193], the use of peripheral and spinal analgesic modalities was effectively able to reduce the need of opioids, decrease the side effects of opioid use, and decrease the length of hospital, all while improving patient satisfaction. In patients with hip fracture undergoing elective repair, Fabi [194] showed that the multimodal analgesic approach provides effective pain relief. This approach was also found to have an “opioid-sparing” effect on patients [194]. Improved analgesic relief resulted in faster ambulation, more productive physical therapy, and faster overall recovery [194]. Concomitant increases in patient satisfaction were also noted in this study [194]. The multimodal therapy approach is likely to guide pain control in the coming years.
Neurokinins, including substance P, when present in the spinal cord, have been shown to augment the response to opioids by attenuating the development of opioid tolerance [174]. Similarly, administration of NOS (increased NO concentration) has been found to increase the receptor density and corresponding efficacy of both opioids and cannabinoids, particularly in the setting of neuropathic pain [149]. The co-administration of H1 agonists and opioids has been shown to produce greater anti-nociception than the administration of morphine alone [112, 118]. Interestingly, cannabinoids have also been found to magnify the analgesic effect of opioids [21, 22]. Co-administration or combination of opioid agents with pharmaceuticals that act in these pathways should be investigated.
Beyond potentiating the analgesic effect of opioids, other significant intersections among transmission pathways have been observed. In rats, Neurokinin (NK1) receptor antagonists have been shown to reduce the hyperalgesic symptoms of opioid withdrawal [175]. Serotonin channels in rats show potential for reversing opioid-induced respiratory depression [161]. Unfortunately, action at these serotonin channels has also been shown to decrease the duration of fentanyl analgesia [161]. It has been proposed that cannabinoids can influence the development of tolerance to opioids [21, 22]. GABA receptors, one study suggested, seem to play a pivotal role in the process of opioid addiction [72]. Research should be conducted to evaluate the possibility of managing or avoiding opioid addictions and side effects via co-administration or combination of these agents with existing opioid analgesics.
6. Key Issues.
Pain transmission pathways are well understood; however, clinical control of pain-related sequelae remains a major challenge.
Pain transmission refers to the process by which a pain signal is communicated from the site of injury or nociceptive stimulus to the brain to be perceived.
Cannabinoids, although somewhat controversial, remain an important frontier for producing novel analgesics, synergistic drugs interactions, and improving the use of opioids.
Opioids remain the gold standard in analgesia. Current research focuses on managing side effects, identifying effective alternatives, and decreasing the dosages required to obtain clinical effect.
Eicosanoids may play an important role in the development of pain chronicity. Additionally, it may be appropriate to re-introduce COX2 inhibitors with no adverse effects into the pharmaceutical market.
Glutamate and GABA pathways represent agents that have potential to modulate excitatory and inhibitory transmission pathways. They are an important eventual source of novel agents.
Norepinephrine and serotonin pathways are becoming more important in alleviating clinical pain.
Acknowledgments
This work was supported by research grants R01 HL116042, R01 HL112597, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
*of interest
**of considerable interest
- 1.Hartvigsen J, Davidsen M, Sogaard K, et al. Self-reported musculoskeletal pain predicts long-term increase in general health care use: A population-based cohort study with 20-year follow-up. Scandanavian Journal of Public Health. 2014:1403494814542263. doi: 10.1177/1403494814542263. [DOI] [PubMed] [Google Scholar]
- 2.Ford A, Castonguay A, Cottet M, et al. Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci. 2015;35(15):6057–67. doi: 10.1523/JNEUROSCI.4495-14.2015. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets – what are the challenges? Nat Rev Drug Discov. 2014;12.4:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imlach W, Bhola R, May L, et al. A positive allosteric modulator of the adenosine A1 receptor selectively inhibits primary afferent synaptic transmission in a neuropathic pain model. Molecular pharmacology. 2015;88(3):460. doi: 10.1124/mol.115.099499. [DOI] [PubMed] [Google Scholar]
- 5.Otsuguro K, Tomonari Y, Otsuka S, et al. An adenosine kinase inhibitor, ABT-702, inhibits spinal nociceptive transmission by adenosine release via equilibrative nucleoside transporters in rat. Neuropharmacology. 2015;97:160–170. doi: 10.1016/j.neuropharm.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Yang JN, Bjorklund O, Lindstrom-Tornqvist K, et al. Mice heterozygous for both A1 and A2A adenosine receptor genes show similarities to mice given long-term caffeine. J Applied Physiology. 2009;106.2:631–639. doi: 10.1152/japplphysiol.90971.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD009281.pub3. doi: 10.1002/14651858.CD009281.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little JW, Ford A, Symons-Liguori AM, et al. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain. 2015;138(1):28–35. doi: 10.1093/brain/awu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janes K, Esposito E, Doyle T, et al. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155(12):2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford A, Castonguay A, Cottet M, et al. Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci. 2015;35(15):6057–6067. doi: 10.1523/JNEUROSCI.4495-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Player M. Bradykinin B1 Receptor Antagonists as Potential Therapeutic Agents for Pain. Journal of Medical Chemistry. 2010;53:5383–5399. doi: 10.1021/jm1000776. [DOI] [PubMed] [Google Scholar]
- 12.Gardes J, Michineau S, Pizard A, et al. Aspirin inhibits human bradykinin B2 receptor ligand binding function. Biochem Pharmacol. 2008;75(9):1807–16. doi: 10.1016/j.bcp.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy WR, Vanhove GF, Lu SP, et al. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain. 2010;11(6):579–87. doi: 10.1016/j.jpain.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Zhang M, Sun G, et al. Calcitonin gene-related peptide promotes the wound healing of human bronchial epithelial cells via PKC and MAPK pathways. Regul Pept. 2013;184:22–29. doi: 10.1016/j.regpep.2013.03.020. doi: 10.1016/j.regpep.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–86. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Reyes M, Pardi V, Akerman S. A potent and selective calcitonin gene-related peptide (CGRP) receptor antagonist, MK-8825, inhibits responses to nociceptive trigeminal activation: Role of CGRP in orofacial pain. Experimental Neurology. 2015 doi: 10.1016/j.expneurol.2015.05.005. doi: 10.1016/j.expneurol.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JM, Hauge AW, Olson J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalgia. 2010;30(10):1179–86. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 18*.Marcus R, Goadsby P, Dodick D, et al. BMS-927711 for the actue treatment of migraine: A double-blind, randomized, placebo controlled, dose-ranging trial. Cephalgia. 2014;34(2):114–125. doi: 10.1177/0333102413500727. [The CGRP antagonist used in this study was known to lack vasodilatory properties. This suggests that CGRP antagonists may treat migraines in a novel, poorly understood way.] [DOI] [PubMed] [Google Scholar]
- 19.Greco R, Mangione A, Siani F, et al. Effects of CGRP receptor antagonism in nitroglycerin-induced hyperalgesia. 2013 Cephalgia. 34(8):594–604. doi: 10.1177/0333102413517776. [DOI] [PubMed] [Google Scholar]
- 20.Ho TW, Ho AP, Ge YJ, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for prevention of headache in women with perimenstrual migraine. Cephalgia. 2015:0333102415584308. doi: 10.1177/0333102415584308. [DOI] [PubMed] [Google Scholar]
- 21.Altun A, Yildirim K, Ozdemir E, et al. Attenuation of morphine anti-nociceptive tolerance by cannabinoid CB1 and CB2 receptor antagonists. J Physiol Sci. 2015 doi: 10.1007/s12576-015-0379-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecina M, Martinez-Jauand M, Hodgkinson C, et al. FAAH selectively influences placebo effects. Molecular Psychiatry. 2014;19(3):385–91. doi: 10.1038/mp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawsey S, Wood M, Browne H, et al. Safety, Tolerability, and Pharmacokinetics of FAAH Inhibitor V158866: A Double-Blind, Randomised, Placebo-Controlled Phase 1 Study in Healthy Volunteers. Drugs R D. 2016;16(2):181–191. doi: 10.1007/s40268-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology. 2013;38(10):1984–92. doi: 10.1038/npp.2013.97. [Interesting comparison of the analgesic effectiveness of oral and inhaled cannabinoids. Supports use of oral cannabinoids.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darmani NA, Izzo AA, Degenhardt B, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology. 2005;48(8):1154–63. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Ostenfeld T, Price J, Albanese M, et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27(8):668–76. doi: 10.1097/AJP.0b013e318219799a. [DOI] [PubMed] [Google Scholar]
- 27.Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136–48. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JR, Lossignol D, Burnell-Nugent M, et al. An open-label extension study to investigate the long-term safety and tolerability of TCH/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46(2):207–18. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Alkaitis MS, Solorzano C, Landry RP, et al. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS One. 2010;5:e10891. doi: 10.1371/journal.pone.0010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landry RP, Martinez E, DeLeo JA, et al. Spinal cannabinoid receptor type 2 agonist reduces mechanical allodynia and induces mitogen-activated protein kinase phosphatases in a rat model of neuropathic pain. J pain. 2012;13(9):836–848. doi: 10.1016/j.jpain.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paszcuk AF, Dutra RC, da Silva KABS, et al. Cannabinoid Agonists Inhibit Neuropathic Pain Induced by Brachial Plexus Avulsion in Mice by Affecting Glial Cells and MAP Kinases. PLoS ONE. 2011;6(9):e24034. doi: 10.1371/journal.pone.0024034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology. 2015;14(2):162–73. doi: 10.1016/S1474-4422(14)70251-0. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438–49. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Hammond V, O'Donnell V. Esterified eicosanoids: generation, characterization and function. Biochimica Et Biophysica Acta. 2012;1818(10):2403–2412. doi: 10.1016/j.bbamem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inceoglu B, Jinks SL, Ulu A, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hennan JK, Huang J, Barrett TD, et al. Effects of Selective Cyclooxygenase-2 Inhibition on Vascular Responses and Thrombosis in Canine Coronary Arteries. Basic Science Reports. 2001;104:820–825. doi: 10.1161/hc3301.092790. doi: 10.1161/hc3301.092790. [DOI] [PubMed] [Google Scholar]
- 37.St-Jacquesa B, Ma W. Peripheral prostaglandin E2 prolongs the sensitization of nociceptive dorsal root ganglion neurons possibly by facilitating the synthesis and anterograde axonal trafficking of EP4 receptors. Experimental Neurology. 2014;261:354–66. doi: 10.1016/j.expneurol.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Hong TT, Huang J, Barrett TD, et al. Effects of cyclooxygenase inhibition on canine coronary artery blood flow and thrombosis. American Journal of Physiology - Heart and Circulatory Physiology. 2008;294(1):H145–H155. doi: 10.1152/ajpheart.00646.2007. DOI: 10.1152/ajpheart.00646.2007. [DOI] [PubMed] [Google Scholar]
- 39.Liua B, Luob W, Zhanga Y, et al. Effect of celecoxib on cyclooxygenase-1-mediated prostacyclin synthesis and endothelium-dependent contraction in mouse arteries. Euro J Pharmacol. 2013;698(1-3):354–61. doi: 10.1016/j.ejphar.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 40**.Coxib and traditional NSAID Trialists' (CNT) Collaboration Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. doi: 10.1016/S0140-6736(13)60900-9. [This study suggests that COX2 inhibitors may not have increased risk of cardiovascular side effects when compared to COX1 inhibitors. The cardiovascular risk between COX1 and COX2 inhibitors does not appear to be statistically different.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizoguchi H, Takagi H, Watanabe C, et al. Involvement of multiple μ-opioid receptor subtypes on the presynaptic or postsynaptic inhibition of spinal pain transmission. Peptides. 2014;51:15–25. doi: 10.1016/j.peptides.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Richards P, Gimbel JS, Minkowitz HS, et al. Comparison of the efficacy and safety of dual -opioid treatment with morphine plus oxycodone versus oxycodone/acetaminophen for moderate to severe acute pain after total knee arthroplasty. Clin Ther. 2013;35.4:498–511. doi: 10.1016/j.clinthera.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Richards P, Riff D, Kelen R, et al. Analgesic and adverse effects of a fixed-ratio morphine-oxycodone combination (MoxDuo) in the treatment of postoperative pain. J Opioid Manage. 2010;7.3:217–228. doi: 10.5055/jom.2011.0064. [DOI] [PubMed] [Google Scholar]
- 44.Imanaka K, Tominaga Y, Etropolski M, et al. Ready conversion of patients with well-controlled, moderate to severe, chornic malignant tumor-related pain on other opioids to tapentadol extended release. Clinical Drug Investigation. 2014;34(7):501–11. doi: 10.1007/s40261-014-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Z, Xu J, Rossi GC, et al. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest. 2015;125.7:2626. doi: 10.1172/JCI81070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang W, Liu YC, Maboudou E, et al. Metoclopramide improves the quality of tramadol PCA indistinguishable to morphine PCA: a prospective, randomized, double blind clinical comparison. Pain Medicine. 2013;14(9):1426–34. doi: 10.1111/pme.12166. [DOI] [PubMed] [Google Scholar]
- 47.Lin SL, Tsai RY, Shen CH, et al. Co-administration of ultra-low dose naloxone attenuates morphine tolerance in rats via attenuation of NMDA receptor neurotransmission and suppression of neuroinflammation in the spinal cords. Pharmacol Biochem Behav. 2010;96.2:236–245. doi: 10.1016/j.pbb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Tsai RY, Cheng YC, Wong CS. (+)-Naloxone inhibits morphine-induced chemotaxis via prevention of heat shock protein 90 cleavage in microglia. Journal of the Formosan Medical Association. 2015;114.5:446–455. doi: 10.1016/j.jfma.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Lin YS, Tsai RY, Shen CH, et al. Ultra-low dose naloxone restores the antinocicepitve effect of morphine in PTX-treated rats: association of IL-10 upregulation in the spinal cord. Life Sci. 2012;91.5:213–220. doi: 10.1016/j.lfs.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Jordan BA, Lakshmi AD. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399.6737:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang HL, Hsu CY, Huang PC, et al. Heterodimerization of opioid receptor-like 1 and μ-opioid receptors impairs the potency of μ receptor agonist. J Neurochem. 2005;92.6:1285–1294. doi: 10.1111/j.1471-4159.2004.02921.x. [DOI] [PubMed] [Google Scholar]
- 52.Pfeiffer M, Kirscht S, Stumm R, et al. Heterodimerization of substance P and μ-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278.51:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 53.Guillemyn K, Starnowska J, Lagard C, et al. Bifunctional peptide-based opioid agonist–nociceptin antagonist ligands for dual treatment of acute and neuropathic pain. J Med Chem. 2016;59.8:3777–3792. doi: 10.1021/acs.jmedchem.5b01976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doehring A, Oertel BG, Sittl R, et al. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain. 2013;154.1:15–23. doi: 10.1016/j.pain.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Chao YC, Xie F, Li X, et al. Demethylation regulation of BDNF gene expression in dorsal root ganglion neurons is implicated in opioid-induced pain hypersensitivity in rats. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.03.007. doi: 10.1016/j.neuint.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Lee CY, Perez FM, Wang W, et al. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. European Journal of Pain. 2011;15(7):669–675. doi: 10.1016/j.ejpain.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Chen SR, Laumet G, et al. Nerve injury diminishes opioid analgesia through lysine methyltransferase-mediated transcriptional repression of mu-opioid receptors in primary sensory neurons. J Biol Chem. 2016 doi: 10.1074/jbc.M115.711812. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grace PM, Strand KA, Galer EL, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences. 2016:201602070. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munro G, Hansen R, Mirza N. GABAa receptor modulation: Potential to deliver novel pain medicines? European Journal of Pharmacology. 2013;716(1-3):17–23. doi: 10.1016/j.ejphar.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 60.Munro G, Erichsen HK, Rae MG, et al. A question of balance - - positive versus negative allosteric modulation of GABA(A) receptor subtypes as a driver of analgesic efficacy in rate models of inflammatory and neuropathic pain. Neuropharmacology. 2011;61(1-2):121–32. doi: 10.1016/j.neuropharm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Mirza NR, Larsen JS, Mathiasen C, et al. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327(3):954–68. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- 62.Munro G, Lopez-Garcia JA, Rivera-Arconada I, et al. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327(3):969–81. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- 63.Hu JH, Yang N, Ma YH, et al. Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice. J Neurosci Res. 2003;73:565–72. doi: 10.1002/jnr.10677. [DOI] [PubMed] [Google Scholar]
- 64.Xu YF, Cai YQ, Cai GQ, et al. Hypoalgesia in mice lacking GABA transporter subtype 1. J Neurosci Res. 2008;86:465–70. doi: 10.1002/jnr.21499. [DOI] [PubMed] [Google Scholar]
- 65.Todorov AA, Kolchev CB, Todorov AB. Tiagabine and gabapentin for the management of chronic pain. Clin J Pain. 2005;21(4):358–61. doi: 10.1097/01.ajp.0000110637.14355.77. [DOI] [PubMed] [Google Scholar]
- 66.Kataoka K, Hara K, Haranishi Y, et al. The Anti-nociceptive Effect of SNAP5114, a Gamma-Aminobutyric Acid Transporter-3 Inhibitor, in Rat Experimental Pain Models. Anesthesia & Analgesia. 2013;116(5):1162–1169. doi: 10.1213/ANE.0b013e318282dda7. [DOI] [PubMed] [Google Scholar]
- 67.Ford A, Castonguay A, Cottet M, et al. Engagment of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci. 2015;35(15):6057–6067. doi: 10.1523/JNEUROSCI.4495-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valencia-de Ita S, Lawand NB, Lin Q, et al. Role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol. 2006;95(6):3553–61. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- 69.Gagnon M, Bergeron MJ, Lavertu G, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19(11):1524–8. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z, Cai YQ, Zou F, et al. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tao W, Chen Q, Wang L, et al. Brainstem brain-derived neurotrophic factor signaling is required for histone deacetylase inhibitor-induced pain relief. Mol Pharmacol. 2015;87(6):1035–1041. doi: 10.1124/mol.115.098186. [DOI] [PubMed] [Google Scholar]
- 72*.Zhang Z, Tao W, Hou YY, et al. Persistent pain facilitates response to morphine reward by downregulation of central amygdala GABAergic function. Neuropsychopharmacol. 2014;39(9):2263–71. doi: 10.1038/npp.2014.77. [Interesting discussion of the intersection between opioid and reward-center pathways. Opioids may be particularly likely to cause addiction to those undergoing painful stimuli via increased action at the reward center.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan WX, Chen SR, Chen H, et al. Stimulation of alpha1-Adrenoreceptors Reduces Glutamatergic Synaptic Input from Primary Afferents through GABAa Receptors and T-type Ca Channels. Neurosci. 2009;158(4):1616–1624. doi: 10.1016/j.neuroscience.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology. 2010;112(5):1259–1265. doi: 10.1097/ALN.0b013e3181d3e1ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Wang H, Xie K, et al. Inhibition of glycogen synthase kinase-3β prevents remifentanil-induced hyperalgesia via regulating the expression and function of spinal N-methyl-D-aspartate receptors in vivo and in vitro. PLoS One. 2013;8(10):e77790. doi: 10.1371/journal.pone.0077790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonnet CS, Williams AS, Gilber SJ, et al. AMAP/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann Rheum Dis. 2015;74:242–251. doi: 10.1136/annrheumdis-2013-203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang XT, Lian X, Xu YM, et al. Alpha2 noradrenergic receptor suppressed CaMKII signaling in spinal dorsal horn of mice with inflammatory pain. Eur J Pharmacol. 2014;724:16–23. doi: 10.1016/j.ejphar.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 78.Wang K, Svensson P, Sessle BJ, et al. Interactions of glutamate and capsaicin-evoked muscle pain on jaw motor functions of men. Clin Neurophysiol. 2010;121(6):950–956. doi: 10.1016/j.clinph.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 79.Baad-Hansen L, Cairns BE, Ernberg M, et al. Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalgia. 2010;30(1):68–76. doi: 10.1111/j.1468-2982.2009.01881.x. [DOI] [PubMed] [Google Scholar]
- 80.Truini A, Piroso S, Pasquale E, et al. N-acetyl-cysteine, a drug that enhances the endogenous activation of group-II metabotropic glutamate receptors, inhibits nociceptive transmission in humans. Mol Pain. 2015;11:14. doi: 10.1186/s12990-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nam M, Kim TH, Kwak J, et al. Discovery and biological evaluation of tetrahydrothieno(2,3-c)pyridine derivatives as selective metabotropic glutamate receptor 1 antagonists for the potential treatment of neuropathic pain. Eur J Med Chem. 2015;97:245–258. doi: 10.1016/j.ejmech.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 82.Castel A, Helie P, Beaudry F, et al. Bilateral central pain sensitization in rats following a unilateral thalamic lesion may be treated with high doses of ketamine. BMC Vet Res. 2013;9:59. doi: 10.1186/1746-6148-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shrestha R, Pant S, Shrestha A, et al. Intranasal ketamine for the treatment of patients with acute pain in the emergency department. World J Emerg Med. 2016;7(1):19–24. doi: 10.5847/wjem.j.1920-8642.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laskowski K, Stirling A, McKay WP, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anesthesia. 2011;58(10):911–23. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- 85.Hu YM, Chen SR, Chen H, et al. Casein Kinase II Inhibition Reverses Pain Hypersensitivity and Potentiated Spinal N-Methyl-D-aspartate Receptor Activity Caused by Calcineurin Inhibitor. J Pharmacol Exp Ther. 2014;349(2):239–247. doi: 10.1124/jpet.113.212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tong W, Maira M, Gagnon M, et al. Ligands binding to cell surface ganglioside GD2 cause src-dependent activation of N-methyl-daspartate receptor signaling and changes in cellular morphology. PLoS One. 2015;10(8):e0134255. doi: 10.1371/journal.pone.0134255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy JD, Paskaradevan JA, Eisler LL, et al. Analgesic efficacy of continuous intravenous magnesium infusion as an adjuvant to morphine for postoperative analgesia: a systematic review and meta-analysis. Middle East Journal of Anesthesiology. 2013;22(1):11–20. [PubMed] [Google Scholar]
- 88.Ko MH, Hsieh YL, Hseih ST, et al. Nerve demyelination increases metabotropic glutamate receptor subtype 5 expression in peripheral painful mononeuropathy. Int J Mol Sci. 2015;16(3):4642–65. doi: 10.3390/ijms16034642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiu S, Zhang M, Liu Y, et al. GluA1 phosphrylation contributes to postsynaptic amplication of neuropathic pain in the insular cortex. J Neurosci. 2014;34(40):13505–15. doi: 10.1523/JNEUROSCI.1431-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen SR, Zhou HY, Byun HS, et al. Nerve Injury Increases GluA2-lacking AMPA Receptor Prevalence in Spinal Cords: Functional Significance and Signaling Mechanisms. J Pharmacol Exp Ther. 2013;347(3):765–772. doi: 10.1124/jpet.113.208363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang CY, Chen YL, Li AH, et al. Minocycline, a microglial inhibitor, blocks spinal CCL2-induced heat hyperalgesia and augmentation of glutamatergic transmission in substantia gelatinosa neurons. J Neuroinflammation. 2014;11:7. doi: 10.1186/1742-2094-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeilhofer HU. The glycinergic control of spinal pain processing. Cellular and Molecular Life Sciences CMLS. 2005;62.18:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Basbaum A, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeilhofer HU, Benke D, Yevenes G. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annual review of pharmacology and toxicology. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 95.MacLeod BA, Wang JT, Chung CC, et al. Analgesic properties of the novel amino acid, isovaline. Anesth Analg. 2010;110(4):1206–1214. doi: 10.1213/ANE.0b013e3181d27da2. [DOI] [PubMed] [Google Scholar]
- 96.Asseri KA, Pull E, Schwarz SK, et al. Group II metabotropic glutamate receptor antagonism prevents the antiallodynic effects of R-isovaline. Neuroscience. 2015;293:151–156. doi: 10.1016/j.neuroscience.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 97.Zhang JY, Gong N, Huang JL, et al. Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait., exhibits potent and specific anti-nociception in chronic pain by acting at spinal α3 glycine receptors. Pain. 2013;154(11):2452–2462. doi: 10.1016/j.pain.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 98.Wu YE, Li YD, Luo YJ, et al. Gelsemine alleviates both neuropathic pain and sleep disturbance in partial sciatic nerve ligation mice. Acta Pharmacol Sin. 2015;36(11):1308–17. doi: 10.1038/aps.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haranishi Y, Hara K, Terada T, et al. The anti-nociceptive effect of intrathecal administration of glycine transporter-2 inhibitor ALX1393 in a rat acute pain model. Anesth Analg. 2010;110(2):615–621. doi: 10.1213/ANE.0b013e3181c7ebbb. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi Y, Hara K, Haranishi Y, et al. Anti-nociceptive effect of intracerebroventricular administration of glycine transporter-2 inhibitor ALX1393 in rat models of inflammatory and neuropathic pain. Pharmacol Biochem Behav. 2015;130:46–52. doi: 10.1016/j.pbb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 101.Edington AR, McKinzie AA, Reynolds AJ, et al. Extracellular loops 2 and 4 of GLYT2 are required for N-arachidonylglycine inhibition of glycine transport. J Biol Chem. 2009;284.52:36424–36430. doi: 10.1074/jbc.M109.017509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morita K, Motoyama N, Kitayama T, et al. Spinal antiallodynia action of glycine transporter inhibitors in neuropathic pain models in mice. J Pharmacol Exp Ther. 2008;326.2:633–645. doi: 10.1124/jpet.108.136267. [DOI] [PubMed] [Google Scholar]
- 103.Mingorance-Le Meur A, Ghisdal P, Mullier B, et al. Reversible inhibition of the glycine transporter GlyT2 circumvents acute toxicity while preserving efficacy in the treatment of pain. Br J Pharmacol. 2013;170.5:1053–1063. doi: 10.1111/bph.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiménez E, Zafra F, Perez-Sen R, et al. P2Y purinergic regulation of the glycine neurotransmitter transporters. J Biol Chem. 2011;286(12):10712–10724. doi: 10.1074/jbc.M110.167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ando RD, Mehesz b, Gyires K, et al. A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br J Pharmacol. 159(5):1106–1117. doi: 10.1111/j.1476-5381.2009.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mobarakeh JI, Sakurada S, Katsuyama S, et al. Role of histamine H 1 receptor in pain perception: a study of the receptor gene knockout mice. Eur J Pharmacol. 2000;391(1):81–89. doi: 10.1016/s0014-2999(00)00060-1. [DOI] [PubMed] [Google Scholar]
- 107.Mahdieh A, Khani MRM. Evaluating the Anti-nociceptive and Anti-inflammatory Effects of Ketotifen and Fexofenadine in Rats. Advanced Pharmaceutical Bulletin. 2014;5.2 doi: 10.15171/apb.2015.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cornbleet T. Use of intravenously given hydroxyzine for simple pain-producing office procedures. Journal of the American Medical Association. 1960;172.1:56–57. doi: 10.1001/jama.1960.63020010004014b. [DOI] [PubMed] [Google Scholar]
- 109.Hupert C, Yacoub M, Turgeon LR. Effect of hydroxyzine on morphine analgesia for the treatment of postoperative pain. Anesth Analg. 1980;59.9:690–696. [PubMed] [Google Scholar]
- 110.Stambaugh JE, Lane C. Analgesic efficacy and pharmacokinetic evaluation of meperidine and hydroxyzine, alone and in combination. Cancer investigation. 1983;1(2):111–118. doi: 10.3109/07357908309042413. [DOI] [PubMed] [Google Scholar]
- 111.Duarte C, Dunaway F, Turner L, et al. Ketorolac versus meperidine and hydroxyzine in the treatment of acute migraine headache: a randomized, prospective, double-blind trial. Annals of emergency medicine. 1992;21.9:1116–1121. doi: 10.1016/s0196-0644(05)80654-7. [DOI] [PubMed] [Google Scholar]
- 112.Erfanparast A, Tamaddonfard E, Taati M, et al. Role of the thalamic submedius nucleus histamine H1 and H2 and opioid receptors in modulation of formalin-induced orofacial pain in rats. Naunyn-Schmiedeberg's Arch Pharmacol. 2015;388(10):1089–1096. doi: 10.1007/s00210-015-1143-0. [DOI] [PubMed] [Google Scholar]
- 113.Erfanparast A Tamaddonfard E, Farshid AA, et al. Effect of microinjection of histamine into the dorsal hippocampus on the orofacial formalin-induced pain in rats. Eur J Pharmacol. 2010;627(1-3):119–23. doi: 10.1016/j.ejphar.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 114.Tamaddonfard E, Rahimi S. Central effect of histamine and peripheral effect of histidine on the formalin-induced pain response in mice. Clin. Exp Pharmacol Physiol. 2004;31:518–522. doi: 10.1111/j.1440-1681.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 115.Mojtahedin A, Tamaddonfard E, Zanbouri A. Anti-nociception induced by central administration of histamine in the formalin test in rats. Indian J Physiol Pharmacol. 2008;52:249–254. [PubMed] [Google Scholar]
- 116.Hamzeh-Gooshchi N, Tamaddonfard E, Farshid EA. Effects of microinjection of histamine into the anterior cingulate cortex on pain-related behaviors induced by formalin in rats. Pharmacological Reports. 2015;67(3):593–599. doi: 10.1016/j.pharep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 117.Tamaddonfard E, Hamzeh-Gooshchi N. Effects of administration of histamine and its H1, H2, and H3 receptor antagonists into the primary somatosensory cortex on inflammatory pain in rats. Iranian journal of basic medical sciences. 2014;17.1:55. [PMC free article] [PubMed] [Google Scholar]
- 118.Tamaddonfard E, Erfanparast A, Farshid AA, et al. Interaction between histamine and morphine at the level of the hippocampus in the formalininduced orofacial pain in rats. Pharmacol Rep. 2011;63.2:423–432. doi: 10.1016/s1734-1140(11)70508-4. [DOI] [PubMed] [Google Scholar]
- 119.Sanna MD, Stark H, Lucarini L, et al. Histamine H4 receptor activation alleviates neuropathic pain through differential regulation of ERK, JNK and P38 MAPK phosphorylation. Pain. 2015 doi: 10.1097/j.pain.0000000000000319. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 120.Kiss R, Keseru GM. Histamine H4 receptor ligands and their potential therapeutic applications: an update. Expert opinion on therapeutic patents. 2012;22(3):205–221. doi: 10.1517/13543776.2012.665447. [DOI] [PubMed] [Google Scholar]
- 121.Yue JX, Wang RR, Yu J, et al. Histamine Upregulates Nav1. 8 Expression in Primary Afferent Neurons via H2 Receptors: Involvement in Neuropathic Pain. CNS Neurosci Ther. 2014;20(10):883–892. doi: 10.1111/cns.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122*.Yu J, Fang Q, Lou GD, et al. Histamine Modulation of Acute Nociception Involves Regulation of Nav1. 8 in Primary Afferent Neurons in Mice. CNS Neurosci Ther. 2013;19(9):649–658. doi: 10.1111/cns.12134. [Histamine up-regulates expression of voltage-gated sodium channels known to be involved in the process of pain transduction. Histamine antagonists, then, may theoretically decrease an individual's ability to sense pain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei H, Jin CY, Viisanen H, et al. Histamine in the locus coeruleus promotes descending noradrenergic inhibition of neuropathic hypersensitivity. Pharmacol Res. 2014;90:58–66. doi: 10.1016/j.phrs.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 124.Hui L, Yuan J, Ren Z, et al. Nerve growth factor reduces apoptotic cell death in rat facial motor neurons after facial nerve injury. Neurosciences. 2015;20(1):65–68. [PMC free article] [PubMed] [Google Scholar]
- 125.Rukwied R, Mayer A, Kluschina O, et al. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersenstivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 126.Ettinger K, Nevo Y, Marcinkiewicz C, et al. Nerve growth factor-induced myoprotection in C2C12 muscle cells is mediated by alpha9beta1 integrin via release of PGE2. J Basic Clin Physiol Pharmacol. 2015;26(4):411–5. doi: 10.1515/jbcpp-2014-0111. [DOI] [PubMed] [Google Scholar]
- 127.Mizumura K, Taguchi T. Delayed onset muscle soreness: involvement of neurotrophic factors. J Phyiol Sci. 2015 doi: 10.1007/s12576-015-0397-0. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen JW, Tong RX, Yang J, et al. Effects of Bushen Wenyang Huayu Recipe on TRPV1 and Sensitization Factor NGF in Experimental Endometriosis. Zhonqquo Zhong Xi Yi Jie He Za Zhi. 2015;35(6):717–23. [PubMed] [Google Scholar]
- 129.Luvone T, Affaitati G, De Filippis D, et al. Ultramicronized Palmitoylethanolamide Reduces Viscero-Visceral Hyperalgesia in a Rat Model of Endometriosis Plus Ureteral Calculosis: Role of Mast Cells. Pain. 2015 doi: 10.1097/j.pain.0000000000000220. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 130.Lopez-Alvarez VM, Modol L, Navarro X, et al. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156(9):1812–25. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 131.Eskander MA, Ruparel S, Green DP, et al. Persistent Nociception Triggered by Nerve Growth Factor (NGF) is Mediated by TRPV1 and Oxidative Mechanisms. J Neurosci. 2015;35(22):8593–603. doi: 10.1523/JNEUROSCI.3993-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Isa IL, Srivastava A, Tiernan D, et al. Hyaluronic Acid Based Hydrogels Attenuate Inflammatory Receptors and Neurotrophins in Interleukin-1beta Induced Inflammation Model of Nucleus Pulposus Cells. Biomacromolecules. 2015;16(6):1714–25. doi: 10.1021/acs.biomac.5b00168. [DOI] [PubMed] [Google Scholar]
- 133.Cirillo G, Cavaliere C, Bianco MR, et al. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol. 2010;30:51–62. doi: 10.1007/s10571-009-9430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154(7):1009–1021. doi: 10.1016/j.pain.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Balanescu AR, Feist E, Wolfram G, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III clinical trial. Ann Rheum Dis. 2014;73(9):1665–1672. doi: 10.1136/annrheumdis-2012-203164. [DOI] [PubMed] [Google Scholar]
- 136.Gow JM, Tsuji WH, Williams GJ, et al. Safety, tolerability, pharmacokinetics, and efficacy of AMG 403, a human anti-nerve growth factor monoclonal antibody, in two phase 1 studies with healthy volunteers and knee osteoarthritis subjects. Arthritis Res Ther. 2015;17:282. doi: 10.1186/s13075-015-0797-9. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stoppiello L, Mapp P, Wilson D, et al. Structural Associations of Symptomatic Knee Osteoarthritis. Arthritis Rheumatol. 2014;66(11):3018–3027. doi: 10.1002/art.38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthritis Cartilage. 2013;21(9):1223–1228. doi: 10.1016/j.joca.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naveilhan P, Hassani H, Lucas G, et al. Reduced anti-nociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409(1):513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- 140.Shi TJ, Li J, Dahlstrom A, et al. Deletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbers. Neuroscience. 2006;140:293–304. doi: 10.1016/j.neuroscience.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 141*.Wang L, Zhang L, Pan H, et al. Levels of neuropeptide Y in synovial fluid relate to pain in patients with knee osteoarthritis. BMC Musculoskeletal Disord. 2014;15:319. doi: 10.1186/1471-2474-15-319. [NPY seems to vary directly with patient reported pain scores in a model of knee pain. As NPY rises, patient pain rises. As NPY falls, patients’ reported pain also fell.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bokarewa M, Erlandsson M, Bjersing J, et al. Smoking is associated with reduced leptin and neuropeptide Y levels and higher pain experience in patients with fibromyalgia. Mediators Inflamm. 2014 doi: 10.1155/2014/627041. doi: 10.1155/kroin2014/627041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boateng EK, Novejarque A, Pheby T, et al. Heterogenous responses of dorsal root ganglion neurons in neuropathies induced by peripheral nerve trauma and the antiretroviral drug stavudine. Eur J Pain. 2015;19(2):236–45. doi: 10.1002/ejp.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sathyanesan M, Girgenti M, Warner-Schmidt J, et al. Indomethacin induced gene regulation in the rat hippocampus. Mol Brain. 2015;8:59. doi: 10.1186/s13041-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chun EH, Kim H, Suh CS, et al. Polymorphisms in neuropeptide genes and bone mineral density in Korean postmenopausal women. Menopause. 2015;22(11):1256–63. doi: 10.1097/GME.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 146.Carvajal C, Dumont Y, Herzog H, et al. Emotional behavior in aged neuropeptide Y (NPY) Y2 knockout mice. J Mol Neurosci. 2006;28:239–246. doi: 10.1385/JMN:28:3:239. [DOI] [PubMed] [Google Scholar]
- 147.Gautam M, Kumar R, Prasoon P, et al. Anti-nociceptive effect of 1400 W, an inhibitor of inducible nitric oxide synthase, following hind paw incision in rats. Nitric Oxide. 2015;50:98–104. doi: 10.1016/j.niox.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 148.Khan S, Shehzad O, Cheng MS, et al. Pharmacological mechanism underlying anti-inflammatory properties of two structurally divergent coumarins through the inhibition of pro-inflammatory enzymes and cytokines. J Inflamm (Lond) 2015;12:47. doi: 10.1186/s12950-015-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hervera A, Negrete R, Leánez S, et al. The Role of Nitric Oxide in the Local Antiallodynic and Antihyperalgesic Effects and Expression of δ-Opioid and Cannabinoid-2 Receptors during Neuropathic Pain in Mice. J Pharmacol Exp Ther. 2010;334(3):887–96. doi: 10.1124/jpet.110.167585. [DOI] [PubMed] [Google Scholar]
- 150.Jin XG, Chen SR, Cao XH, et al. Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. J Biol Chem. 2011;286:33190–33202. doi: 10.1074/jbc.M111.270967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maire JJ, Close LN, Heinricher MM, et al. Distinct pathways for norepinephrine- and opioid-triggered anti-nociception from the amygdala. Eur J Pain. 2015 doi: 10.1002/ejp.708. doi: 10.1002/ejp.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Singh RB, Chopra N, Choubey S, et al. Role of Clonidine as adjuvant to intrathecal bupivicaine in patients undergoing lower abdominal surgery: A randomized control study. Anesthesia Essays and Research. 2014;8(3):307–12. doi: 10.4103/0259-1162.143119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sen J, Sen B. Response to low-dose intrathecal clonidine in septuagenarians undergoing sub-umbilical surgeries: A study. Saudi Journal of Anesthesia. 2015;9(2):142–7. doi: 10.4103/1658-354X.152840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Blaudszun G, Lysakowski C, Elia N, et al. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116(6):1312–22. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- 155.Surendar MN, Pandey RK, Saksena AK, et al. A comparative evaluation of intranasal dexmedetomidine, midazolam and ketamine for their sedative and analgesic properties: a triple blind randomized study. Journal of Clinical Pediatric Dentistry. 2014;38(3):255–61. doi: 10.17796/jcpd.38.3.l828585807482966. [DOI] [PubMed] [Google Scholar]
- 156.Soto N, Fauber AE, Ko JC, et al. Analgesic effect of intra-articularly administrered morphine, dexmedetomidine, or a morphine-dexmedetomidine combination immediately following stifle joint surgery in dogs. Journal of the American Veterinary Medicine Associ ation. 2014;244(11):1291–7. doi: 10.2460/javma.244.11.1291. [DOI] [PubMed] [Google Scholar]
- 157.Gao Y, Guo X, Han P, et al. Treatment of patients with diabetic peripheral neuropathic pain in China: a double-blind randomised trial of duloxetine vs. placebo. International Journal of Clinical Practice. 2015 doi: 10.1111/ijcp.12641. doi: 10.1111/ijcp.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhosale UA, Khobragade R, Naik C, et al. Randomized, double-blind, placebo-controlled study to investigate the pharmacodynamic interaction of 5-HT3 antagonist ondansetron and paracetamol in postoperative patients operated in an ENT department under local anesthesia. Journal of Basic Clinical Physiology and Pharmacology. 2015;26(3):217–22. doi: 10.1515/jbcpp-2014-0070. [DOI] [PubMed] [Google Scholar]
- 159.Pickering G, Esteve V, Loriot MA, et al. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84(1):47–51. doi: 10.1038/sj.clpt.6100403. [DOI] [PubMed] [Google Scholar]
- 160.Pickering G, Loriot MA, Libert F, et al. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79(4):371–8. doi: 10.1016/j.clpt.2005.12.307. [DOI] [PubMed] [Google Scholar]
- 161.Ren J, Ding X, Greer JJ. 5-HT1A receptor agonist Befiradol reduces fentanyl-induced respiratory depression, analgesia, and sedation in rats. Anesthesiology. 2015;122(2):424–34. doi: 10.1097/ALN.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 162.Kim JM, Jeong SW, Yang J, et al. Spinal 5-HT1A, not the 5-HT1B or 5-HT3 receptors, mediates descending serotonergic inhibition for late-phase mechanical allodynia of carrageenan-induced peripheral inflammation. Neuroscience Letters. 2015;600:91–97. doi: 10.1016/j.neulet.2015.05.058. 29. [DOI] [PubMed] [Google Scholar]
- 163.Yesilyurt O, Seyrek M, Tasdemir S, et al. The critical role of spinal 5-HT7 receptors in opioid and non-opioid type stress-induced analgesia. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.04.020. doi:10.1016/j.ejphar.2015.04.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 164.Leombruni P, Miniotti M, Colonna F, et al. A randomised controlled trial comparing duloxetine and acetyl L-carnitine in fibromyalgic patients: preliminary data. Clinical Experimental Rheumatology. 2015;33:S82–5. [PubMed] [Google Scholar]
- 165.Walitt B, Urrutia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database System Review. 2015:CD011735. doi: 10.1002/14651858.CD011735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Regoli D, Boudon A, Fauchere JL. Receptors and antagonists for substance P and related peptides. Pharmacol Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- 167.Rupniak NMJ, Webb JK, Williams AR, et al. Anti-nociceptive activity of the tachykinin NK-1 receptor antagonist, C-99, 994, in conscious gerbils. Br J Pharmacol. 1995;116(2):1937–1943. doi: 10.1111/j.1476-5381.1995.tb16686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gonzalez MI, Field MJ, Hughes J, et al. Evaluation of selective NK-1 receptor antagonist CI-1021 in animal models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2000;294(2):444–450. [PubMed] [Google Scholar]
- 169.Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post- treatment with a neurokinin-1 antagonist. Pain. 2002;95(3):277–285. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 170.Dionne RA, Max MB, Gordon SM, et al. The substanc P receptor antagonist CP-99, 994 reduces acute postoperative pain. Clin Pharmacol Ther. 1998;64(5):562–568. doi: 10.1016/S0009-9236(98)90140-0. [DOI] [PubMed] [Google Scholar]
- 171.Chizh BA, Gohring M, Troster A, et al. Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth. 2007;98(2):246–254. doi: 10.1093/bja/ael344. [DOI] [PubMed] [Google Scholar]
- 172.Goldstein DJ, Wang O, Gitter BD, et al. Dose-response study of the analgesic effect of lanepitant in patients with painful diabetic neuropathy. Clin Neuropharmacol. 2001;24(1):16–22. doi: 10.1097/00002826-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 173.Chen W, McRoberts JA, Marvizon JCV. μ-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain. Neuroscience. 2014;267:67–82. doi: 10.1016/j.neuroscience.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174*.Liu NJ, Gintzler AR. Facilitative interactions between vasoactive intestinal polypeptide and receptor type-selective opioids: implications for sensory afferent regulation of spinal opioid action. Brain res. 2003;959(1):103–110. doi: 10.1016/s0006-8993(02)03734-4. [VIP expression was increased in association with decreased pain thresholds prior to parturition in female rats. VIP is associated with the pain of childbirth.] [DOI] [PubMed] [Google Scholar]
- 175.Tumati S, Largent-Milnes TM, Keresztes AI, et al. Tachykinin NK 1 receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation. Eur J Pharmacol. 2012;684(1):64–70. doi: 10.1016/j.ejphar.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Uematsu T, Sakai A, Ito H, et al. Intra-articular administration of tachykinin NK 1 receptor antagonists reduces hyperalgesia and cartilage destruction in the inflammatory joint in rats with adjuvant-induced arthritis. Eur J Pharmacol. 2011;668(1):163–168. doi: 10.1016/j.ejphar.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 177.Camargo LL, Denadai-Souza A, Yshii LM, et al. Peripheral Neurokinin-1 Receptors Contribute to Kaolin-Induced Acute Monoarthritis in Rats. Neuroimmunomodulation. 2015;22(6):373–84. doi: 10.1159/000381549. [DOI] [PubMed] [Google Scholar]
- 178.Chauhan VS, Kluttz JM, Bost KL, et al. Prophylactic and therapeutic targeting of the neurokinin-1 receptor limits neuroinflammation in a murine model of pneumococcal meningitis. J Immunol. 2011;186(12):7255–7263. doi: 10.4049/jimmunol.1100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Simavli S, Thompson IR, Maguire CA, et al. Substance P Regulates Puberty Onset and Fertility in the Female Mouse. Endocrinology. 2015;156(6):2313–2322. doi: 10.1210/en.2014-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Niedermair T, Kuhn V, Doranehgard F, et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biology. 2014;38:22–35. doi: 10.1016/j.matbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 181.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123:98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 182.Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP 6–28 reduces nociception in an animal model of osteoarthritis. Osteoarthritis and cartilage. 2006;14(11):1155–1162. doi: 10.1016/j.joca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 183.Cernuda-Morollón E, Martinez-Camblor P, Alvarez R, et al. Increased VIP levels in peripheral blood outside migraine attacks as a potential biomarker of cranial parasympathetic activation in chronic migraine. Cephalalgia. 2015;35(4):310–316. doi: 10.1177/0333102414535111. [DOI] [PubMed] [Google Scholar]
- 184.Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 2014;137(pt3):779–94. doi: 10.1093/brain/awt369. [DOI] [PubMed] [Google Scholar]
- 185.Shehab S, Al-Deen S. Fifth lumbar spinal nerve injury causes neurochemical changes in corresponding as well as adjacent spinal segments: a possible mechanism underlying neuropathic pain. Journal of chemical neuroanatomy. 2014;55:38–50. doi: 10.1016/j.jchemneu.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 186.Son SJ, Lee KM, Jeon SM, et al. Activation of transcription factor c-jun in dorsal root ganglia induces VIP and NPY upregulation and contributes to the pathogenesis of neuropathic pain. Experimental Neurology. 2007;204(1):467–472. doi: 10.1016/j.expneurol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 187.Kim HJ, Back SK, Kim J, et al. Increases in spinal vasoactive intestinal polypeptide and neuropeptide Y are not sufficient for the genesis of neuropathic pain in rats. Neuroscience letters. 2003;342(1):109–113. doi: 10.1016/s0304-3940(03)00254-4. [DOI] [PubMed] [Google Scholar]
- 188.Li J, Wang X, Liu H, et al. In Silico Classification and Prediction of VIP Derivatives as VPAC1/VPAC2 Receptor Agonists/Antagonists. Comb Chem High Throughput Screen. 2015;18(1):33–41. doi: 10.2174/1386207317666141128104031. [DOI] [PubMed] [Google Scholar]
- 189.Smith MT, Muralidharan A. Targeting angiotensin II type 2 receptor pathways to treat neuropathic pain and inflammatory pain. Expert Opinion on Therapeutic Targets. 2015;19(1):25–35. doi: 10.1517/14728222.2014.957673. [DOI] [PubMed] [Google Scholar]
- 190.Gris G, Portillo-Salido E, Aubel B, et al. The selective sigma-1 receptor antagonist E-52862 attentuates neuropathic pain of different aetiology in rats. Scientific Reports. 2016;6:24591. doi: 10.1038/srep24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gilron I, Moore RA, Wiffen PJ, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. The Cochrane Library. 2010 doi: 10.1002/14651858.CD008307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Clarke H, Bonin RP, Orser BA, et al. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428–42. doi: 10.1213/ANE.0b013e318249d36e. [DOI] [PubMed] [Google Scholar]
- 193.Kerfeld MJ, Hambsch ZJ, McEntire DM, et al. Physiologic Advantages of Peripheral Nerve Blockade Translate to Decreased Length of Stay and Improved Patient Satisfaction. Open Anesthesiology. 2016;1:1. [Google Scholar]
- 194.Fabi DW. Multimodal Analgesia in the Hip Fracture Patient. Journal of orthopaedic trauma. 2016;30:S6–S11. doi: 10.1097/BOT.0000000000000561. [DOI] [PubMed] [Google Scholar]