Abstract
Stress-activated transcription factors influence T-cell function in different physiopathologic contexts. NFAT5, a relative of nuclear factor κB and the calcineurin-activated NFATc transcription factors, protects mammalian cells from hyperosmotic stress caused by the elevation of extracellular sodium levels. In T cells exposed to hypernatremia, NFAT5 not only induces osmoprotective gene products but also cytokines and immune receptors, which raises the question of whether this factor could regulate other T-cell functions in osmostress-independent contexts. Here we have used mice with a conditional deletion of Nfat5 in mature T lymphocytes to explore osmostress-dependent and -independent functions of this factor. In vitro experiments with CD4 T cells stimulated in hyperosmotic medium showed that NFAT5 enhanced the expression of IL-2 and the Th17-associated gene products RORγt and IL-23R. By contrast, NFAT5-deficient CD4 T cells activated in vivo by anti-CD3 antibody exhibited a different activation profile and were skewed towards enhanced interferon γ (IFNγ) and IL-17 expression and attenuated Treg responses. Using a model of experimental colitis, we observed that mice lacking NFAT5 in T cells exhibited exacerbated intestinal colitis and enhanced expression of IFNγ in draining lymph nodes and colon. These results show that NFAT5 can modulate different T-cell responses depending on stress conditions and stimulatory context.
The ability to integrate the sensing of stress signals with inputs from diverse receptors is important for leukocytes working at sites of intense immune activity, such as inflamed tissues, infected wounds or tumors, where conditions of nutrient availability, oxygen pressure and ionic balance can differ considerably from blood and lymphoid organs.1, 2 Different stress responsive transcription factors have been shown to regulate both adaptation to stress and T-cell activation and polarization responses. For instance, FOXO transcription factors confer protection against oxidative stress and nutrient limitation in lymphocytes.3 They also regulate Th1 and Treg polarization, and effector to memory differentiation in CD8 T cells.3, 4, 5 Hypoxia-inducible factor-1α (HIF-1α) is critical for T-cell adaptation to hypoxia but also promotes Th17 polarization, under both hypoxia and normoxia.6, 7 The transcription factor NFAT5, also known as TonEBP, is related to nuclear factor κB and the calcineurin-activated NFATc proteins, and protects mammalian cells from hyperosmotic stress by inducing gene products that allow them to resist prolonged hyperosmotic conditions.8, 9, 10, 11 In addition, in leukocytes exposed to osmotic stress NFAT5 induces diverse cytokines, receptors and enzymes: tumor necrosis factor-α, lymphotoxin-β and CD24 in T cells, BAFF in B cells and inducible nitric oxide synthase (iNOS) in macrophages.2, 12, 13 Recently, NFAT5 was shown to enhance Th17 polarization in human T cells that were exposed to hypernatremia during stimulation with Th17-inducing cytokines.14
The osmostress response is essential in the kidney medulla, where cells are continuously exposed to substantial elevations of ambient osmotic pressure.15 Besides the renal medulla, systemic hypertonicity with elevated sodium concentrations in blood has been described in certain pathological conditions,16, 17, 18 and in mouse models such as NFAT5-null mice19 or mice mutant for aquaporin 1 and the V2 vasopressin receptor.20, 21 The relevance of hypernatremia and osmotic stress in immune responses in vivo has been addressed in various works. In this regard, we showed that blood hypernatremia enhanced the expression of the homeostatic proliferation regulator CD24 in NFAT5-competent T cells and impaired the proliferation of NFAT5-deficient ones.19 NFAT5-deficient T cells were also biased towards acquiring a memory phenotype under persistent hypernatremia.19 Machnik et al.22 found that skin tissues in rodents subjected to prolonged high-salt diet accumulated sodium at a concentration high enough to elicit NFAT5-mediated osmostress responses in local immune cells, and additional works have shown that a high-salt diet can exacerbate pathogenic autoimmune Th17 responses in mouse models of experimental autoimmune encephalitis.14, 23 More recently, a direct connection between osmotic stress and immune defenses has been shown by the finding that sodium accumulates rapidly in infected wounds and enhances inflammatory functions in local macrophages through NFAT5.2
NFAT5 shares a recognizably conserved DNA-binding domain with NFATc proteins but differs significantly from them in several key features. In contrast to NFATc, whose nuclear translocation is tightly regulated by calcineurin, NFAT5 lacks the calcineurin-binding motifs that define NFATc proteins, does not depend on calcineurin for its activation, and is found in both the nucleus and the cytosol in unstimulated cells.8, 12, 24 Nonetheless, calcineurin can indirectly stimulate NFAT5 by enhancing its expression, for instance during lymphocyte activation by T-cell receptor signaling25 or in mitogen-activated lymphocytes exposed to osmotic stress.12 Activation of NFAT5 by osmostress in different cell types, including lymphocytes, is mainly regulated by the mitogen and stress-activated activated kinase p38,14, 26, 27 with other kinases such as the DNA damage and stress response ataxia telangiectasia mutated,28 Fyn,26 protein kinase A29 and the growth regulator mammalian target of rapamycin30 also contributing to NFAT5 activation by osmotic stress.
While NFAT5 has been characterized as a general regulator of osmostress responses in multiple cell types, other works have identified osmostress-independent functions of this factor in different cells, leukocytes included.31 NFAT5 is activated in macrophages in response to Toll-like receptor stimulation to regulate the induction of diverse gene products involved in inflammatory functions and antipathogen defense, and is particularly critical for macrophage responsiveness to low concentrations of pathogen-derived products.32 In macrophages, NFAT5 has also been shown to exacerbate inflammatory responses in a model of Toll-like receptor-induced arthritis,33 and to enhance HIV replication.34 In thymocytes, NFAT5 regulates the transition through the double-negative stage, and suppression of NFAT5 impairs thymocyte progression to double-positive cells and leads to a reduced pool of mature CD4 and CD8 peripheral T cells.35 By contrast, suppressing NFAT5 after thymocytes have become double positive does not impair T-cell maturation or alter the homeostatic proportions of T-cell subsets in the periphery.19, 35 In view of these observations we asked whether the role of NFAT5 in mature T cells was limited to osmostress responses or could extend to a wider set of functions. In this work we identify osmostress-dependent and -independent roles for NFAT5 in activated T cells. Results presented here show that NFAT5 regulated different responses depending on stress and stimulatory context. In CD4 T cells activated in hypertonic conditions in vitro NFAT5 enhanced the expression of IL-2 and the Th17-associated gene products RORγt and IL-23R, whereas in vivo-activated NFAT5-deficient CD4 T cells showed a skewing towards enhanced IFNγ and IL-17A expression. We also found that mice lacking NFAT5 specifically in T cells exhibited worsened intestinal pathology in an experimental model of colitis, and enhanced IFNγ mRNA expression in draining lymph nodes and colon. These results revealed a previously unsuspected capacity of NFAT5 to limit pathogenic inflammatory responses in osmostress-independent contexts.
Results
Regulation of IL-2 and Th17-associated genes by NFAT5 in T lymphocytes exposed to hypernatremia
To assess how the lack of NFAT5 in mature T cells might affect their function in osmostress and non-stress conditions we used two approaches. One was to activate CD4 T cells in culture with antibodies to CD3 and CD28 in isotonic or hypertonic medium, and the other was to stimulate T cells in vivo by injecting anti-CD3 antibody in mice. We used NFAT5 conditional knockout mice, Cd4CreNfat5fl/fl, which lacked NFAT5 specifically in mature T cells.19 We had previously described that loss of NFAT5 in T cells in this mouse model did not cause any obvious phenotype in the T-cell compartment in homeostatic conditions.19, 35
Exposure of CD4 T cells to hypernatremia during stimulation with antibodies to CD3 and CD28 enhanced the expression of the mRNA of IL-2 and the Th17 cell markers RORγt, IL-23R and IL-17A by 48 h (Figure 1 and Supplementary Figure S1). High salt also caused a mild increase in T-bet mRNA expression without enhancing IFNγ, did not affect the Treg cell marker FOXP3 and inhibited the expression of the Th2 markers GATA3 and IL-4 (Supplementary Figure S1). NFAT5-deficient T cells showed reduced induction of IL-2, RORγt and IL-23R mRNAs in response to hypernatremia, but induced IL-17A mRNA comparably to wild-type cells (Figure 1a). NFAT5 did not affect T-bet, IFNγ and FOXP3 under hypernatremia, nor contributed to the downregulation of GATA3 and IL-4 (Supplementary Figure S1). In these experiments, we did not detect any obvious effect of NFAT5 in the genes analyzed when T cells were not exposed to osmotic stress. We also asked whether NFAT5 could regulate Th17 induction by the polarizing cytokines IL-6 and TGFβ in isotonic conditions, and found that lack of NFAT5 did not impair the induction of RORγt, IL-17A and IL-23R (Figure 1b). Altogether, these results showed that NFAT5 promoted a Th17-like phenotype when T cells were activated under hypernatremia, but not in the absence of osmotic stress.
Figure 1.
Effect of NFAT5 deficiency on the expression of Th17 cell-associated gene products in CD4 T cells under hypernatremia. (a) Expression of the indicated mRNAs in wild-type (Cd4CreNfat5WT (WT)) or NFAT5-deficient CD4 T cells (Cd4CreNfat5fl/fl (KO)) activated with antibodies to CD3 and CD28 in isotonic (300 mOsm kg−1) or hypertonic medium (+NaCl, final 420 mOsm kg−1) for 48 h. Circles represent independent experiments each with a different pair of wild type and NFAT5-deficient CD4 T-cell cultures. Bars within groups represent the mean±s.e.m. (n=6–8). Significance was determined by an unpaired t-test, with Welch's correction when variances differed significantly between sample groups (NFAT5, RORγt, IL-23R). ***P<0.001, **P<0.01 and *P<0.05. (b) mRNA levels of the indicated gene products in wild type or NFAT5-deficient CD4 T cells activated with antibodies to CD3 and CD28 in Th17-inducing conditions (TGFβ plus IL-6) in isotonic medium for 48 h. (c) Wild type or NFAT5-deficient CD4 T cells were activated in isotonic (300 mOsm kg−1) of hypertonic medium (+NaCl, 420 mOsm kg−1), with or without the RORγt inhibitor digoxin (Dig) during 48 h. mRNA levels are represented relative to the sample of wild-type CD4 T cells (WT) in hypertonic conditions (+NaCl, shown as 100%). Bars represent the mean±s.e.m. (n=5 independent experiments). Significance was determined by a one-sample t-test for comparisons between each point and its respective hypertonicity-treated wild-type control, which was given a value of 100% in each individual experiment. An unpaired t-test was used for comparisons between other groups of samples. ***P<0.001, **P<0.01 and *P<0.05. (d) Wild-type (WT) or NFAT5-deficient CD4 T cells (KO) were expanded for 3 days and reestimulated with antibodies to CD3 and CD28 in isotonic (300 mOsm kg−1) or hypertonic (500 mOsm kg−1) medium for 12 h. Formaldehyde crosslinked chromatin was immunoprecipitated with preimmune rabbit serum or a mixture of two rabbit polyclonal antibodies specific for NFAT5. Graphics represent the percentage of immunoprecipitated chromatin for Rorc (intron 2) or Akr1b3 (aldose reductase gene promoter) with respect to the chromatin input in each sample. Bars represent the mean±s.e.m. (n=3–5 independent experiments for Rorc and 2–4 experiments for Akr1b3). Statistical significance was determined by an unpaired t-test (*P<0.05).
The observation that IL-17A mRNA was induced by hypernatremia independently from NFAT5 led us to analyze the potential role of RORγt, as this factor is critical for the induction of Th17 genes in response to polarizing cytokines. Inhibition of RORγt with digoxin36 revealed that induction of IL-17A in both wild type and NFAT5-deficient T cells was RORγt-dependent (Figure 1c). This factor was also needed to induce IL-23R but not NFAT5 or IL-2 (Figure 1c). This experiment suggested that even if RORγt was less strongly induced in NFAT5-deficient T cells under osmotic stress, it sufficed to induce IL-17A, and that NFAT5 could contribute to IL-23R expression under hypernatremia by enhancing RORγt induction. We noticed that the Rorc region between exons 2 and 3 that regulates its induction by Th17-promoting transcription factors such as STAT337 contained potential NFAT5-binding sites (5′-(A/G)TGGAAA(C/A/T)-3′).8 Chromatin imunoprecipitation showed that NFAT5 bound to this Rorc region in CD4 T cells stimulated under hypertonic stress (Figure 1d). Collectively, these experiments suggested that NFAT5 could facilitate the acquisition of Th17 features under hypernatremia by enhancing the expression of the main Th17-promoting factor RORγt.
Recent works have shown that cytokine-induced Th17 polarization is enhanced by hypernatremia in a manner dependent on the osmostress responsive kinases p38 and SGK1.14, 23 Besides these pathways, we and others had shown previously that calcineurin and mammalian target of rapamycin complex 1 (mTORC1), which regulate Th17 induction downstream the T-cell receptor, are required for an efficient osmostress response in T lymphocytes.27, 30, 38, 39 We analyzed the contribution of calcineurin and mTORC1 to the Th17-like response induced by hypernatremia (Supplementary Figure S2a). The calcineurin inhibitor FK506 impaired the expression of IL-2, RORγt, IL-17A and aldose reductase (AR), a hallmark osmotic stress response gene product, but enhanced IL-23R induction. The mTORC1 inhibitor rapamycin strongly reduced the induction of IL-2, IL-17A, IL-23R and AR, and caused a partial inhibition of RORγt expression (Supplementary Figure S2a). We also found that STAT3, an essential inducer of RORγt and IL-17 in response to IL-6,40 was inhibited by hypernatremia in CD4 T lymphocytes stimulated through CD3 plus CD28, which suggested that it was not a main regulator of the Th17-like response induced by hypertonic stress (Supplementary Figure S2b).
Altered Th/Treg balance in NFAT5-deficient T lymphocytes in the absence of osmotic stress
We next analyzed whether NFAT5-deficient T cells showed differences in cytokine expression in response to in vivo stimulation. We injected mice intraperitoneally with an activating antibody to CD3, followed by a recall injection 2 days later, after which we isolated CD4 T cells from mesenteric lymph nodes. CD3-mediated T-cell stimulation in vivo has been shown to elicit an acute inflammatory response and the activation of Th17 cells.41, 42 This experiment showed enhanced IFNγ and IL-17A mRNA induction in NFAT5-deficient CD4 T cells, together with a decrease in FOXP3 expression (Figure 2a). We did not find significant differences in the expression of IL-2 and IL-10, the Th1-associated markers T-bet and IL-12R and the Th2 markers GATA3 and IL-4 (Figure 2a). Although in this experiment we found reduced levels of FOXP3 but not lower CTLA4, pooled analysis of successive independent experiments revealed a bias towards reduced FOXP3 and CTLA4 expression relative to IFNγ and IL-17A in NFAT5-deficient lymphocytes (Figure 2b). Following these results we analyzed the respective proteins by intracellular staining and flow cytometry in mice injected with the Golgi inhibitor brefeldin A at the time of the second anti-CD3 injection.43 This experiment showed a mild increase in intracellular IFNγ and reduced levels of FOXP3 and CTLA4 protein in NFAT5-deficient CD4 T cells (Figure 2c and Supplementary Figure S3). This result was consistent with the skewing in mRNA expression observed in our previous experiments (Figures 2a and b). However, when we analyzed the mRNA levels of these markers in T cells from the brefeldin A-treated mice, which we had separated before processing for intracellular staining, we found that they did not match the changes in protein levels (Figure 2c, bottom panels). Although we do not have a straightforward explanation for this mismatch, it is possible that brefeldin A, which can activate inflammatory functions in macrophages by triggering the endoplasmic reticulum unfolded protein response and production of IL-1β,44, 45 might have displaced the timing of mRNA induction. This, together with our having used a fixed time point for all our in vivo assays, and that the magnitude of mRNA changes between wild-type and NFAT5-deficient T cells was moderate (Figure 2a) might have made us miss potential mRNA differences in this set of samples.
Figure 2.
Expression of Th and Treg markers in NFAT5-deficient CD4 T cells activated in vivo. (a) Mice were left untreated or injected with antibody to CD3 at days 0 and 2, and mesenteric lymph node (mLN) CD4 T cells were isolated 4 h after the second injection. Circles show individual mice and bars within sample groups represent the mean±s.e.m. Data were pooled from two independent experiments, the first with 5 mice of each genotype injected with antibody to CD3, and the second with 5 untreated controls of each genotype and 10 wild type and 8 Cd4CreNfat5fl/fl mice injected with antibody to CD3. Significance was determined by an unpaired t-test, except for IL-17A whose distribution was not Gaussian and was analyzed with a nonparametric Mann–Whitney test. *P<0.05. (b) mRNA expression ratios for the indicated gene products in wild type and NFAT5-deficient CD4 T cells activated in vivo as in (a). Result shows the pooled analysis of three independent experiments with 5–10 mice of each genotype per experiment. Significance was determined by an unpaired t-test. *P<0.05. (c) Upper panels show the percentage of in vivo-activated mLN wild type and NFAT5-deficient CD4 T cells expressing intracellular proteins IFNγ, FOXP3, CTLA4, doubly positive for FOXP3 and CTLA4, and the ratio of IFNγ-positive cells to FOXP3 and CTLA4-double positive (see Supplementary Figure S3). Mice were treated as in (a), except that they received intraperitoneal brefeldin A with the second anti-CD3 injection (4 h). Significance was determined by an unpaired t-test. **P<0.01, *P<0.05. The bottom panels show a parallel analysis of mRNA levels of samples of CD4 T cells from the same experiment.
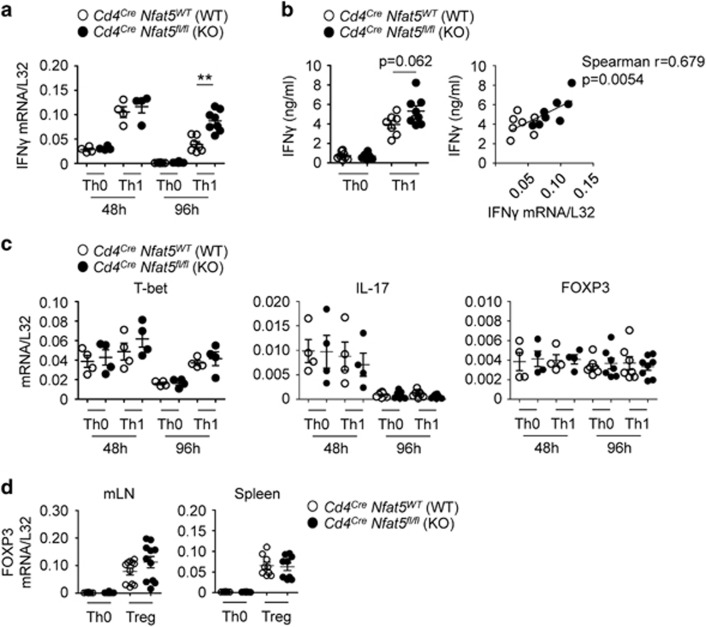
Our initial in vitro experiments had not revealed enhanced IFNγ or IL-17A mRNA induction in NFAT5-deficient T cells in unpolarized and Th17-stimulatory culture conditions (Figures 1a and b). We then asked whether stimulation with the Th1-promoting cytokine IL-12 or induction of Treg cells with TGFβ plus IL-2 might reveal differences between wild-type and NFAT5-deficient T cells. Stimulation of wild-type CD4 T cells in the presence of exogenous IL-12 (Th1 conditions) induced a robust expression of IFNγ, but also a mild increase in IL-10, while stimulation with TGFβ plus IL-2 (Treg) induced FOXP3 and CTLA4 and downregulated IL-10 (Supplementary Figure S4). This result indicated that IFNγ, FOXP3 and CTLA4 discriminated Th1 from Treg cells, whereas IL-10 was better expressed by Th1 than Treg cells. This result agrees with earlier reports showing that IL-10, which has immunosuppressive properties, can be produced by Th1 cells as a mechanism to limit excessive inflammatory responses.46, 47 In view of this result we analyzed IFNγ and FOXP3 as respective Th1 and Treg markers. CD4 T cells stimulated with IL-12 induced IFNγ comparably to wild-type ones in the first 48 h, but maintained a more prolonged expression of it up to 96 h (Figure 3a). We also found a near significant increase in IFNγ protein in supernatants of NFAT5-deficent Th1 cultures after 96 h (Figure 3b). In the absence of IL-12, both wild-type and NFAT5-deficient T lymphocytes expressed similar levels of IFNγ. We did not detect differences between lymphocytes of both genotypes in mRNA expression of T-bet, IL-17A and FOXP3 in the absence or presence of IL-12 at 48 or 96 h (Figure 3c), nor differences in FOXP3 expression under Treg-inducing cytokines (Figure 3d). These results indicated that in vitro stimulation of T cells with IL-12 could reproduce to some extent the enhanced induction of IFNγ observed in vivo in NFAT5-deficient T cells.
Figure 3.
Expression of Th and Treg markers in NFAT5-deficient CD4 T cells activated in culture. (a) Spleen CD4 T cells were activated with antibodies to CD3 and CD28 in neutral conditions (Th0) or plus IL-12 (Th1). Significance was determined by an unpaired t-test. **P<0.01. (b) IFNγ ELISA of CD4 T cells stimulated in neutral conditions or plus IL-12 for 96 h. The panel on the right shows the correlation between IFNγ mRNA and protein induction in wild-type and NFAT5-deficient CD4 T cells stimulated with IL-12 during 96 h. (c) Expression of T-bet, IL-17 and FOXP3 mRNA in the same samples of Th0 and Th1-stimulated cells from (a). (d) Spleen or mLN CD4 T cells were activated during 96 h with antibodies to CD3 and CD28 in neutral conditions (Th0) or plus TGFβ and IL-2 (Treg).
In summary, analysis of several diagnostic Th and Treg markers throughout various independent in vivo experiments indicated that NFAT5-deficient CD4 T cells exhibited a modest but consistent pattern of skewing towards enhanced IFNγ and IL-17A expression relative to FOXP3 and CTLA4. Our culture experiments showed a more sustained expression of IFNγ in NFAT5-deficient cells under IL-12 stimulation, but we did not find changes in IL-17A and FOXP3 in Th17 and Treg cultures. This suggests that the in vivo phenotype of NFAT5-deficient CD4 T cells could depend on a richer context of regulatory cytokines and microenvironment variables than tested in our simpler culture conditions. Nonetheless, our collective results suggested that in the absence of osmostress NFAT5 could balance Th and Treg pro-and anti-inflammatory responses in T cells differently from its Th17-promoting effect observed under hypernatremia.
NFAT5 deficiency in T cells can exacerbate dextran sodium sulfate-induced colitis
After having observed that NFAT5 could influence the expression of different T-cell activation genes in different contexts, we decided to explore whether T cells lacking NFAT5 might alter the outcome of an inflammatory response in vivo using the model of colitis induced by dextran sodium sulfate (DSS).48 DSS disrupts the intestinal epithelial barrier, leading to the activation of pathogenic Th17 and Th1 responses.49, 50, 51, 52, 53 We first did an exploratory experiment in which mice were given 3% DSS in their drinking water during 4 days and then allowed to recover with normal water up to 6 more days. We found that mice whose T cells lacked NFAT5 (Cd4CreNfat5fl/fl mice) had shorter colons than wild-type ones after DSS (Supplementary Figure S5a), although we did not observe differences in body weight loss between both mouse genotypes (Supplementary Figure S5b). We also found higher expression of IFNγ and IL-17A mRNA in mesenteric lymph nodes (mLN) of Cd4CreNfat5fl/fl mice than in wild-type ones 4 days after DSS, a difference that disappeared by day 6 post-DSS (Supplementary Figure S5c). Both mouse genotypes showed similar expression of FOXP3, CD4 and CD8 mRNA in mLNs, but we found increased mRNA levels of CTLA4 at day 4 and a near significant decrease in IL-2 at day 6 in mLN of Cd4CreNfat5fl/fl mice.
We repeated the experiment, this time using a longer DSS treatment of 5 days to induce a stronger response, and analyzed mice 2 and 4 days later. We again observed a more pronounced shortening of the colons in Cd4CreNfat5fl/fl mice, which also exhibited a delayed recovery of body weight after the DSS treatment (Figures 4a and b). Histopathology analysis of colon sections revealed similar types of lesions in both mouse genotypes at day 2 post-DSS, with severe inflammation, a diffused distribution involving mucosa and submucosa, epithelial erosion and crypt disappearance, loss of goblet cells, and areas of mucosal ulceration (Figure 4c). Whereas wild-type mice began to recover by day 4 post-DSS, Cd4CreNfat5fl/fl mice had a poorer recovery and exhibited a significantly higher extension of colon lesions (Figure 4c and d). As in our first experiment, we detected elevated levels of IFNγ and CTLA4 mRNA in mLN of Cd4CreNfat5fl/fl mice 4 days after DSS treatment (Figure 5a), but not earlier at day 2 post-DSS. FOXP3 mRNA levels in mLN did not vary between both mouse genotypes in mLN and colon. We also found increased expression of IL-10 and reduced levels of IL-2 mRNA in mLN of Cd4CreNfat5fl/fl mice. We did not observe differences in the mRNA levels of various T-cell subset markers: CD4, CD8, TCRα and TCRγ (Figure 5a). As mRNA levels of these markers could be considered a surrogate for the abundance of the respective T-cell subsets, this result suggested that these populations were comparably represented in wild type and Cd4CreNfat5fl/fl mice, and that changes in IFNγ and CTLA4 mRNA reflected a shift in their activation or polarization profile. Regarding IL-17A mRNA, both genotypes expressed comparable levels in mLN, but Cd4CreNfat5fl/fl mice had elevated IL-17A, together with higher IFNγ, in the proximal colon at day 4 post-DSS (Figure 5b). Samples from distal colon did not show differences between both mouse genotypes for these cytokine mRNAs (Figure 5b).
Figure 4.
DSS-induced colitis in wild type and Cd4CreNfat5fl/fl mice. (a) Colon length of wild type and Cd4CreNfat5fl/fl mice before a 5-day DSS treatment, and 2 or 4 days after stopping DSS administration. Each circle shows an individual mouse, and bars within sample groups represent the mean±s.e.m. (8–9 mice). Significance was determined by an unpaired t-test. ***P<0.001 and **P<0.01. (b) Mouse body weight shown relative to each mouse just before DSS administration (day 0). Mean±s.e.m. of 18 mice of each genotype at day 0, 18 mice after 5 days of DSS, 17 wild type and 18 Cd4CreNfat5fl/fl mice at day 2 post-DSS, and 8 wild type and 9 Cd4CreNfat5fl/fl mice at day 4 post-DSS. Significance was determined by an unpaired t-test. *P<0.05. (c) Representative colon histology (magnification × 10) with hematoxylin and eosin in untreated mice, and at days 2 and 4 post-DSS. (d) Extent of colon lesions at days 2 (left) and 4 post-DSS (right). Circles indicate individual mice and bars represent the mean±s.e.m. (n=8), with significance determined by an unpaired t-test with Welch's correction. **P<0.01.
Figure 5.
Cytokine mRNA expression in mesenteric lymph nodes and colon of DSS-treated wild type and Cd4CreNfat5fl/fl mice. (a) Wild-type and Cd4CreNfat5fl/fl mice were treated with DSS during 5 days, and analyzed 2 or 4 days after stopping DSS administration. Mesenteric lymph nodes were isolated and lysed for mRNA analysis. Results are from the same groups of mice shown in Figure 4. Graphics show expression of the indicated mRNAs relative to L32 in untreated mice or mice analyzed 2 and 4 days after the 5-day DSS treatment. Circles indicate individual mice and bars represent the mean±s.e.m. (n=7–8 mice). Significance was determined by an unpaired t-test. **P<0.01. (b) Schematic diagram of a mouse colon with proximal and distal sections indicated. Expression of the indicated mRNAs in sections of proximal (upper panels) and distal (lower panels) colon in wild type (WT) and Cd4CreNfat5fl/fl (KO) mice before and after DSS treatment. Each circle shows an individual mouse, and bars within sample groups represent the mean±s.e.m. (n=8–9 mice). Significance was determined by an unpaired t-test with Welch's correction (IFNγ), and a nonparametric Mann–Whitney test when samples did not follow Gaussian distributions (IL-17A). *P<0.05. NA, not analyzed.
In both experiments we found comparable expression of the macrophage marker CD11b in mLN of DSS-treated mice of both genotypes, but higher expression of inducible nitric oxide synthase (iNOS) mRNA and reduced expression of mannose receptor (MRC1) in Cd4CreNfat5fl/fl mice, which was consistent with a pro-inflammatory shift in local macrophages (Figure 5a and Supplementary Figure S5c). In these samples, arginase 1 (Arg1) expression was also enhanced. Arg1 is considered a marker of macrophages associated with healing responses in inflammatory contexts, but can be induced by pro-inflammatory stimuli in tissue-resident macrophages.54, 55, 56 The simultaneous elevation of both Arg1 and iNOS in DSS-treated Cd4CreNfat5fl/fl mice could reflect a heightened state of inflammation in these mice.
We also analyzed the expression of aldose reductase (AR), a general hypertonic stress marker in lymphocytes and other cell types,10, 30 as an indirect way to monitor whether the local tissue exhibited and osmostress response. We did not find increased expression of AR in colons of DSS-treated mice (Figure 5b), which suggested that enhanced cytokine expression in Cd4CreNfat5fl/fl mice did not coincide with a sustained osmotic stress response.
The results of the DSS experiments suggest that whereas lack of NFAT5 in T cells led to a more pronounced Th1 and Th17 response, accompanied by enhanced expression of macrophage inflammatory markers, it also produced a progressive elevation of CTLA4 by day 4 post-DSS. An enhanced regulatory response could result from the initially heightened inflammation, and reflect its resolution towards recovery. In this regard, Th and Treg markers were both downregulated by day 6 post-DSS as mice recovered body weight and colon length (Supplementary Figure S5). These data, together with the in vitro Th1 cultures and in vivo T-cell stimulation with anti-CD3, depict a repeatedly altered pattern of enhanced pro-inflammatory markers associated with the lack of NFAT5 in T cells. Altogether, these results suggest that NFAT5 could play a role in T cells as attenuator of potentially pathogenic responses in vivo.
Discussion
In this work we show that NFAT5 may play different roles as a regulator of CD4 T cells depending on the stimulatory context, and we identify an osmostress-independent capacity of this factor to regulate Th and Treg features in activated T cells and attenuate pathogenic inflammatory responses.
Under hypernatremia, NFAT5 enhanced the expression of IL-2 and several Th17 gene products. Early works had shown that osmotic stress enhanced IL-2 induction in activated T cells,57 but its dependence on NFAT5 had not been tested. NFAT5 also enhanced expression of RORγt and IL-23R, but was not necessary for hypernatremia-induced IL-17A expression. Instead, Il-17A induction under osmotic stress was likely sustained by RORγt even in NFAT5-deficient T cells that expressed reduced RORγt levels. Hypernatremia also impaired the induction of the Th2-associated markers GATA3 and IL-4, but this inhibitory effect did not involve NFAT5. Recent works have described the costimulatory effect of osmotic stress on cytokine-induced Th17 responses.14, 23 Our results further show that even in the absence of exogenous polarizing cytokines, sustained hypernatremia can bias T-cell activation towards a Th17-like phenotype, in part through NFAT5-mediated induction of RORγt.
In contrast to osmostress conditions, a deficiency of NFAT5 in T cells led to enhanced IFNγ and IL-17A expression in different in vivo experimental settings. In DSS-induced colitis, Cd4CreNfat5fl/fl mice exhibited an extended window of elevated IFNγ and IL-17A mRNA in mLNs and colon, paralleled by increased CTLA4 expression. Simultaneously enhanced expression of IFNγ, IL-17A and CTLA4 in day 4 post-DSS samples in Cd4CreNfat5fl/fl mice could reflect a heightened inflammatory state progressing towards resolution, since these markers were all downregulated by day 6 post-DSS as mice recovered body weight and colon length. In this regard, the inflammatory macrophage marker inducible nitric oxide synthase was more expressed in mLN of DSS-treated Cd4CreNfat5fl/fl mice while expression of MRC1 was reduced, an observation consistent with the effect of IFNγ on macrophages and a pro-inflammatory shift in them.55 On the other hand, arginase 1 expression was also enhanced in Cd4CreNfat5fl/fl mice. Although arginase 1 is often considered a marker of anti-inflammatory or M2-spectrum macrophages, particularly in macrophages differentiated from bone marrow in vitro (BMDM), it can be induced by pro-inflammatory stimuli, such as Toll-like receptor ligands and in the presence of IFNγ in tissue-resident macrophages.54, 55, 56 It is likely that these changes in different macrophage activity markers reflect a state of ongoing unresolved inflammation, which would be consistent with the exacerbated intestinal pathology in Cd4CreNfat5fl/fl mice.
While we found increased IFNγ and IL-17A mRNA in NFAT5-deficient T cells in both in vivo stimulation with anti-CD3 and the DSS experiments, these assays showed a divergence in FOXP3 and CTLA4 expression. This could reflect differences between the two types of experiments in the kinetics of T-cell activation or in stimulatory conditions to which T cells were exposed in vivo, and suggests that the impact of NFAT5 on T-cell gene profiles could vary depending perhaps on the cytokine microenvironment or stress conditions. This possibility agrees with our in vitro assays, in which we found that NFAT5-deficient T cells induced more IFNγ than wild-type ones upon prolonged stimulation with IL-12, had no obvious defects in FOXP3 or Th17 markers under respective Treg- or Th17-inducing conditions, and exhibited reduced expression of Th17 markers and IL-2 under osmotress.
Our findings suggest that NFAT5 does not regulate a fixed program of T-cell polarization. While we observed a repeated trend of NFAT5 attenuating IFNγ expression in cultured Th1 cells and in vivo, we also found it to regulate different gene patterns in DSS-induced colitis than in anti-CD3 treated mice, or in vitro under hypernatremia. Our results open the obvious question of how can NFAT5 regulate diverse genes in different stimulatory settings. One possibility could be that NFAT5 acted as an opportunistic, context-dependent regulator. Unlike the calcineurin-activated NFATc proteins, whose nuclear translocation is tightly controlled in a stimulus-dependent manner, NFAT5 is distributed in both the nucleus and cytoplasm in T cells.8, 12, 24 The constitutive nuclear residency of NFAT5 could facilitate its access to target genes, although subordinated to a permissive chromatin configuration conferred by specific stimulatory and stress conditions. This dependence has been shown in macrophages, where NFAT5 is constitutively bound to the Tnf promoter already in unstimulated conditions, but depends on Toll-like receptor stimulation and histone deacetylation to bind to the Nos2 promoter.32 One example of a T-cell transcription factor dependent on the chromatin configuration context is STAT5. In response to IL-2 signaling STAT5 translocates to the nucleus but cannot activate transcription unless simultaneous TCR signaling decondenses chromatin to allow its access to target genes.58 Another one is c-Myc, which does not control a fixed set of genes in lymphocytes but regulates those that have been previously activated and exhibit an open chromatin configuration.59 In line with these observations, it would not be unexpected that in different in vivo microenvironments, where T cells are subjected to multiple inputs, some of them instructing them in different directions, NFAT5 could influence different T cells features asymmetrically depending on the stimulatory and stress context. Further research will be needed to identify direct NFAT5 target genes in various phases of T-cell activation or polarization by different inducers, as well as exploring its ability to regulate gene patterns indirectly by influencing other signaling and transcription regulators in specific stimulatory contexts.
Our finding that lack of NFAT5 in T cells could worsen inflammatory processes is in line with the recent characterization of the first human patient identified to date with an NFAT5 haploinsufficiency,60 who presented with an autoimmune enterocolopathy with symptoms resembling inflammatory bowel disease. T lymphocytes of this patient expressed NFAT5 to about 20% of the level found in healthy individuals and had reduced capability to resist hypertonic stress. Intriguingly, in the absence of hypernatremia the deficiency in NFAT5 affected CD4 and CD8 T cells differently: while the patient's CD4 T cells induced tumor necrosis factor-α and IFNγ normally after short-term unpolarized stimulation, his CD8 T cells had reduced production of both cytokines.60 Although this study did not analyze the patient's T-cell responses at longer time points, the lack of defects in early CD4 T-cell responses coincide with our results in NFAT5-deficient T cells. The patient also had fewer NK cells and an altered distribution of B cell subsets in comparison with healthy controls, indicating that insufficient expression of NFAT5 was associated with perturbations in diverse leukocyte populations. The same study also found that patients with ulcerative colitis and Crohn's disease showed reduced levels of NFAT5 mRNA in intestinal tissue compared with healthy controls.60 The association observed in this study, between reduced NFAT5 expression and enhanced intestinal inflammation, is intriguing in view of our results showing that a deficiency in NFAT5 in T cells could exacerbate colitis in an experimental mouse model. Although the intestinal inflammatory disease in this patient likely involves alterations in other cell subsets besides T lymphocytes and may not be directly comparable with our T-cell-specific Nfat5 deletion mouse model, it is nonetheless suggestive that in both scenarios a deficiency in NFAT5 was associated with T-cell dysfunctions in the absence of osmotic stress and exacerbation of inflammatory processes.
The ability of NFAT5 to regulate osmostress-independent functions in T cells has remained relatively unexplored. Our results here indicate that in non-osmostress conditions NFAT5 can modulate different T-cell functions than in response to hypernatremia, and play a significant role in attenuating pathologic inflammatory responses. These findings, together with recent works identifying NFAT5 as a regulator of early stages of thymocyte differentiation 35 and links between NFAT5 and autoimmune disease in humans,60 are beginning to uncover new roles for this factor in T-cell biology, as well as opening questions for future studies.
Methods
Mice
Cd4CreNfat5wt/wt (wild type) and Cd4CreNfat5fl/fl mice19, 61 were bred and housed in specific pathogen-free conditions at the animal facility of the Biomedical Research Park of Barcelona (PRBB). For DSS-induced colitis experiments, mice were kept at the animal facility of Universidad de Cantabria. Animal handling and experiments were in accordance with protocols, respectively, approved by the ethics committee of the PRBB Animal Care and Use Committee, or the Cantabria University Institutional Laboratory Animal Care and Use Committee, and carried out in accordance with the Declaration of Helsinki and the European Communities Council Directive (86/609/EEC).
CD4 T-cell isolation and culture
Mouse CD4+ T cells were obtained from spleens and lymph nodes of Cd4CreNfat5wt/wt (wild type) and Cd4CreNfat5fl/fl mice. Spleen and lymph nodes were disaggregated and CD4+ T cells were isolated with the CD4+ isolation magnetic bead system Dynabeads/Detachabead (Invitrogen Dynal AS, Oslo, Norway; catalog 11445D and 12406D, respectively) according to the manufacturer's instructions. Cells were activated with hamster anti-mouse CD3 (clone 145-2C11) (1 μg per 106 cells) plus hamster anti-mouse CD28 antibodies (1 μg per 106 cells) (BD Biosciences, San Jose, CA, USA; catalog 553058 and 553295) on 12-well plates coated with goat anti-hamster-IgG (9.5 μg cm−2, CAPEL; MP Biomedicals, Solon, OH, USA; catalog 55397) and cultured in DMEM culture medium (Life Technologies, Rockville, MD, USA) supplemented with 10% fetal bovine serum (Life Technologies), non-essential amino acids (Life Technologies), 2 mm l-glutamine (Life Technologies), 50 μm β-mercaptoethanol (Life Technologies), 10 mm HEPES (Lonza, Basel, Switzerland), 1 mm sodium pyruvate (Life Technologies) and penicillin and streptomycin (Life Technologies).19 For cultures lasting longer than 48 h, 5 ng ml−1 recombinant human IL-2 (Proleukin; Chiron, formerly Eurocetus, Emeryville, CA, USA) was added at 48 h. For Th17 conditions, cells were stimulated with antibodies specific for CD3 and CD28 plus 10 ng ml−1 IL-6 (ImmunoTools, Friesoythe, Germany; catalog 12340063) and 2.5 ng ml−1 TGFβ (PeproTech, Rocky Hill, NJ, USA; catalog 100-21). For Th1 conditions 5 ng ml−1 IL-12 (PeproTech; catalog 210-12) was added. For Treg conditions 5 ng ml−1 TGFβ (PeproTech; catalog 100-21) and 5 ng ml−1 recombinant human IL-2 were added. Rapamycin (Calbiochem, San Diego, CA, USA) was used at 50 nm, FK506 (Calbiochem) at 100 nm and digoxin (Sigma-Aldrich, St Louis, MO, USA) at 10 μm.
Hypertonic stress
The osmolality of the culture medium was measured in a VAPRO 5520 vapor pressure osmometer (Wescor, Logan, UT, USA). As the osmolality of the T-cell medium with supplements was 330 mOsm kg−1, 10% sterile endotoxin-free water (Grifols, Barcelona, Spain) was added for adjusting it to 300 mOsm kg−1. This medium was made hipertonic by adding NaCl from a 4 m sterile stock solution. Over the baseline of 300 mOsm kg−1 (isotonic conditions), addition of 60 mm NaCl raised the osmolality to 420 mOsm kg−1. This value is comparable to that measured in the skin of rodents after a prolonged high-salt-diet or in the interstitial fluid of infected wounds in mice and humans.2, 22
Chromatin immunoprecipitation
CD4+ T cells stimulated with anti-CD3 and anti-CD28 antibodies were grown for 3 days in IL-2-containing medium, then cleared of dead cell and debris by centrifugation on Lymphoprep (StemCell Technologies, Vancouver, BC, Canada; catalog 07801), and activated again for 12 h with hamster anti-mouse CD3 (1 μg per 106 cells) plus hamster anti-mouse CD28 antibodies (1 μg per 106 cells) in plates coated with goat anti-hamster IgG, in media at 300 or 500 mOsm kg−1. Cells (16 × 106) were fixed with 1% formaldehyde for 10 min at room temperature. Formaldehyde was quenched with glycine (final concentration of 125 mm) for 5 min. After washing cells once with cold phosphate-buffered saline and once with phosphate-buffered saline with 1 mm phenylmethylsulfonyl fluoride (PMSF), cells were lysed in 175 μl of lysis buffer (50 mm Tris-HCl pH 8, 10 mm EDTA, 1% SDS, 1 mm PMSF, 5 μg ml−1 leupeptin, 5 μg ml−1 aprotinin, 1 μg ml−1 pepstatin A, 10 mm NaF, 10 mm sodium orthovanadate and 10 mm β-glycerophosphate) for 30 min on ice and stored at −80 °C for at least 1 h. Lysates were sonicated with a bath sonicator (Bioruptor; Diagenode, Liège, Belgium) for six cycles of 30 s on and 30 s off on high power to obtain DNA fragments between 500 and 1000 bp, and centrifuged at 16 000 g to remove insoluble debris. Supernatants were collected and 6% of each sample was separated to use as a measure of chromatin imput for normalization. The rest of the sample was diluted 10 times in ChIP dilution buffer (1% TX-100, 20 mm Tris-HCl pH 8, 2 mm EDTA, 150 mm NaCl, 1 mm PMSF, 5 μg ml−1 leupeptin, 5 μg ml−1 aprotinin, 1 μg ml−1 pepstatin A, 10 mm NaF, 10 mm sodium orthovanadate and 10 mm β-glycerophosphate). Samples were precleared for 1 h with protein A-sepharose beads (Amersham Biosciences, Uppsala, Sweden; catalog 17-0780-01) that were previously preadsorbed with fish sperm DNA (Roche, Indianapolis, IN, USA; catalog 11 467 001) and bovine serum albumin (New England Biolabs, Beverly, MA, USA; catalog B9001S) overnight at 4 °C. After removing the preclearing beads, 20 μl preimmune serum, or a mixture of two rabbit polyclonal NFAT5-specific antibodies that recognize its N-terminal and DNA-binding domain regions8 (10 μl of each), were added to the lysates and incubated overnight at 4 °C. Preadsorbed protein A-sepharose beads were then added, incubated for 1 h at 4 °C and then washed three times with washing buffer (0.1% SDS, 0.1% TX-100, 20 mm Tris-HCl pH 8, 2 mm EDTA and 150 mm NaCl) and once with final washing buffer (0.1% SDS, 1% TX-100, 20 mm Tris-HCl pH 8, 2 mm EDTA and 500 mm NaCl). For DNA elution, beads were incubated with elution buffer (1% SDS and 100 mm NaHCO3) for 15 min at room temperature in a shaker. To reverse the crosslinking, samples were incubated overnight at 65 °C with 6 ng μl−1 RNase (Roche; catalog 11 119 915 001) and DNA was purified using the phenol/chloroform protocol. DNA was subjected to real-time quantitative PCR. DNA from each sample was normalized to its respective chromatin input. Primer sequences for the PCR reactions were: Rorc intron 2, forward: 5′-TgT ACC ACA CTg gTT ATg gC-3′, reverse: 5′-CTT CAC CAA gTg ACATCA gg-3′ Akr1b3 (AR) promoter, forward: 5′-CAC CAg AAT TTC CAC ATg CC-3′, reverse: 5′-Agg gAC AAC TgC ATC TgC AA-3′.
Real-time quantitative PCR and mRNA analysis
Total RNA was isolated using the High Pure RNA isolation kit (Roche; catalog 11 828 665 001), quantified in a NanoDrop (ND-1000) spectrophotometer and total RNA (100 ng to 1 μg) was retro-transcribed to cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche; catalog 04 897 030 001). Real-time quantitative PCR was performed with LightCycler 480 SYBR Green I mix (Roche; catalog 04 887 352 001) and analyzed with LightCycler Software, version 1.5 (Roche). Samples were normalized to L32 mRNA. The list of primers used is provided in Supplementary Table S1.
Enzyme-linked immunosorbent assay
Cell-free supernatants from cultures of isolated CD4 T cells (1 × 106 cells per ml) were harvested and IFNγ was measured by enzyme-linked immunosorbent assay (eBiosciences, San Diego, CA, USA; catalog 88-7314).
Protein sample preparation and western blot analysis
CD4+ T cells grown for 5 days were cleared of dead cell and debris by centrifugation on Lymphoprep (StemCell Technologies; catalog 07801), activated with hamster anti-mouse CD3 (1 μg per 106 cells) plus hamster anti-mouse CD28 antibodies (1 μg per 106 cells) and cultured in plates coated with goat anti-hamster IgG in isotonic medium, hypertonic medium, or isotonic medium plus TGFβ and IL-6 (Th17 stimulation). Cells were washed twice with cold phosphate-buffered saline at the same osmolality as the culture medium and lysed (8 × 106 cells in 50 μl) for 30 min at 4°C in Nonidet P-40 lysis buffer (50 mm HEPES pH 7.4, 80 mm NaCl, 5 mm MgCl2, 10 mm EDTA, 5 mm sodium pyrophosphate, 1% Nonidet P-40, 2 mm PMSF, 5 μg ml−1 leupeptin, 5 μg ml−1 aprotinin, 1 μg ml−1 pepstatin A, 10 mm sodium orthovanadate and 20 mm β-glycerophosphate). Lysates were cleared by centrifugation at 16 000 g for 8 min at 4 °C and supernatants were collected. Protein concentration was measured using the BCA Protein Assay (Pierce, Rockford, IL, USA; catalog 23227). Lysates were then boiled in reducing Laemmli buffer and proteins (50 μg per lane) were separated in sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto nitrocellulose Protran membranes (Whatman, Dassel, Germany; catalog 10401396) in 25 mm Tris, 192 mm glycine and 20% methanol. Membranes were blocked in Tris-buffered saline (20 mm Tris-HCl pH 7.6, 150 mm NaCl) containing 5% bovine serum albumin (BSA) for 1 h at room temperature. Membranes were probed overnight at 4°C with specific antibodies: rabbit polyclonal antibody anti-phospho-STAT3(Tyr705) (Cell Signaling Technology, Danvers, MA, USA; catalog 9131) or goat polyclonal antibody anti-pyruvate kinase (Chemicon, Temecula, CA, USA; catalog AB1235). Proteins were detected with HPR-labeled secondary antibodies and enhanced chemiluminescence (Supersignal West Pico Chemiluminescent Substrate, Pierce; catalog; 34080).
In vivo treatment with anti-CD3 antibody
Cd4CreNfat5wt/wt (wild type) and Cd4CreNfat5fl/fl mice were injected intraperitoneally with a stimulatory antibody to CD3 (clone 145-2C11) (0.8 mg kg−1) at 0 and 48 h. CD4+ T cells were isolated from mesenteric lymph nodes 4 h after the second injection with the CD4-positive isolation magnetic bead system (Dynabeads, Invitrogen Dynal AS; catalog 11445D).
Intracellular cytokine staining
Mice were injected intraperitoneally with antibody to CD3 as described above at day 0 and again 48 h later. Mice received intraperitoneal brefeldin A (4 mg kg−1)43 (Sigma-Aldrich; catalog B7651) together with the second anti-CD3 injection. Mesenteric lymph nodes were harvested 4 h later and processed for intracellular cytokine staining using the eBioscience ICS kit and protocol (catalog 00-5123-43 and 00-5233-56). Before fixing cells, an aliquot was separated for isolating CD4+ lymphocytes for RNA extraction. Cells used for intracellular cytokine staining were stained with PECy5-conjugated rat anti-mouse CD4 antibody (eBioscience; 15-0042-83) before fixing and permeabilizing. Antibodies used for flow cytometry were PE-conjugated rat anti-mouse IFNγ antibody (BD Biosciences; catalog 554412), PE-conjugated rat anti-mouse FOXP3 antibody (eBioscience; catalog 12-5773-80) and APC-conjugated hamster anti-mouse CTLA4 antibody (eBioscience; catalog 17-1522-80). Samples were analyzed with a BD LSR-II flow cytometer (BD Biosciences) and cytometry software FlowJo 7.5 (FlowJo, LLC, Ashland, OR, USA).
DSS-induced colitis
Colitis was induced by 3% (w/v) DSS (molecular weight 36000–50000; ICN Biochemicals, Aurora, OH, USA) added to the drinking water for 4 or 5 days as indicated in the figures. Mice were weighted every day during and after DSS treatment, and were killed for further analysis at days 4 and 6 after DSS treatment for the first experiment, and days 2 and 4 post-DSS for the second experiment. Colon length was measured for assessing inflammation and proximal and distal regions were removed for histology and mRNA analysis. Sections (4 μm) of paraffin-embedded tissues were stained with hematoxylin and eosin. For the histological analyses, images were captured and quantified with an Axio Scan Z1 equipped with the ZEN 2012 (blue edition) software (Zeiss Iberica, Madrid, Spain). Whole mesenteric lymph nodesand colon sections were also obtained for mRNA analysis.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Significance of the differences between sets of experimental data was determined with an unpaired Student's t-test, with Welch's correction whenever variances were significantly different between the compared groups, with a one-sample t-test for comparison of samples in one experimental condition with a reference control sample, or with a nonparametric Mann–Whitney test when samples did not follow Gaussian distributions.
Acknowledgments
We especially acknowledge Katherine Drews-Elger for initial experiments characterizing NFAT5-deficient T cells. We also thank Iván Gómez for technical assistance, and members of the JA and CL-R groups for stimulating and helpful discussions. JA was funded by the Spanish Ministry of Economy and Competitiveness (SAF2011-24268, cofunded by the European Regional Development Fund), and Fundació la Marató TV3 (122530). CL-R was funded by the Spanish Ministry of Economy and Competitiveness (SAF2012-36535, SAF2015-71363-R, cofunded by the European Regional Development Fund). CL-R is a recipient of an ICREA Acadèmia award from Institució Catalana de Recerca i Estudis Avançats (ICREA, Generalitat de Catalunya). JA and CL-R also acknowledge funding support from Generalitat de Catalunya (2009SGR601, 2014SGR1153) and the Spanish Ministry of Economy and Competitiveness, through the 'María de Maeztu' Program for Units of Excellence in R&D (MDM-2014-0370). RM was funded by the Spanish Ministry of Economy and Competitiveness (SAF2011-22463 and SAF2014-55088-R, cofunded by the European Regional Development Fund). MA was supported by predoctoral fellowships from Generalitat de Catalunya (FI-DGR program 2009) and the Spanish Ministry of Education, Culture and Sports (FPU program, AP2010-5411). MI was partially supported by a grant from the Spanish Ministry of Economy and Competitiveness (IPT2011-1527-010000). ST is the recipient of a predoctoral fellowship of the Spanish Ministry of Economy and Competitiveness (BES-2013-062670).
Author contributions
MA performed most of the experiments, analyzed them, generated the data for figures and prepared the initial figure drafts; MI performed the DSS treatments, collected data on mouse weight and colon length, and helped MA in harvesting tissue samples for mRNA analysis and histology; ST performed in vivo experiments with anti-CD3, intracellular flow cytometry and contributed gene expression analyses in various experiments; RM did the histopathology analysis of colon samples and provided guidance on DSS treatment protocols and data interpretation; CL-R helped in setting up T lymphocyte assays, provided expert background on NFAT5-deficient mouse models and feedback in data interpretation and project planning; JA designed the study, analyzed the data, prepared figure layout and wrote the manuscript. All authors actively contributed with discussion and feedback throughout the drafting of the manuscript to its final version.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I et al. Cutaneous na(+) storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 2015; 21: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM, Michelini RH, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol 2012; 12: 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med 2013; 210: 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Oh S, Lee D, Oh HJ, Park JY, Lee SB et al. Mst1-FoxO signaling protects naïve T lymphocytes from cellular oxidative stress in mice. PLoS ONE 2009; 4: e8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang H-YY, Jinasena D, Yu H, Zheng Y et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011; 146: 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011; 208: 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA 1999; 96: 7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 1999; 96: 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Drews-Elger K, Estrada-Gelonch A, Minguillon J, Morancho B, Santiago V et al. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol 2006; 72: 1597–1604. [DOI] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J Biol Chem 2008; 283: 7309–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity 2001; 15: 47–58. [DOI] [PubMed] [Google Scholar]
- Kino T, Takatori H, Manoli I, Wang Y, Tiulpakov A, Blackman MR et al. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal 2009; 2: ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013; 496: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA 2004; 101: 2392–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka T, Takahashi S, Tsutsumi Z, Moriwaki Y, Yamamoto T, Fukuchi M. Hyperosmolar non-ketotic diabetic syndrome associated with rhabdomyolysis and acute renal failure: a case report and review of literature. Diabetes Nutr Metab 2003; 16: 317–322. [PubMed] [Google Scholar]
- Dogan E, Erkoc R, Sayarlioglu H, Buyukbese A. Nonketotic hyperosmolar coma in a patient with type 1 diabetes-related diabetic nephropathy: case report. Adv Ther 2005; 22: 429–432. [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Kipourou K, Manta C, Tapaki G, Philippidis P. Adipsic hypernatremia syndrome in infancy. J Pediatr Endocrinol Metab 1997; 10: 547–550. [DOI] [PubMed] [Google Scholar]
- Berga-Bolanos R, Drews-Elger K, Aramburu J, Lopez-Rodriguez C. NFAT5 regulates T lymphocyte homeostasis and CD24-dependent T cell expansion under pathologic hypernatremia. J Immunol (Baltimore, MD 1950) 2010; 185: 6624–6635. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 1998; 273: 4296–4299. [DOI] [PubMed] [Google Scholar]
- Yun J, Schoneberg T, Liu J, Schulz A, Ecelbarger CA, Promeneur D et al. Generation and phenotype of mice harboring a nonsense mutation in the V2 vasopressin receptor gene. J Clin Invest 2000; 106: 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009; 15: 545–552. [DOI] [PubMed] [Google Scholar]
- Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013; 496: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol 2002; 169: 5477–5488. [DOI] [PubMed] [Google Scholar]
- Trama J, Lu Q, Hawley RG, Ho SN. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol 2000; 165: 4884–4894. [DOI] [PubMed] [Google Scholar]
- Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS. Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP). J Biol Chem 2002; 277: 46085–46092. [DOI] [PubMed] [Google Scholar]
- Morancho B, Minguillon J, Molkentin JD, Lopez-Rodriguez C, Aramburu J. Analysis of the transcriptional activity of endogenous NFAT5 in primary cells using transgenic NFAT-luciferase reporter mice. BMC Mol Biol 2008; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irarrazabal CE, Liu JC, Burg MB, Ferraris JD. ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 2004; 101: 8809–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris JD, Persaud P, Williams CK, Chen Y, Burg MB. cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/osmotic response element-binding protein. Proc Natl Acad Sci USA 2002; 99: 16800–16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortells MC, Morancho B, Drews-Elger K, Viollet B, Laderoute KR, Lopez-Rodriguez C et al. Transcriptional regulation of gene expression during osmotic stress responses by the mammalian target of rapamycin. Nucleic Acids Res 2012; 40: 4368–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JA, Kwon HM, Wamhoff BR. Tonicity-independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). AJP Cell Physiol 2011; 302: C1–C8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxade M, Lunazzi G, Minguillon J, Iborra S, Berga-Bolanos R, Del Val M et al. Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J Exp Med 2012; 209: 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N-H, Choi S, Han E-J, Hong B-K, Choi SY, Kwon HM et al. The xanthine oxidase-NFAT5 pathway regulates macrophage activation and TLR-induced inflammatory arthritis. Eur J Immunol 2014; 44: 2721–2736. [DOI] [PubMed] [Google Scholar]
- Ranjbar S, Tsytsykova AV, Lee SK, Rajsbaum R, Falvo JV, Lieberman J et al. NFAT5 regulates HIV-1 in primary monocytes via a highly conserved long terminal repeat site. PLoS Pathog 2006; 2: e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berga-Bolaños R, Alberdi M, Buxadé M, Aramburu J, López-Rodríguez C. NFAT5 induction by the pre-T-cell receptor serves as a selective survival signal in T-lymphocyte development. Proc Natl Acad Sci USA 2013; 110: 16091–16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Leung MWL, Huang P, Ryan DA, Krout MR, Malapaka RR V et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 2011; 472: 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F et al. A validated regulatory network for Th17 cell specification. Cell 2012; 151: 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 2009; 31: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009; 30: 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol (Baltimore, MD 1950) 2007; 178: 4901–4907. [DOI] [PubMed] [Google Scholar]
- Merger M, Viney JL, Borojevic R, Steele-Norwood D, Zhou P, Clark DA et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut 2002; 51: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY et al. Control of TH17 cells occurs in the small intestine. Nature 2011; 475: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood 2011; 118: 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis 2012; 3: e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nuñez G, He Y et al. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 2015; 43: 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 2009; 31: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med 2007; 204: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 2009; 15: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez-Andrade N, Lamana A, Sancho D, Gisbert JP, Gonzalez-Amaro R, Sanchez-Madrid F et al. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J Pathol 2011; 224: 212–221. [DOI] [PubMed] [Google Scholar]
- Brown JB, Cheresh P, Zhang Z, Ryu H, Managlia E, Barrett TA. P-selectin glycoprotein ligand-1 is needed for sequential recruitment of T-helper 1 (Th1) and local generation of Th17 T cells in dextran sodium sulfate (DSS) colitis. Inflamm Bowel Dis 2012; 18: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama M, Hokari R, Hozumi H, Kurihara C, Ueda T, Watanabe C et al. HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J Leukoc Biol 2012; 91: 901–909. [DOI] [PubMed] [Google Scholar]
- Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol 2006; 146: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity 2010; 32: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 2008; 9: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem 2003; 278: 4590–4596. [DOI] [PubMed] [Google Scholar]
- Rawlings JS, Gatzka M, Thomas PG, Ihle JN. Chromatin condensation via the condensin II complex is required for peripheral T-cell quiescence. EMBO J 2011; 30: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W et al. c-Myc Is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 2012; 151: 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland BS, Widjaja CE, Banno A, Zhang B, Kim SH, Stoven S et al. Immunodeficiency and autoimmune enterocolopathy linked to NFAT5 haploinsufficiency. J Immunol 2015; 194: 2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews-Elger K, Ortells MC, Rao A, Lopez-Rodriguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS ONE 2009; 4: e5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.